Abstract
The aim of this research was to determine the main hydrochemical processes, quality change and groundwater pollution resulted from various human activities in the suburban area of Urmia city, located in the northwest of Iran. For this purposes, 62 groundwater samples were collected from urban, suburban and rural areas in September 2015. Furthermore, for determining the effect of anthropogenic activities on urban and suburban groundwater quality, a comparison was made between rural, suburban and urban groundwater. The results indicate that the main type of groundwater is Ca–HCO3, and the important processes controlling groundwater chemistry are mineral weathering, ion exchange and anthropogenic activity. The effect of anthropogenic activity such as increase in urbanization and change in land use caused the increase in the concentration of Ca, HCO3, Cl, Na, Mg, NO3 and pollution of groundwater in the suburban area. In suburban zone and especially in areas with low-depth groundwater, runoff infiltration, domestic effluent sewages and application of fertilizers caused an increase in nitrate in groundwater. In urban areas with impermeable surfaces, due to less infiltration, the amount of nitrate in groundwater is low, and groundwater is not contaminated. The results obtained from groundwater sustainability indicators (index of damages, index of pollution and groundwater quality index) show that the suburban area has low-hazard pollutant problem. Also, quality of 41% groundwater samples in the suburban zone and 13% in the rural zone is poor. In this paper, to determine the water quality for irrigation uses, sodium percent (Na%), sodium absorption ratio, residual sodium carbonate and permeability index were all calculated. The calculation of the irrigation water quality indices indicated that the quality of water for irrigation purposes can be classified as excellent to permissible categories.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Population growth and immigration to urban areas have caused uncontrolled expanding of Iranian cities in recent decades. According to information from Iran statistics center in 2011, about 71.4% of the 75 million population of Iran has been urbanized. Expanding of industrial, agricultural activities, change in land use, the increase in impermeable surface, change in topographic and surface water drainage system in the urban and suburban area have all resulted in changes in quality and quantity of groundwater. Therefore, quality management of groundwater in urban and suburban areas in many countries even in Iran is of great importance.
The study of hydrochemical processes controlling water quality, determining the factors affecting water quality and identifying groundwater pollution factors can all help us have a better understanding of the governing mechanism of the hydrochemical system. This would also help us manage urban groundwater resources. For analyzing the extent of natural processes and human impacts on groundwater systems in space and time, we can use groundwater quality sustainability indices, because these indices provide simplified information about the current status and future trends in the groundwater systems (Anbazhagan and Jothibasu 2016. The evaluation of groundwater quality is not only necessary to know the suitability but also for planning the management of groundwater in a more sustainable way to meet the existing and future demands for drinking and irrigation uses (Islam et al. 2017).
Up to now, most of the hydrogeological and hydrochemical studies conducted in the world and Iran have been related to investigations on groundwater in plains (e.g., Jalali 2007; Aghazadeh and Mogaddam 2010; Thivya et al. 2013; Pazand and Javanshir 2014; Singaraja, et al. 2014; Amiri et al. 2016; Fijani et al. 2016; Li et al. 2016; Sahu et al. 2016; Sethy et al. 2016; Islam et al. 2017; Xiao et al. 2017). Also, there are only a few studies on hydrogeology and hydrochemistry in urban areas and groundwater qualities in these regions. The results of the recent studies on quality and pollution of groundwater in urban and suburban areas indicate that groundwater contamination is the result of urban storm water infiltration, irrigation of green spaces, industrial activities and the uncontrolled leakage of wastewater and septic tanks (Jeong 2001; Kazemi 2011; Dong et al. 2013; Haque et al. 2013; Arunprakash et al. 2014; Nasrabadi and Abbasi 2014; Oiste 2014; Martin et al. 2014; Chung et al. 2015; Howard 2015; Jabal et al. 2015; Hassane et al. 2016; Attard et al. 2016; Yan et al. 2016; Elangoyan et al. 2017). Due to the importance of groundwater for various usages in Iran, many investigations have been conducted on groundwater quality and suitability for agriculture and drinking purposes (Sarikhani et al. 2015; Aghazadeh et al. 2016). However, urban groundwater quantity and quality in Iranian urban areas have not been fully investigated.
