Abstract
Groundwater is an important resource for domestic and irrigation purposes in the Yinchuan Region, northwest China. However, the groundwater quality in this area is degrading because of human activities, and this adversely affects human health. In an effort to quantitatively describe the pollution status of groundwater and its influence on human health, comprehensive water quality index was used for drinking water quality assessment, and salinity–alkalinity method was applied to evaluate the irrigation water quality. Furthermore, the human health risk of the drinking groundwater has been evaluated with the model recommended by the United States Environmental Protection Agency. The results reveal that most of groundwater samples are suitable for irrigation except a few with high salinity and alkalinity. However, over 70 % of the phreatic water samples and nearly half of the confined water samples do not conform to the drinking water quality standard, and many parameters such as total dissolved solids, total hardness, and Na+ and SO4 2− exceed their limits. Noncarcinogenic risks of all collected water samples are relatively negligible. While carcinogenic risks in nearly 30 % of phreatic water samples and over 20 % of confined water samples surpass the acceptable level recommended by ICRP (5 × 10−5 per year), Cr6+ is the main influencing factor on the risk. In addition, the study shows that the severe groundwater pollution in the study area is conjunctively caused by industry and agricultural activities. Therefore, effective measures must be implemented to prevent further deterioration of water quality and reduce the health risks for local residents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an important resource for human survival, which, compared with surface water resource, has some advantages in that it usually has more even and stable spatiotemporal distribution, better water quality, and more constant water temperature. Thus, many countries and regions take groundwater as the main source for their water supply. It is estimated that about 50 % of domestic drinking water in France comes from groundwater, while 70 % of drinking water in China is groundwater (Tai et al. 2012). However, with the increase of population and economy, natural environment is changed, many industrial plants are built, and land cover is also altered, posing significant influences on water environment (Li et al. 2014a). Actually, groundwater pollution are mostly of human origin, such as industrial effluent, wastewater irrigation, land cover change, and excessive use of fertilizer and pesticide (Li et al. 2014a, 2016a). Common groundwater pollution includes mainly heavy metal pollution (Momot and Synzynys 2005; Wongsasuluk et al. 2014), nitrogen pollution (Deutsch et al. 2006; Jalali 2011; Kuhr et al. 2013; Li et al. 2016b; Mahvi et al. 2005), fluorine pollution (Daniele et al. 2013; Li et al. 2014b; Wu et al. 2015), arsenic pollution (Akter and Ali 2011; Nasrabadi and Bidabadi 2013; Uddin et al. 2006) as well as organic contamination (Manecki and Gałuszka 2012; Han et al. 2013). Especially, the huge land creation projects in loess areas of China and the revival of the Silk Road economic belt proposed by China may pose notable impacts on groundwater quantity and quality (Li et al. 2014a, 2015) which requires urgent and comprehensive research.
There are more and more cities and regions becoming short of water due to groundwater pollution. The human health risks due to water pollution are also becoming increasingly severe. According to the World Health Organization (WHO), 80 % of the diseases suffered by the citizens in the third world are caused by water pollution (WHO 2011). Most of the cities in northwest China use groundwater as the only supply of drinking water. Pollution of groundwater will have direct influences on the health of the citizens who drink contaminated water. Therefore, the prevention and control of groundwater pollution has gained much attention from the government and the public in recent years. For example, in October 2011, the Ministry of Land and Resources, the Ministry of Environmental Protection and the Ministry of Water Resources jointly issued the “National Planning of Groundwater Pollution Control (2011–2020) (Ministry of Environmental Protection of the PRC 2011), requiring all regions to build a system of groundwater pollution control within the time specified, and assess the pollution risk (Tai et al. 2012). Due to the increasing importance of groundwater quality evaluation and risk assessment, scholars have carried out a lot of studies on quality and risk assessment for different purposes by different techniques (Aksoy and Scheytt 2007; Bouderbala 2015). For example, water quality index (Li et al. 2010a), entropy weighted osculating value method (Li et al. 2010b), and rough sets attribute reduction (Li et al. 2012) have all been applied to evaluate groundwater quality. These studies have provided abundant techniques and experience for rational water quality management.