Urmia city, located at the center of West Azerbaijan Province, Iran, is very well known for its agricultural activities. Thus, groundwater is the primary source of providing the demand for water in this area. More than 40% of drinking water in this city and more than 90% of irrigation water in suburban and rural areas are obtained from groundwater (MRUDI 2015). Expansion of Urmia city in recent years has been so rapid that urban area (59.4 km2) in 1986 has been changed to (100 km2) in 2016. Uncontrolled expanding of urban areas, change in land use, developing of industrial and agricultural activities around the city and uncontrolled intake of groundwater water in the mentioned area have changed groundwater quality. However, there has not been any serious study on urban groundwater quality. Therefore, it is necessary to determine hydrochemical processes and factors affecting urban groundwater pollution. The aims of this study are to identify the main hydrochemical processes, quality change and groundwater pollution resulted from various human activities. The study also seeks to determine groundwater quality for different purposes. To this end, groundwater samples were collected from urban, suburban and rural areas. Based on the results of the chemical analyses of samples, different hydrochemical investigations were carried out. Furthermore, for determining the effect of anthropogenic activities on urban and suburban groundwater quality, a comparison was made between rural, suburban and urban groundwater.
2 Description of the study area
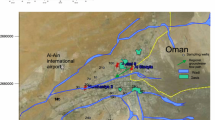
The study area is located in 18 km to the west of Urmia lake between longitude 44°59′ to 45°15′ and latitude 37°29′ to 37°39′ (Fig. 1). The climate of the study area is semiarid cold with annual average precipitation and temperature 360 mm and 11°, respectively. In the study area, the main precipitation occurs in May in the form of rain and in January and February in the form of snow. Urmia plain with 1000 km2 area and the average elevation of 1336 m.a.s.l m have four permanent rivers, namely Nazloochay, Rozehchay, Shahrchay and Barandoozchay. The Shahrchay River with the width of 20–150 m extends from west to east and passes through the middle of the city.
Urmia city with 100 km2 and almost one million populations is the main city in Urmia plain. About 66.5% of the total 100 km2 of Urmia city has been used for residential, transportation and industrial purposes, and 33.5% are gardens, farms, cemeteries, open areas and arid lands (NWWI 2015). In this city, urban sewerage system has covered 58%, and water distribution network has covered more than 90% of the Urmia city (NWWI 2015). Most of the urban expansion has been happening toward the southern and northwestern side of the city (Fig. 2), making the agriculture areas to use for urbanization purposes.
Based on the structural division of Iran, the study area is a part of Alborz–Azerbaijan zone that has various geologic units with different lithology from Cambrian to Quaternary (Nabavi, 1976). The main lithologic units around Urmia plain include alternation of limestone, sandstone, conglomerate and marl with age ranging from Miocene to Oligocene (Fig. 1; Aghazadeh et al. 2012). According to well and borehole logs and geophysical results, the aquifer in the study area is unconfined and consists of gravel, silty sand and clay sediments with medium grained (ARWA 2015). The depth of groundwater in plain varies from 1 to 52 m, which in the urban and suburban area reaches to 5 m. The minimum depth of groundwater in the suburb area is 1 m. The general groundwater flow direction in urban and suburb area is from W to E. To investigate the effects of anthropogenic activity on groundwater quality, the study area in the present research, for comparison purpose, has been subdivided into three zones: urban zone (UZ), suburban zone (SZ) and rural zone (RZ).
3 Materials and methods
To investigate the changes in urban, suburb and rural groundwater quality and pollution, the sampling stations were first determined. Fifteen samples from water wells and boreholes in urban areas, 24 samples from drinking and irrigating water wells in suburban areas and 23 samples from drinking and irrigating water wells in the rural areas were collected for comparative study in September 2015. The position of sampling stations is shown in Fig. 1. All samples were collected after 10 min of pumping wells in polyethylene bottles and were transferred to the laboratory to analyze the physicochemical parameters immediately. Electrical conductivity (EC), acidity (pH) and temperature (T) were measured in the field with WTW portable instrument. The groundwater samples were analyzed in the Environmental Protection Agencies of West Azerbaijan Laboratory to determine the anions (HCO3, Cl, NO3, SO4 and CO3) and cations (Ca, Mg, Na and K) according to standard methods (APHA 1998). Specific methods of estimation of different hydrochemical parameters of groundwater samples are shown in Table 1. For statistical and hydrochemical analysis, SPSS 11 software and AQQA software were used. The ArcGIS 9.3 software was used to create the database and to prepare the maps. In this research, to determine the water quality for drinking uses and analyze the extent of natural processes and the impact of anthropogenic activity on groundwater quality were calculated groundwater quality sustainability indexes GWQI, Id and Ip by equations. Also, to determine for agricultural purposes were calculated hydrochemical parameters such as Na%, SAR, RSC and PI. Then, according to the results, groundwater quality was classified.
4 Results and discussion
4.1 Groundwater chemistry
The descriptive statistics results related to hydrochemical parameters of groundwater samples in various zones of the study areas are presented in Table 2. Figure 3 shows the pip diagram of anions and cations. Investigations show that pH and concentration of some ions such as K in various zones of the study areas do not have much change. Average concentration of Ca, Mg, Na in UZ are 95.7, 23.3, 21.8 mg/l, in SZ are 106, 38, 44.2 mg/l and in RZ are 87.6, 38, 34 mg/l, respectively. High range of Ca and Mg concentration in the study areas may be due to water rock interaction. However, in the case of high concentration of Na, SO4 and Cl (samples SZ1, SZ13 and RZ 14) anthropogenic sources are considered.
Average concentration of SO4, HCO3 and Cl in UZ are 57.4, 317 and 33.5 mg/l, in SZ are 103, 365 and 64.6 mg/l and in RZ are 105.3, 336 and 50 mg/l, respectively. Comparing the concentration of various ions in the study areas shows that the average concentration of Ca, HCO3, Cl, Na, Mg, in SZ is higher than other zones because of the effect of anthropogenic activity. The index of pollution of chloride in the suburban area is high. This index varies from 0.2 to 0.86. The used road salt and saline water have caused an increase in chloride concentration in groundwater of the Urmia city (Aghazadeh et al. 2012). Therefore, in the suburban area, the high Cl in groundwater is due to the impact of urban runoff infiltration.
The average amount of TDS in RZ and UZ is 543 and 486, respectively. The average amount of TDS in the suburban zone (SZ) is 663 mg/l and reaches to 1128 mg/l. Based on the TDS classification, more than 97% of samples of each three zones are placed in freshwater group (TDS less than 1000 mg/l) (Fig. 4). Samples with more than 1000 mg/l (brackish) are located in SZ and RZ (Fig. 4). Total hardness in groundwater samples of UZ varies between 200 and 463 mg/l, in SZ 205 and 605 and in RZ between 150 and 803 mg/l. Due to Sawyer and McCarty (1978) classification, 24% of samples in UZ, 8% in SZ and 20% in RZ are hard, and the remaining are very hard (Fig. 4).
In this research, box plots were used to assess the intensity of variation of different ions concentration. The pattern of variation of the various ions concentration shows that there is much difference between the average and the maximum in ions of Na, Cl and SO4 (Fig. 5). Such pattern of variation is due to local contamination of groundwater in the study areas.
Nitrate is one of the main parameters in investigation of water pollution. This ion does not have lithologic source and mainly is produced by anthropogenic activities such as fertilizers, leakage of septic tanks, sewage and domestic waste (Subrahmanyam and Yadaiah 2000). Nitrate can be considered as low-hazard pollutants. The maximum permissible concentration (MPC) limit of nitrate is 45 mg/l. Distribution of nitrate in groundwater samples of the study areas shows that in suburban and industrial areas the concentration of NO3 is high (Fig. 6). The results of the calculation of the groundwater damages (Id) (Eq. 1) indicate that this index in the urban, suburban and rural area is 0.1, 0.4 and 0.13, respectively. The index of pollution of nitrate (Ip) (Eq. 2) in the urban area varies from 0.3 to 0.8, in the suburban area from 0.4 to 3 and in the rural area from 0.1 to 0.5. In suburban area, about 30% of the area exceeds the maximum permissible concentration of nitrate.