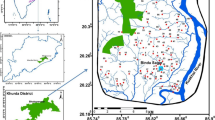
The Yinchuan Plain belongs to the midstream and upstream of the Yellow River and extends from the east foot of the Helan Mountains to the west bank of the Yellow River (Fig. 1). The area is wide and flat, with a nearly bimillennial reclamation history (Qian et al. 2013a). The area is studded with lakes of different sizes, and is an important production area for agriculture, forestry, animal husbandry, and fishery. Influenced by geographical and climatic factors, the ecological environment in the study area is very fragile (Qian et al. 2012). In recent years, rapid increases in urban sewage and waste discharge because of the expediting industry, agriculture, and urbanization have occurred in the plain, producing ecological damage, water resource shortage, and water pollution. Previous study by Ji et al. (2005) found that the concentrations of Fe and NH4 + in the confined groundwater significantly exceed the permissible limit for drinking water. Actually, groundwater pollution within the Yinchuan Region is not only limited to iron and nitrogen, but also carcinogens such as arsenic and chromium. Considering the importance of groundwater in this area, an assessment on the quality of drinking water and irrigation water is conducted in the study area, and the health risk assessment is also performed.
Study Area
The Yinchuan Region has a typical continental climate characterized by little rain, strong evaporation, and dry climate (Qian and Li 2011; Qian et al. 2013b). The rainy season is July, August, and September, and the rain in the rainy season accounts for 70 % of the total rainfall throughout the year. The landscape can be divided into inclined pluvial plain, pluvial–alluvial plain, alluvial–lacustrine plain (Qian et al. 2012). The Yellow River is a perennial river with large sediment concentration and an average salinity of 0.4 g/L. The stratum exposed in the study area is mainly quaternary stratum, divided into early, middle, and late Pleistocene and Holocene Series according to their ages. There are mainly four large fractures passing through the study area. In cross section, the Yinchuan basin sinks toward the center. The quaternary formation is deepest in the middle, and gradually becomes shallower toward the east and west sides.
The groundwater in the study area is pore-water within the unconsolidated sediments, which can be divided into phreatic and confined aquifers according to its formation conditions and distribution. The hydrogeological condition in the study area is clearly controlled by the lithological characteristics of the formations. Two zones can be divided. In the west, the inclined pluvial plain forms the single phreatic aquifer, and in the east it transits to multilayer structures (Fig. 1). The single phreatic aquifer is typically 230–265 m in thickness and gradually becomes thicker from south to north. The thickness of the phreatic aquifer within multilayer structure zone is only 20–60 m. Compared to phreatic aquifer, the confined water has deeper burial depth and better water quality. Therefore, the confined aquifers are the major abstracted aquifers for industrial and domestic water supply. The confined aquifer is mostly composed of fine sand, silty fine sand, and a small amount of medium sand. The confined aquifers are thin in the middle, and gradually become thicker toward the east and west.
Materials and Methods
Sampling and Measurements
To obtain representative data covering the study area, twenty-two phreatic water samples and eighteen confined water samples were collected from different boreholes (depth: 10–20 and 20–50 m, respectively) drilled for rural water supply (see Fig. 1). All the boreholes were sampled once between March to June, at the beginning of irrigation and just before summer rains occur. The collection process, storage, transport, and measurement of the samples were all conducted following APHA (2005). Tested parameters included total bacteria count (TBC), total hardness (TH), pH, potassium (K+), sodium (Na+), cadmium (Ca2+), Mg2+, nitrate-nitrogen (NO3-N), ammonia nitrogen (NH4-N), total iron (Fe), chloride (Cl−), sulfate (SO4 2−), HCO3 −, fluorine (F), total dissolved solids (TDS), volatile phenol, prussiate, arsenic (As), chromium (Cr6+), copper (Cu), zinc (Zn), manganese (Mn), and silicate (H2SiO3 −). pH was measured in the field with a portable pH meter (PHSJ-4A), Na+ and K+ were analyzed by flame photometer, SO4 2−, HCO3 −, Cl−, and F− were determined by ion chromatograph. The concentrations of NO3-N and NH4-N were measured by visible spectrophotometer. Multi-functional analyzer of heavy metals and ICP-MS were used to detect the heavy metals (Cu, Zn, Mn, Fe, and Cr6+) in the water samples, the results of these two methods are essentially in agreement with each other. In this study, the analyzed results of ICP-MS were adopted. In addition, As was determined by atomic fluorescence spectrometry method and the concentrations of volatile phenol and prussiate were measured by extraction spectrophotometric method and silver nitrate titration method, respectively. The total bacteria counts were analyzed by plate colony-counting method. Detailed measurement methods and instruments are shown in Table 1. Two parallel double samples have been tested for each sample. And eight of the forty samples were extracted and the recovery ratio of standard addition was tested. The results indicated that the relative standard deviation of parallel double samples were all below their maximum allowable values and the recovery ratio varies between 91 and 108 %, which showed the results were of good precision and accuracy (Ministry of Land and Resources of the People’s Republic of China 2006). Furthermore, the charge balance errors for the analyzed data were less than 5 % in most samples except few samples which have been eliminated in the study.