In the rural areas, a few samples have nitrate above the standard limit. In urban and suburban areas, the source of nitrate in groundwater is because of domestic sewages, industrial waste waters, nitrogenous fertilizers, infiltration of surface water, return sewage water and agricultural sewage usage (Yan et al. 2016). In urban areas with impermeable surfaces, because of less infiltration, the amount of nitrate in groundwater is low, but in permeable surface areas, nitrate concentration is high. The average concentration of nitrate in groundwater of UZ is 22 mg/l and varies between 13 and 38 mg/l. In suburban zone and especially in areas with low-depth groundwater, the concentration of nitrate in groundwater is high. In this zone, the concentration of nitrate in groundwater samples ranges from 8.6 to 137 mg/l with the mean of 59 mg/l. About 46% of groundwater samples have NO3 concentration above the tolerance limit of 45 mg/l (Fig. 6). In some parts of the suburban areas, urban runoff spreads on the ground and can penetrate into groundwater and change its quality. Also, in suburban zones, most types of the land use are for agriculture so that in these areas chemical fertilizers were mostly used. Therefore, runoff infiltration, domestic effluent sewages and application of fertilizers can cause an increase in the nitrate in groundwater. Nosrati and Van Den Eeckhaut (2012) suggested similar sources of contamination in groundwater of Hashtgerd plain (north Iran). Results show that in rural areas (RZ) concentration of nitrate in groundwater samples is low and ranges from 4 to 57 mg/l with the mean of 12.5 mg/l. In these areas, a few samples (RZ10) have nitrate above the standard limit. These groundwater samples are located in agricultural areas so that the use of fertilizers can cause an increase in the nitrate in this groundwater samples.
The comparison of nitrate concentration in groundwater samples of the three zones with groundwater depth shows that, in low groundwater depth areas, nitrate concentration is high (Fig. 7). In groundwater samples with nitrate above the standard limit (45 mg/l), the average depth of groundwater is 4 m. In groundwater of suburban areas with high nitrate (Sample 1 and 21), the depth of groundwater is 3.3 and 2 m, respectively. Also, in sample RZ 10, with high nitrate (57 mg/l) related to rural areas, the depth of groundwater is 4.1 m.
4.2 Hydrogeochemical process
4.2.1 Hydrochemical facies and water–rock interaction
For hydrochemical investigation, for hydrochemical classification and determined hydro-chemical facies was commonly used Piper (1944) trilinear diagram. In this study, for the determination of predominant ions, hydrochemical facies and water type, Piper diagram was used. Based on this diagram (Fig. 8), the main type of groundwater in UZ, SZ and RZ areas is Ca–HCO3, Ca–HCO3 and Ca, Mg–HCO3, respectively. Also, in the three zones of the study areas, the major cations are Ca and Mg and the anion is HCO3. In groundwater samples, Ca and Mg significantly exceed the Na and K, and the HCO3 and CO3 exceed Cl- and SO4. In some urban and suburban samples, because of ion exchange and anthropogenic activities, the concentration of Na, Cl and sulfate increased.
The water–rock interaction has the main role in groundwater quality. To determine the chemistry mechanism of the groundwater (precipitation dominance, rock dominance and evaporation dominance), Gibbs diagram (Gibbs 1970) was commonly used. Due to the location of groundwater samples of study areas in Gibbs diagram (Fig. 9), the water–rock interaction and weathering of rocks are the main processes in providing ions of groundwater.
The equilibrium state of the water with respect to a mineral phase can be determined by calculating a saturation index (SI) using analytical data. Changes in saturation state are useful to distinguish different stages of hydrochemical evolution and help identify which geochemical reactions are important in controlling water chemistry (Aghazadeh and Mogaddam 2010). The saturation index of a mineral is obtained from Eq. 2.
where IAP is the ion activity product of the dissociated chemical species in solution, Kt is the equilibrium solubility product for the chemical involved at the sample temperature. An index (SI), less than zero, indicates that the groundwater is undersaturated with respect to that particular mineral. An index (SI), greater than zero, specifies that the groundwater is being supersaturated with respect to the particular mineral phase and therefore incapable of dissolving more of the mineral. Such an index value reflects groundwater discharging from an aquifer containing ample amount of the mineral with sufficient resident time to reach equilibrium (Langmuir 1997). The results indicated that the values of SI calcite and dolomite are from −0.4 to 1.65 and 0.1 to 2.8, respectively. The 90% of the groundwater samples are saturated and supersaturated with respect to dolomite and 73% with respect to calcite. Nearly all samples undersaturated with respect to gypsum and anhydrite minerals, suggesting that these carbonate mineral phases may have influenced the chemical composition of the study area.
The calcium, magnesium and bicarbonate are the major ions in groundwater of study areas. The solution of carbonate minerals such as calcite and dolomite can be the origin of these ions. In hydrochemical consideration to determine the minerals solution process and its impact on groundwater chemistry, Ca + Mg versus HCO3 + SO4 diagram was commonly used. Due to this diagram, if the solution of minerals such as calcite, dolomite and gypsum would be dominant, then samples fell near the 1:1 equiline. In samples with number of HCO3 + SO4 less than 10 meq/l, the groundwater chemistry is related to carbonated minerals and more than 10 meq/l it is related to the solution of sulfate minerals like gypsum. In samples above 1:1 equiline, hydrochemical ion exchange process is dominant.
The position of the groundwater samples of the study areas in this diagram (Fig. 10) shows that the solution processes of carbonate minerals and ion exchange are critical hydrochemical processes. According to Fig. 10, some of the urban and suburban samples fall above the 1:1 line, which indicated that Ca and Mg are more than HCO3 and SO4, and the ion exchange process has been occurred. Therefore, ion exchange is one of the important hydrochemical processes that control the chemistry and quality of the groundwater.
4.2.2 Ion exchange process
Ion exchange is one of the main geochemical processes that control the chemistry and quality of the groundwater. To determine the ion exchange process, chloro-alkaline indices (Eqs. 4, 5) were usually used. The negative amount of these indices shows normal ion exchange, and positive amounts show reversion ion exchange.
In these equations, all ionic concentrations are expressed in meq/l. The calculation of chloro-alkaline indices (CAI 1, 2) by Eqs. 4 and 5 for groundwater samples from the study areas shows that these indices vary between −4 and 0.9. Therefore, normal ion exchange is the dominant geochemical process. Also, based on Fig. 11, when normal ion exchange is a significant geochemical process, the slope of the liner was less than −1. Regarding the sample locations on this diagram (Fig. 11), normal ion exchange is dominantly a geochemical process. In samples that are located up and down the linear trend, in addition to ion exchange, anthropogenic activities are also affecting the process on the groundwater quality of the reign.
4.3 Water quality
4.3.1 Drinking water quality
The groundwater mostly has good quality and suitable for the various purpose. However, human activity in recent decades caused pollution and degradation of quality this resource. Therefore, quality control of this resource is necessary. Groundwater quality index (GWQI) is an effective tool for measuring the suitability of drinking water, and it is one of the important indices in investigating and quality management of groundwater (Singh et al. 2011; Islam et al. 2017).
The GWQI is calculated according to the suitability of water for drinking uses. In this research, we used GWQI for assessment of the change in groundwater quality and the potential effects of urbanization on the groundwater quality in the urban and suburban area. To calculate GWQI have been used acidity (pH), total dissolved solids (TDS), nitrates (NO3), total hardness (TH), magnesium (Mg), bicarbonate (HCO3), calcium (Ca), sulfate (SO4) and chloride (Cl) parameters. Then for each parameter, weight (wi) is appointed based on its relative importance in the overall quality of water for drinking purposes (Table 4). The GWQI is computed by Eqs. 6–9 (Oiste 2014).
In this formula, Sli shows the subindex for each parameter.
where wi = weight of each parameter and n = number of parameter
where qi = quality rating, Ci = concentration of each chemical parameter, for each water sample, and Si = WHO standard.
The GWQI values are classified into five classes. In the study area, GWQI values range from 54 to 80 in UZ, 64 to 143 in SZ and 43 to 131 in RZ. The GWQI map is shown in Fig. 12. According to the GWQI values, 59% samples of the SZ, 87% RZ and 100% of UZ fit into the high-quality class, and the rest of the samples in three zones fit into the poor class (Fig. 13). The groundwater samples with poor quality are placed at the exit point of city and suburb area and the parks and agricultural area of the suburb. In suburb area, intensive agricultural activities associated with domestic effluent sewages and industrial wastewaters have an important role in the pollution of groundwater.
4.3.2 Irrigation water quality
In the study area, agriculture activity is high, and the groundwater is main water resource for irrigation uses. To determine the quality of water for agricultural uses, various parameters were used. The sodium absorption ratio (SAR), percentage of sodium (Na%), residual sodium carbonate (RSC) and permeability index (PI) are main parameters to assess its suitability for agriculture purpose.