Water Quality Assessment
Drinking Water Quality Assessment
The underground water resources in the study area are used for domestic and irrigation purposes. The Comprehensive Water Quality Index (CWQI) recommended by the Quality Standard for Groundwater of the People‘s Republic of China (PRC) (Bureau of Quality and Technical Supervision of China 1994) was applied for drinking water quality assessment in the present study. The computing formula is as follows (Li et al. 2014c):
where F i is the evaluation value for each single component; \(\bar{F}\) is the average value of F i ; F max is the maximal value of F i ; n is the number of items. Table 2 shows the groundwater quality classification criteria using Eq. 1.
Irrigation Water Quality Assessment
Groundwater typically contains several different dissolved substances, some of which are necessary nourishment for crop growth, while some can be harmful in excessive, deteriorating the physicochemical property of the soil and constraining crop growth. Here, the salinity and alkalinity of the irrigation water were assessed. The salinity and alkalinity can be computed as follows (Li et al. 2013):
-
(1)
Salinity The saline level of the groundwater for irrigation is expressed by salinity in mmol/L, which represents the maximal hazardous content of aqueous NaCl and Na2SO4.
When [Cl−] + 2[SO4 2−] < [Na+], salinity = [Cl−] + 2[SO4 2−]
When [Na+] < [Cl−] + 2[SO4 2−], salinity = [Na+]
where all ions are expressed in mmol/L.
-
(2)
Alkalinity The alkali level of the groundwater for irrigation is expressed by alkalinity in mmol/L, which refers to the maximal hazardous content of aqueous Na2CO3 and NaHCO3.
Alkalinity = ([HCO3 −] + 2[SO4 2−]) − 2([Ca2+] + [Mg2+])
where, all ions are expressed in mmol/L.
When alkalinity < 0, salinity plays the leading role.
Health Risk Assessment
Health risk assessment refers to the process of estimating the nature and probability of adverse health effects in humans because of exposure to contaminants (Li and Qian 2011; Li et al. 2016c). The US Environmental Protection Agency (USEPA) puts forward a health risk assessment method for drinking water. The assessment mainly considers two exposure paths via ingestion and skin, and takes citizens’ daily water intake and the chemical elements contained in the water as the basic input data in the assessment. The assessment model is used in the present study to calculate the dosage of chemical carcinogens and noncarcinogens ingested by the human body when citizens drink the groundwater. The human health risk through drinking water intake can be calculated by the following equation (Li and Qian 2011):
where the \(R_{i}^{c}\) is carcinogenic risk value of single carcinogen; D is daily average exposure dosage of unit weight of the contaminants through drinking water intake [mg/(kg d’)]; \(R_{j}^{n}\) is the risk value of single noncarcinogen; D iRf is a reference dosage for noncarcinogenic risk through drinking exposure pathway [mg/(kg d)]; and S f is the carcinogenic slope factor of pollutants in [mg/(kg d)]. The specific values are shown in Table 3 (Li and Qian 2011; Ni et al. 2009; Geng et al. 2006; Ministry of Environmental Protection of the PRC 2014).
The daily average exposure dosage of unit weight of contaminants through drinking water intake can be calculated by Eq. 6 (Li et al. 2016c; Wu and Sun 2015):
where AT is the average exposure time (days, AT = 70 years = 25,550 days for carcinogenic risk and 10,950 days (30 years) for noncarcinogenic risk); C w is the pollutant concentration in the water; IR is the ingestion rate (L/d, IR = 2L/day in the study); EF is the exposure frequency of the exposed people (EF = 365 days/year); ED is the exposure period. The ED is 70 years for carcinogenic risk and 30 years for noncarcinogenic risk; BW is the average weight of the exposed person with 65 kg in the present study. The values for the AT, IR, EF, ED, and BW are derived from the field survey in Yinchuan of China. And before the final values were determined, the recommended values of Ministry of Environmental Protection of PRC (2011) have been referred to.
Results and Discussion
Hydrogeochemical Characteristics
Geologic structures and hydrogeological settings may cause variations in hydrochemical characteristics of groundwater in different locations. Groundwater in the Yinchuan Region has been heavily exploited, which has an important influence on the hydrochemical characteristics of the groundwater (Sun et al. 2007). As shown in Fig. 2, the main hydrochemical types for the phreatic water are SO4-Mg, Cl–Na, HCO3-Mg, and mixed type, while more than 80 % of the confined groundwater samples are classified as the HCO3 type, but with no dominated cations. In addition, the value of HCO3/SiO2 can be used to approximately estimate the main source of chemical components in the groundwater. When the value of HCO3/SiO2 is greater than 10, the chemical constituents in groundwater mainly come from the dissolution of carbonates, when HCO3/SiO2 is <5, the chemical constituents come from the dissolution of silicate rock, and when HCO3/SiO2 is 5–10, the chemical constituents are from both (a mixed type) (Kortatsi et al. 2008). The groundwater samples analyzed here all show a ratio of HCO3/SiO2 greater than ten, except C3 (7.80), indicating that the main constituents in the groundwater are from the dissolution of carbonate except C3 that can be considered a mixed origin. Furthermore, the sampling location of C3 is near a paper mill, and the groundwater is of Cl–Na·Mg type, indicating that the groundwater in this location is subject to serious industrial pollution.