4.3.2.1 Sodium adsorption ratio
Sodium adsorption ratio (SAR) is an important parameter for determining the suitability of groundwater for irrigation because it is a measure of alkali/sodium hazard to crops (Jalali 2007). SAR is calculated by Eq. 6.
where all ionic concentrations are expressed in meq/l. SAR values in study area ranged from 0.06 to 2.4 (Table 3), and according to SAR classification (Table 3), most of the samples in three zones of study area fall in excellent category and are suitable for irrigation uses (Table 4).
4.3.2.2 Sodium percent
The sodium percent (%Na) is obtained by Eq. 11.
where all ionic concentrations are expressed in meq/l. The result shows that in UZ, SZ and RZ the Na% values ranged 9–21, 6.5–38 and 1.8–35, respectively. According to water classification based on Na% (Table 3), most of the samples in three zones of study area fall in the right category and are suitable for irrigation uses.
4.3.2.3 Residual sodium carbonate
When the HCO3 and CO3 are greater than the Ca and Mg, a precipitation of Ca and Mg happens in soils, which damages the permeability of the soil. A relation of Ca and Mg with HCO3 and CO3 is expressed in terms of residual sodium carbonate (RSC). Also, Na% and SAR are used for determining the quality of water for agriculture purposes (Li et al. 2016). The RSC is calculated by difference between alkaline earths and weak acids (Eq. 12). In study area, the result shows that RSC values ranged from −5.9 to 0.8 (Table 3). According to RSC classification (Table 3), all of the samples fall in the good category.
4.3.2.4 Permeability index
The permeability index (PI) also is used to determine the suitability of groundwater for irrigation. PI is defined as Eq. 13.
In this equation, all ionic concentrations are expressed in meq/l. According to PI values, the groundwater of in the study area can be designated as class II (25–75%), indicating that all of the groundwater is suitable for irrigation. From the above investigation, it is evident that the most of the groundwater of the study area is suitable for drinking or irrigation purpose.
5 Conclusion
Analysis of hydrochemical data indicated that in the study area, the main type of groundwater is Ca–HCO3 and the main processes controlling groundwater chemistry are mineral weathering, ion exchange and anthropogenic activity. The increase in urbanization and change in land use caused the decline of groundwater quality in urban and suburban of the study area in recent years. Result revealed that the average concentration of Ca, HCO3, Cl, NO3, Na and Mg in the suburban zone is higher than other zones because of the effect of anthropogenic activity. The results obtained from groundwater sustainability indicators show that, in the suburban area especially in areas with low depth groundwater, the concentration of nitrate in groundwater and some samples chloride are high. This indicated that groundwater has been pollulted by NO3 and Cl. The existence of high NO3 in groundwater might originate from wastewater and fertilizer application, and the existence of high Cl is due to the infiltration of urban runoff result of snowmelts in winter. In rural areas, the use of chemical fertilizers contributes to the pollution of groundwater resources, especially by nitrate. In urban areas with impermeable surfaces, because of less infiltration, the amount of nitrate in groundwater is low, and groundwater is not contaminated. According to the GWQI values, quality of 41% groundwater samples in the suburban zone and 13% in the rural zone is poor. The groundwater samples with high nitrate and with poor quality based on GWQI values are located in the industrial, park and agricultural area. Based on permissible limits prescribed by WHO for drinking uses, most of the groundwater in the urban and rural area is suitable for drinking purpose, but in 46% groundwater samples in the suburban zone nitrate is above the permissible limit. The calculated values of SAR, RSC, Na% and PI index indicated that the quality of water for irrigation uses can be ranged as excellent to the permissible category.
References
Aghazadeh, N., Chitsazan, M., & Golestan, Y. (2016). Hydrochemistry and quality assessment of groundwater in the Ardabil area, Iran. Applied Water Science. doi:10.1007/s13201-016-0498-9.
Aghazadeh, N., & Mogaddam, A. A. (2010). Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environmental Monitoring and Assessment, 176, 183–195.
Aghazadeh, N., Nojavan, M., & Mogaddam, A. A. (2012). Effects of road deicing salt (NaCl) and saline water on water quality in the Urmia area, Northwest of Iran. Arabian Journal of Geosciences, 5(4), 565–570.
Amiri, V., Nakhaei, M., Lak, R., & Kholghi, M. (2016). Investigating the salinization and freshening processes of coastal groundwater resources in Urmia aquifer, NW Iran. Environmental Monitoring and Assessment, 188, 233.