From the analysis of hydrochemical types, it can be preliminarily judged that the groundwater samples from the phreatic aquifer are subject to more severe pollution than the confined samples. To further assess its pollution level and to determine whether it is suitable for human consumption, water quality assessment should be conducted.
Water Quality Assessment
Statistical Analysis of the Groundwater Quality
Statistical analysis results of the phreatic water samples are shown in Table 4. According to the statistical analysis, the highest value of the total bacteria count (TBC) in the phreatic water samples is 2900 cfu/mL and the average value is 540 cfu/mL. The TBC in 50 % of the phreatic water samples exceed the drinking water standard (100 cfu/mL), therefore the phreatic water samples in the study area should be boiled for disinfection before drinking. For the confined samples, as shown in Table 5, the highest value of TBC is 570 cfu/mL, and the average value is 72 cfu/mL. The TBC in 11.1 % of the confined samples exceeds the established limit. The TBC is influenced, to a greater extent, by human activities. In addition, for the parameters such as TDS, TH, Na+, SO 24 , and Fe, over 20 % of the phreatic water samples exceed the established limits. While in the confined aquifer, NH4-N exceeds the standard, accounting for 22.22 % of the total samples, and the exceeding rates for other parameters are all less than 20 %. The groundwater quality would be affected because so many parameters exceed their standard. Therefore, the assessment of groundwater quality is necessary according to the main application including drinking and irrigating in the study area.
Drinking Water Assessment
Drinking water quality was assessed using the CWQI, and the results are listed in Table 6 and delineated in Figs. 3 and 4. Table 6 shows that the values of F are in the range of 2.17–7.44, ranging from good quality water to extremely poor quality water subjected to the phreatic aquifer. Of the 22 unconfined samples, only seven are of good quality which can be used for drinking readily. Poor and extremely poor quality samples accounts for 68.18 % for the phreatic water. The severely pollution also has been plotted in Fig. 3 which shows that the water samples with extremely poor quality are mainly distributed in the southern and northern sides of the study area, and samples with good quality are mainly distributed in regions of single phreatic aquifer, while being poor in other regions. None of the samples within the entire study area meet the requirements of excellent water. To some extent, phreatic water is not quite suitable as a source of drinking water.
Compared with phreatic water samples, the overall quality of confined groundwater samples is much better, and nearly 50 % of confined groundwater samples were classified as “good quality”. Table 6 reflects that the values of F range from 2.15 to 7.65 with the average value of 3.87. There are two samples accounting for 11.12 % of the confined samples with F > 7.20, which means extremely poor water for drinking. As shown in Fig. 4, with the confined groundwater flowing from west to east, groundwater quality deteriorates gradually. It can be seen that there are very close relations and relatively consistent trend between the spatial distribution of groundwater levels and the hydrogeological conditions in confined aquifer. The water samples with good quality are mainly distributed in the western region of the study area. In this area, the topography is much steeper than any other places, and therefore the groundwater flow is faster here, indicating a shorter residence time, which weakened the interaction between the water and rock and produce less dissolution of minerals. The samples with extremely poor water quality are mainly distributed in the discharge region of groundwater, which is also the region with higher concentration of NH4-N. It is indicated that agricultural irrigation is the main factor causing groundwater quality deterioration (Ji et al. 2005). Furthermore, the topography is relatively flatter and evaporation is intense in this area, which are the nature factors leading to the deterioration of the groundwater quality.
Irrigation Water Quality Assessment
Groundwater is not only the main source for drinking water in the study area, but also an important irrigation water source. The irrigation water quality assessment can provide basis for the prevention and control of soil salinization and alkalization. The quality of groundwater for irrigation is determined by salinity and alkalinity and the classification is shown in Fig. 5 (Li et al. 2013). High salinity will constrain plant growth and increase Na+ accumulation in the roots and lower the stems of the plants (Sperling et al. 2014). High alkalinity irrigation will gradually cause growth inhibition and reduce stomata conductance of the plants (Aguilar 2004). In general, extremely high saline-alkali water is not suitable for irrigation (Esmaeili et al. 2014). Moreover, saline–alkali water can only be suitable for irrigation when it is used in some special ways (for example dilution) or for some specific plant species. While medium and good quality water is suitable for agricultural purpose and is helpful to prevent soil salinization if proper irrigation method is adopted.
The results of irrigation water quality assessment show (Fig. 5) that more than 80 % of the groundwater samples were plotted in zones A and B except only a few in zones C and D, indicating a good-to-medium quality of irrigation water. Three phreatic water samples and one confined sample plotted in zone C contain alkalinity in the range of 10–20 mmol/L and alkalinity in 4–8 mmol/L, suggesting that the water quality for these samples is influenced by alkalinity and salinity simultaneously and is unsuitable for irrigation. One confined groundwater sample C17 (located in XiGang Township, Henlan County) plotted in zone D contains high salinity (47.67 mmol/L) but negative alkalinity, indicating that salinity hazard is the main factor affecting the suitability of confined water for agricultural purpose in this location. The alkalinities of two phreatic groundwater samples (all located in an oil refinery in ShiliBu) plotted in zone D are 20.65 and 21.27 mmol/L, respectively, and the salinities are 5.15 and 10.84 mmol/L, respectively, indicating the unsuitability of quality water for irrigation.
Overall, the quality of groundwater in the study area is generally suitable for agricultural purpose, especially the confined groundwater. But some samples, especially phreatic groundwater samples out of Yinchuan City, contain high salinity and alkalinity and are unsuitable for irrigation, suggesting that proper measures need to be taken to prevent the soil salinization and alkalization.
Health Risk Assessment of Groundwater
According to the above analysis, the groundwater in the study area has been polluted to some degree. Main contaminants are TDS, Na+, SO4 2−, As, and Cr6+. Moreover, prussiate, F−, and volatile phenol were also detected in the study area. Based upon the measurements, a health risk assessment of groundwater was conducted. After comparison of the various constituents in the samples with the standard, the hazardous effects of primary pollutants including physicochemical properties, toxicological toxic effects, etc. can be identified, The parameters selected for health risk assessment include noncarcinogens (including NO3-N, NH4-N, F, Cu, Mn, Zn, and Fe) and carcinogens (As and Cr6+) (Ministry of Environmental Protection of the PRC 2014). The International Committee on Radiation Protection (ICRP) recommends that the acceptable maximum level of health risk is 5.0 × 10−5 (per year) (Li and Qian 2011; Li et al. 2014d; Ni et al. 2009; Wu et al. 2012; Wei et al. 2008).
The results (as shown in Tables 7, 8) reveal that the risks of noncarcinogens (either in confined water samples or phreatic water samples) are mainly caused by Zn, with an average value of 2.07 × 10−8 per year for phreatic water and 1.93 × 10−8 per year for confined groundwater samples. Besides, F− is the second most influential factor parameter, with a maximum of 3.81 × 10−8 per year. The order of the other parameters in terms of noncarcinogenic risk value is Fe > NO3-N > Cu > Mn > NH4-N.
Compared to noncarcinogens, carcinogens in the groundwater caused much more risks to human health. The most influential factor is Cr6+, which ranges from 1.67 × 10−5 to 7.18 × 10−4 per year and with a mean value of 1.24 × 10−4 per year for phreatic water samples. With respect to confined aquifer, Cr6+ ranges from 1.67 × 10−5 to 1.99 × 10−4 and with an average value of 8.59 × 10−5 per year. It is obviously to find that the phreatic water is under higher carcinogenic health risk than the confined groundwater. The total carcinogenic risk ranges from 1.67 × 10−5 to 7.18 × 10−4 per year for phreatic water samples while ranges from 1.67 × 10−5 to 2.24 × 10−4 per year for the confined water samples. The similar ranges of Cr6+ and carcinogenic risks can also reveal that Cr6+ is the main factor caused the highest risk for carcinogens.
Similarly, the total health risk range and the carcinogenic risk range almost unanimously (as shown in Tables 7, 8), which indicates that the risk caused by noncarcinogens can be neglected to some extent. Meanwhile, the total risks water samples in 73 % of the phreatic water samples and 78 % of the confined exceed the acceptable level recommended by ICRP. The average of the total risk is 1.32 × 10−4 for the phreatic water, which is nearly three times higher than the acceptable value. With respect to the confined water, the average value of the total risk is 9.89 × 10−5, which is nearly two times higher than the acceptable value recommended by ICRP. The lowest total health risk for the phreatic water samples is U14 (1.67 × 10−5 per year), and highest point is U15 (7.18 × 10− 4 per year). The lowest total health risk of the confined samples is C3 (1.67 × 10−5 per year), and highest is C13 (2.24 × 10−4 per year). The sampling location of U15 is in Team 9 in Liangqushao, around which there are diverse industrial pollution sources including petrochemical industry enterprises. Similarly, sampling location of C13 has a paper mill nearby which drains wastewater into the environment.