Anbazhagan, S., & Jothibasu, A. (2016). Groundwater sustainability indicators in parts of Tiruppur and Coimbatore Districts, Tamil Nadu. Journal Geological Society of India, 87, 161–168.
APHA. (1998). Standard methods for the examination of water and wastewater (20th ed., pp. 1–467). Washington: American Public Health Association.
Arunprakash, M., Giridharan, L., Krishnamurthy, R. R., & Jayaprakash, M. (2014). Impact of urbanization in groundwater of south Chennai City, Tamil Nadu, India. Environmental Earth Sciences, 71, 947–957.
Attard, G., Winiarski, T., Rossier, Y., & Eisenlohr, L. (2016). Review: Impact of underground structures on the flow of urban groundwater. Hydrogeology Journal, 24, 5–19.
Azerbaijan Regional Water Authority (ARWA). (2015). Evaluation of groundwater in Urmia Plain, Urmia, Iran.
Chung, S. Y., Venkatramanan, S., Kim, T. H., Kim, D. S., & Ramkumar, T. (2015). Influence of hydrogeochemical processes and assessment of suitability for groundwater uses in Busan City, Korea. Environment, Development and Sustainability, 17(3), 423–441.
Dong, D., Sun, W., Zhu, Z., Xi, S., & Lin, G. (2013). Groundwater risk assessment of the third aquifer in Tianjin City, China. Water Resources Management, 27, 3179–3190.
Elangoyan, N. S., Lavanya, V., Arunthathi, S. (2017). Assessment of groundwater contamination in a suburban area of Chennai, Tamil Nadu, India. Environment, Development and Sustainability 1–13.
Fijani, F., Moghaddam, A. A., Tsai, F. T. C., & Tayfur, G. (2016). Analysis and assessment of hydrochemical characteristics of Maragheh-Bonab Plain Aquifer, Northwest of Iran. Water Resources Management. doi:10.1007/s11269-016-1390-y.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 17, 1088–1090.
Haque, S. J., Onodera, S., & Shimizu, Y. (2013). An overview of the effects of urbanization on the quantity and quality of groundwater in South Asian megacities. Limnology, 14, 135–145.
Hassane, A. B., Leduc, C., Favreau, G., Bekins, B. A., & Margueron, T. (2016). Impacts of a large Sahelian city on groundwater hydrodynamics and quality: Example of Niamey (Niger). Hydrogeology Journal, 24, 407–423.
Howard, K. W. F. (2015). Sustainable cities and the groundwater governance challenge. Environmental Earth Sciences, 73, 2543–2554.
Islam, A. R. M. T., Shen, S., Haque, M. A., Bodrud-Doza, M., Maw, K. W., & Habib, M. A. (2017). Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches. Environment, Development and Sustainability. doi:10.1007/s10668-017-9971-3.
Jabal, M. S. A., Abustan, I., Rozaimy, M. R., & Najar, H. E. (2015). Groundwater beneath the urban area of Khan Younis City, southern Gaza Strip (Palestine): hydrochemistry and water quality. Arabian Journal of Geosciences, 8, 2203–2215.
Jalali, M. (2007). Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah basin in western Iran. Environmental Monitoring and Assessment, 130, 347–364.
Jeong, C. H. (2001). Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. Journal of Hydrology, 253, 194–210.
Kazemi, G. A. (2011). Impacts of urbanization on the groundwater resources in Shahrood, Northeastern Iran: Comparison with other Iranian and Asian cities. Physics and Chemistry of the Earth, 36, 150–159.
Langmuir, D. (1997). Aqueous environmental geochemistry (p. 601). Upper Saddle River: Prentice Hall Inc.
Li, P., Wu, J., & Qian, H. (2016). Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County China. Arabian Journal of Geosciences, 9, 15.
Martin del Campo, M. A., Esteller, M. V., Exposito, J. L., & Hirata, R. (2014). Impacts of urbanization on groundwater hydrodynamics and hydrochemistry of the Toluca Valley aquifer (Mexico). Environmental Monitoring and Assessment, 186, 2979–2999.
Ministry of Roads and Urban Development of Iran (MRUDI). (2015). Development of urban areas in Urmia city, Urmia, Iran. (in Persian)
Nabavi, M. H. (1976). Preface geology of Iran. Geology Survey Iran, Tehran. (in Persian)
Nasrabadi, T., & Abbasi, M. P. (2014). Groundwater quality degradation of urban areas (case study: Tehran city, Iran). International Journal of Environmental Science and Technology, 11, 293–302.