Groundwater contamination is a worldwide problem and health risk assessments have been taken in many regions (Wu et al. 2015). In the adjacent plains formed by Yellow River, health risk assessments had been conducted in an industrial area on Weining plain (Li et al. 2014d) and Shizuishan city (Li and Qian 2011). Shizuishan is in the upstream of Yinchuan area, while Weinning Plain is in the downstream. Compared with the present study, the maximum value of total human risk is much higher for the phreatic aquifer in the Yinchuan Region than that of in their research regions. In the studies carried out by domestic scholars (Li and Qian 2011; Li et al. 2014d), the maximum value of the total health risk was 2.94 × 10−4 and 3.31 × 10−4 per year in the industrial areas of Zhongwei and Shizuishan city, respectively, and the average value were, respectively, 8.41 × 10−5 and 7.72 × 10−5. While in the present study, the maximum and average values of the human health risk are 7.18 × 10−4 and 1.32 × 10−4 for the phreatic water samples, 2.24 × 10−4 and 9.89 × 10−5 for the confined water samples. Obviously, Yinchuan Region has the higher total health risk, especially the phreatic water. Moreover, they have similar influential factors due to carcinogens and noncarcinogens. Cr6+ is the most influential factor due to the carcinogens and Zn due to the noncarcinogens in all the three regions, indicating the similar pollution sources (intense agricultural and industrial activities attributed) for the groundwater. In general, residents living in Yinchuan Region are at higher health risk, especially at high carcinogenic risk through drinking unconfined groundwater.
It should be pointed that the health risk assessment results are associated with uncertainty, because the citizens in the study area often drink boiled groundwater and some chemical compositions change accordingly. In addition, the assessment did not consider the exposure pathway of dermal contact through the body skin.
Conclusions
Phreatic water samples in the study area can be classified into four groups according to the hydrochemical type including SO4–Mg, Cl–Na, HCO3–Mg, and mixed type. On the contrary, the chemical types of the confined groundwater in the area are relatively simple, mainly including HCO3 type and the mixed type with no dominated cations. Drinking water quality assessment shows that unconfined groundwater is severely contaminated, and only three out of the 22 samples are classified as good quality water. The major contaminants are TBC, TDS, TH, Na+, SO 2–4 , and Fe, indicating that the unconfined groundwater may not quite be suitable for drinking. About 50 % of confined groundwater samples are classified as “good water quality,” and NH4-N is the main pollutant which reveals that agricultural activities play an important role on the groundwater deterioration.
Most of the groundwater quality is suitable for irrigation except a few with high salinity or with both high salinity and alkalinity. More than 77 % of phreatic water samples and nearly 90 % of confined groundwater samples are classified as good or medium water quality for agricultural purpose. Others are not suitable for irrigation mainly by the industrial pollution (such as oil refineries, paper mills, etc.) or the impact of human activities.
Compared to carcinogenic risk, the noncarcinogenic risks are relatively negligible in the study area. The average values of the noncarcinogenic risk in phreatic and confined water are 3.20 × 10−8 and 2.55 × 10−8 per year, respectively, which is nearly four orders of magnitude lower than that of carcinogenic risk value (1.32 × 10−4 and 9.89 × 10−5, respectively). The total health risks in 73 % of the phreatic water samples and 78 % of the confined samples exceed the acceptable level recommended by the ICRP (5.0 × 10−5 per year). The noncarcinogenic health risk is mainly due to Zn in groundwater, while the carcinogenic health risk is mainly determined by Cr6+. In the present study, contamination from the industry is the main reason of the high risk in groundwater, and therefore urgent actions should be taken to reduce the risk and guarantee the human health.
References
Aguilar L (2004) Effect of alkalinity in irrigation water on selected greenhouse crops. A & M University, College Station
Aksoy A, Scheytt T (2007) Assessment of groundwater pollution around Torbali, Izmir, Turkey. Environ Geol 53(1):19–25. doi:10.1007/s00254-006-0614-5
Akter A, Ali M (2011) Arsenic contamination in groundwater and its proposed remedial measures. Int J Environ Sci Technol 8(2):433–443
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Bouderbala A (2015) Assessment of groundwater quality and its suitability for agricultural uses in the Nador Plain, north of Algeria. Water Qual Expo Health 7:445–457. doi:10.1007/s12403-015-0160-z
Bureau of Quality and Technical Supervision of China (1994) National standard of the People’s Republic of China: quality standard for groundwater, GB/T 14848-93. Bureau of Quality and Technical Supervision of China, Beijing (in Chinese)
Daniele L, Corbella M, Vallejos A, Díaz-Puga M, Pulido-Bosch A (2013) Geochemical simulations to assess the fluorine origin in Sierra de Gador groundwater (SE Spain). Geofluids 2(13):194–203
Deutsch B, Kahle P, Voss M (2006) Assessing the source of nitrate pollution in water using stable N and O isotopes. Agron Sustain Dev 26(4):263–267. doi:10.1051/agro:2006025
Esmaeili A, Moore F, Keshavarzi B (2014) Nitrate contamination in irrigation groundwater, Isfahan, Iran. Environ Earth Sci 72(7):2511–2522
Geng F, Xue L, Lu G, Wu Y (2006) Water quality health-hazard risk assessment on drinking water supply sources. J Hydraul Eng 37(10):1242–1245 (in Chinese)
Han D, Tong X, Jin M, Hepburn E, Tong C, Song X (2013) Evaluation of organic contamination in urban groundwater surrounding a municipal landfill, Zhoukou, China. Environ Monit Assess 185:3413–3444. doi:10.1007/s10661-012-2801-z
Jalali M (2011) Nitrate pollution of groundwater in Toyserkan, western Iran. Environ Earth Sci 62:907–913. doi:10.1007/s12665-010-0576-5
Ji Y, Zhang L, Qian H (2005) Reason and prevention measures of nitrogen pollution in groundwater at Yinchuan area. J Earth Sci Environ 27(3):100–103
Kortatsi B, Tay C, Anornu G, Hayford E, Dartey G (2008) Hydrogeochemical evaluation of groundwater in the lower Offin basin, Ghana. Environ Geol 53(8):1651–1662
Kuhr P, Haider J, Kreins P, Kunkel R, Tetzlaff B, Vereecken H, Wendland F (2013) Model based assessment of nitrate pollution of water resources on a federal state level for the dimensioning of agro-environmental reduction strategies: the North Rhine-Westphalia (Germany) case study. Water Resour Manag 27:885–909. doi:10.1007/s11269-012-0221-z
Li P, Qian H (2011) Human health risk assessment for chemical pollutants in drinking water source in Shizuishan City, Northwest China. Iran J Environ Health Sci Eng 8(1):41–48
Li P, Qian H, Wu J (2010a) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. J Chem 7(S1):S209–S216. doi:10.1155/2010/451304
Li P, Wu J, Qian H (2010b) Groundwater quality assessment based on entropy weighted osculating value method. Int J Environ Sci 1(4):621–630
Li P, Wu J, Qian H (2012) Groundwater quality assessment based on rough sets attribute reduction and TOPSIS method in a semi-arid area, China. Environ Monit Assess 184(8):4841–4854. doi:10.1007/s10661-011-2306-1
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225. doi:10.1007/s12665-012-2049-5
Li P, Qian H, Wu J (2014a) Accelerate research on land creation. Nature 510(7503):29–31. doi:10.1038/510029a
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014b) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145. doi:10.1007/s12665-013-2691-6
Li P, Wu J, Qian H (2014c) Hydrogeochemistry and quality assessment of shallow groundwater in the southern part of the Yellow River alluvial plain (Zhongwei section), China. Earth Sci Res J 18(1):27–38. doi:10.15446/esrj.v18n1.34048
Li P, Wu J, Qian H (2014d) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36:693–712. doi:10.1007/s10653-013-9590-3
Li P, Qian H, Howard KWF, Wu J (2015) Building a new and sustainable “Silk Road economic belt”. Environ Earth Sci 74(10):7267–7270. doi:10.1007/s12665-015-4739-2
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016a) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health. doi:10.1007/s12403-016-0193-y
Li P, Wu J, Qian H (2016b) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9(1):15. doi:10.1007/s12517-015-2059-1
Li P, Li X, Meng X, Li M, Zhang Y (2016c) Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Expo Health. doi:10.1007/s12403-016-0205-y
Mahvi A, Nouri J, Babaei A, Nabizadeh R (2005) Agricultural activities impact on groundwater nitrate pollution. Int J Environ Sci Technol 2(1):41–47
Manecki P, Gałuszka A (2012) Groundwater quality as a geoindicator of organochlorine pesticide contamination after pesticide tomb reclamation: a case study of Franciszkowo, Northwestern Poland. Environ Earthsci 67(8):2441–2447. doi:10.1007/s12665-012-1694-z