National Water and Wastewater Engineering Company of Iran (NWWI). (2015). Development of water and sewage network system in Urmia city, Urmia, Iran. (in Persian)
Nosrati, K., & Van Den Eeckhaut, M. (2012). Assessment of groundwater quality using multivariate statistical techniques in Hashtgerd Plain, Iran. Environmental Earth Sciences, 65(1), 331–344.
Oiste, A. M. (2014). Groundwater quality assessment in urban environment. International Journal of Environmental Science and Technology, 11, 2095–2102.
Pazand, K., & Javanshir, A. (2014). Geochemistry and water quality assessment of groundwater around Mohammad Abad Area, Bam District, SE Iran. Water Quality, Exposure and Health, 6, 225–231.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analysis. American Geophysical Union Transplantation, 25, 914–928.
Richards, L. A. (1954). Diagnosis and improvement of saline alkali soils: Agriculture (vole 160. Handbook 60). Washington, DC: US Department of Agriculture.
Sahu, P., Sikdar, P. K., & Chakraborty, S. (2016). Geochemical evolution of groundwater in southern Bengal Basin: The example of Rajarhat and adjoining areas, West Bengal, India. Journal of Earth System Science, 125(1), 129–145.
Sarikhani, R., GhassemiDehnavi, A., Ahmadnejad, Z., & Kalantari, N. (2015). Hydrochemical characteristics and groundwater quality assessment in Bushehr Province, SW Iran. Environmental Earth Sciences, 74, 6265–6281.
Sawyer, C. N., & McCarty, P. L. (1978). Chemistry for environmental engineering (p. 532). New York: McGraw-Hill.
Sethy, S. N., Syed, T. H., Kumar, A., & Sinha, D. (2016). Hydrogeochemical characterization and quality assessment of groundwater in parts of Southern Gangetic Plain. Environmental Earth Sciences, 75, 232.
Singaraja, C., Chidambaram, S., Anandhan, P., & Prasanna, M. V. (2014). Determination of the utility of groundwater with respect to the geochemical parameters: a case study from Tuticorin District of Tamil Nadu (India). Environment, Development and Sustainability, 16, 689–721.
Singh, C. K., Shashtri, S., Mukherjee, S., Kumari, R., Singh, A., & Singh, R. P. (2011). Application of GWQI to assess effect of land use change on groundwater quality in lower Shiwaliks of Punjab: Remote sensing and GIS based approach. Water Resource Management, 25, 1881–1898.
Subrahmanyam, K., & Yadaiah, P. (2000). Assessment of the impact of industrial effluents on water quality in Patancheru and environs, Medak district, Andhra Pradesh, India. Hydrogeology Journal, 9(3), 297–312.
Thivya, C., Chidambaram, S., Singaraja, C., Thilagavathi, R., Prasanna, M. V., Anandhan, P., et al. (2013). A study on the significance of lithology in groundwater quality of Madurai district, Tamil Nadu (India). Environment, Development and Sustainability, 15, 1365–1387.
WHO. (2011). Guidelines for drinking-water quality (4th ed.). Geneva: Switzerland.
Wilcox, L. V. (1955). Classification and use of irrigation water (Circular 969). Washington, DC: USDA.
Xiao, Y., Shao, J., Cui, Y., Zhang, G., & Zhang, Q. (2017). Groundwater circulation and hydrogeochemical evolution in Nomhon of Qaidam Basin, northwest China. Journal of Earth System Science, 126, 26.
Yan, B., Xiao, C., Liang, X., & Fang, Z. (2016). Impacts of urban land use on nitrate contamination in groundwater, Jilin City, Northeast China. Arabian Journal of Geosciences, 9, 105.
Acknowledgements
The authors of this study gratefully acknowledge the research vice-chancellery of Shahid Chamran University in Ahvaz and the Environmental Protection Agencies of West Azerbaijan for providing the existing relevant data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chitsazan, M., Aghazadeh, N., Mirzaee, Y. et al. Hydrochemical characteristics and the impact of anthropogenic activity on groundwater quality in suburban area of Urmia city, Iran. Environ Dev Sustain 21, 331–351 (2019). https://doi.org/10.1007/s10668-017-0039-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-017-0039-1