Ministry of Environmental Protection of the PRC (2011) National plan of groundwater pollution prevention (2011–2020). http://www.zhb.gov.cn/gkml/hbb/bwj/201111/W020111109376922920938.pdf. Accessed 18 Apr 2015 (in Chinese)
Ministry of Environmental Protection of the PRC (2014) Technical guidelines for risk assessment of contaminated sites, HJ 25.3-2014. China Environmental Science Press, Beijing (in Chinese)
Ministry of Land and Resources of the People’s Republic of China (2006) Industry standard of the People’s Republic of China: the specification of testing quality management for geological laboratories, DZ/T 0130.1-2006. Ministry of Land and Resources of the People’s Republic of China, Beijing (in Chinese)
Momot O, Synzynys B (2005) Toxic aluminium and heavy metals in groundwater of middle Russia: health risk assessment. Int J Environ Res Public Health 2(2):214–218. doi:10.3390/ijerph2005020003
Nasrabadi T, Bidabadi N (2013) Evaluating the spatial distribution of quantitative risk and hazard level of arsenic exposure in groundwater, case study of Qorveh County, Kurdistan Iran. Iran J Environ Health Sci Eng. doi:10.1186/1735-2746-10-30
Ni F, Liu G, Ren H, Yang S, Ye J, Lu X (2009) Health risk assessment on rural drinking water safety—a case study in rain city district of Ya’an city of Sichuan Province. J Water Resour Prot 2:128–135. doi:10.4236/jwap.2009.12017
Qian H, Li P (2011) Hydrochemical characteristics of groundwater in Yinchuan plain and their control factors. Asian J Chem 23(7):2927–2938
Qian H, Li P, Howard KWF, Yang C, Zhang X (2012) Assessment of groundwater vulnerability in the Yinchuan Plain, Northwest China using OREADIC. Environ Monit Assess 6(184):3613–3628. doi:10.1007/s10661-011-2211-7
Qian H, Wu J, Zhou Y, Li P (2013a) Stable oxygen and hydrogen isotopes as indicators of lake water recharge and evaporation in the lakes of the Yinchuan plain. Hydrol Process 28(10):3554–3562. doi:10.1002/hyp.9915
Qian H, Li P, Wu J, Zhou Y (2013b) Isotopic characteristics of precipitation, surface and ground waters in the Yinchuan plain, Northwest China. Environ Earth Sci 70(1):57–70. doi:10.1007/s12665-012-2103-3
Sperling O, Lazarovitch N, Schwartz A, Shapira O (2014) Effects of high salinity irrigation on growth, gas-exchange, and photoprotection in date palms (Phoenix dactylifera L., cv. Medjool). Environ Exp Bot 99(3):100–109
Sun Y, Qian H, Wu X (2007) Hydrogeochemical characteristics of groundwater depression cones in Yinchuan city, Northwest China. Chin J Geochem 4(26):350–355
Tai T, Wang J, Wang Y, Bai L (2012) Groundwater pollution risk evaluation method research progress in our country. J Beijing Norm Univ Nat Sci 06:648–653 (in Chinese)
Uddin M, Harun-Ar-Rashid A, Hossain S, Hafiz M, Nahar K, Mubin S (2006) Slowarsenic poisoning of the contaminated groundwater users. Int J Environ Sci Technol 3(4):447–453
Wei J, Mi W, He T (2008) Assessment of the safety status for the drinking water supply sources and its management strategies in Yinchuan city. Water Resour Water Eng 19(3):65–68 (in Chinese)
Wongsasuluk P, Chotpantarat Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province. Environ Geochem Health Thail. doi:10.1007/s10653-013-9537-8
World Health Organization (WHO) (2011) Guidelines for drinking-water quality 4th edn. http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf. Accessed 31 March 2013
Wu J, Sun Z (2015) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 2015:1–19. doi:10.1007/s12403-015-0170-x
Wu J, Li P, Qian H (2012) Study on the hydrogeochemistry and non-carcinogenic health risk induced by fluoride in Pengyang County, China. Int J Environ Sci 2(3):1127–1134. doi:10.6088/ijes.00202030001
Wu J, Li P, Qian H (2015) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73(12):8575–8588. doi:10.1007/s12665-015-4018-2
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41502234 and 41572236), Natural Science Foundation of Science and Technology Department in Hebei Province (13274520 and D2016403044), Natural Science Foundation of Education Department in Heibei Province (D2015149), Youth Natural Science Foundation of Shijiazhuang University of Economics (QN201508), Teaching Reform Research Project of Shijiazhuang University of Economics (2014J04), Open foundation of Key Laboratory of groundwater resources of Ministry of land and resources of the People’s Republic of China (KF201509), and Natural Science Foundation of Ministry of Water Resources in Hebei Province (201501). The authors are grateful to the anonymous reviewers and the editors who helped us in improving the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Y., Wei, A., Li, J. et al. Groundwater Quality Evaluation and Health Risk Assessment in the Yinchuan Region, Northwest China. Expo Health 8, 443–456 (2016). https://doi.org/10.1007/s12403-016-0219-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-016-0219-5