Abstract
Potentially harmful elements (PHEs) manganese (Mn), cobalt (Co), copper (Cu), lead (Pb), nickel (Ni) and zinc (Zn) were measured in human hair/nails, staple crops and drinking water to ascertain the level of exposure to dust transference via wind and rain erosion for members of the Mugala community living near a mine waste dump in the Zambian Copperbelt. The mean PHE concentrations of hair in decreasing order were Zn (137 ± 21 mg/kg), Cu (38 ± 7 mg/kg), Mn (16 ± 2 mg/kg), Pb (4.3 ± 1.9 mg/kg), Ni (1.3 ± 0.2 mg/kg) and Cr (1.2 ± 0.2 mg/kg), Co (0.9 ± 0.2 mg/kg) and Cd (0.30 ± 0.02 mg/kg). Whilst for toenails the decreasing order of mean concentrations was Zn (172 ± 27 mg/kg), Cu (30 ± 5 mg/kg), Mn (12 ± 2 mg/kg), Pb (4.8 ± 0.5 mg/kg), Ni (1.7 ± 0.14 mg/kg) and Co (1.0 ± 0.02 mg/kg), Cr (0.6 ± 0.1 mg/kg) and Cd (0.1 ± 0.002 mg/kg). The concentration of these potentially harmful elements (PHEs) varied greatly among different age groups. The results showed that Mn, Co, Pb, Cd and Zn were above the interval values (Biolab in Nutritional and environmental medicine, Hair Mineral Analysis, London, 2012) at 0.2–2.0 mg/kg for Mn, 0.01–0.20 mg/kg for Co, < 2.00 mg/kg for Pb, < 0.10 mg/kg for Cd and 0.2–2.00 mg/kg for Zn, whilst Ni, Cu and Cr concentrations were within the normal range concentrations of < 1.40 mg/kg, 10–100 mg/kg and 0.1–1.5 mg/kg, respectively. Dietary intake of PHEs was assessed from the ingestion of vegetables grown in Mugala village, with estimated PHE intakes expressed on a daily basis calculated for Mn (255), Pb (48), Ni (149) and Cd (33) µg/kg bw/day. For these metals, DI via vegetables was above the proposed limits of the provisional tolerable daily intakes (PTDIs) (WHO in Evaluation of certain food additive and contaminants, Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives, 2011) for Mn at 70 µg/kg bw/day, Pb at 3 µg/kg bw/day, Ni and Cd 5 µg/kg bw/day and 1 µg/kg bw/day, respectively. The rest of the PHEs listed were within the PTDIs limits. Therefore, Mugala inhabitants are at imminent health risk due to lead, nickel and cadmium ingestion of vegetables and drinking water at this location.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potentially harmful elements (PHEs) are natural constituents of the Earth’s crust (Vogel 1989) and are non-biodegradable persistent pollutants found in the environment and can possibly enter the human body via different routes such as food, drinking water and air in small amounts and then accumulate in the body (Stihi et al. 2011).
Living organisms and humans require trace amounts of some elements which are referred to as trace elements that include Zinc (Zn), Copper (Cu), Iron (Fe), Cobalt (Co) and others. These elements are naturally found in foodstuffs, fruits and vegetables and in commercially available multivitamin products, but these can have a negative health effect at excessive levels (Mcbride 1994). Some non-essential metals such as cadmium (Cd), mercury (Hg) and lead (Pb) are toxic at very low concentrations (Nies 1999; Kaewsarn and Yu 2001) and pose serious risk to human and ecosystem health (Chan et al. 2003; Gao 2012). For instance, chronic Cd intoxication may give rise to renal tubular dysfunction, anaemia and skeletal damage (itai–itai disease) (Horiguchi et al. 1994; Jarup 2013). Prolonged exposure to Pb may cause kidney and liver damage and has an adverse effect on the central and peripheral nervous systems, haemopoietic and cardiovascular system (Liu et al. 2014). Exposure routes for a wider panel of PHEs associated with exposure routes from mining activities combining geochemical and health disciplines are essential to provide appropriate information for mitigation and protection of health (Middleton et al. 2019a). Therefore, it is important to carry out an investigation on PHEs to assess the potential health risks of PHEs to humans, particularly where exposure associated with mining activities exists (Zhuang et al. 2009; Kaninga et al. 2019; Stewart 2019).
Hair and nails are metabolic end products that incorporate metals into their structure during the growth process (Ionescu et al. 2006) (Romanowicz-Makoska et al. 2011). These two biomarkers have been reported frequently for use in assessing exposure to PHEs (Goulle et al. 2009; Button et al. 2009; Oyoo-Okoth et al. 2012, 2013; Alves et al. 2014; Middleton et al. 2016a, b, c; Bakri et al. 2017). Rashed and Hossam (2007) reported using human scalp hair and fingernails in Aswan, Egypt, as a biological indicator for the assessment of heavy metal pollution (Rashed and Hossam 2007). Hair and nail analyses provide a measure of exposure over 4–8 weeks (Button et al. 2009), smoothing out day-to-day variations which occur in blood and urine measurements for which measurements can vary on an hourly basis and provide an exposure assessment over a 24-h period (Chojnacka et al. 2005; Middleton et al. 2016b, c, 2019b; Wongsasuluk et al. 2018). Hair and nails are an appropriate biomarker for exposure since trace elements and PHEs bind to the keratin in hair and nails in much larger concentrations than in any other biological samples, providing measurable quantities, particularly for inductively coupled plasma mass spectrometry (ICP-MS) (Zhunk and Kist 1995). These matrices are also useful for determining deficiencies of essential trace elements in the diet and therefore indicators of metabolic or physiological disorders (Dombovari and Papp 1998; Katz and Chatt 1998; Dombovari et al. 1999). Hair and nails are practical biological materials owing to the collection and preservation of the sample compared to blood and urine, in addition to biosafety for the analyst handling the sample. Hair analysis for determination of contents (concentration) of trace elements has been a continual activity in the field of biomedical and environmental studies over the past three decades (Ciszewski et al. 1997).
The consummation of PHEs can be estimated through the calculation of the dietary intake of staple foodstuffs and drinking water following their elemental analyses, either assuming full or measurement of bioaccessibility and consideration of inhibitors for absorption, such as phytic acid in grains (O’Reilly et al. 2010; Reason et al. 2015; Zia et al. 2017; Gibson et al. 2018).
A recent unpublished study conducted in 2016 in the Zambian Copperbelt showed that crops grown near a tailings dump contain concentrations of Cu and Co above the recommended limits prescribed by FAO/WHO (1999). However, the exposure to PHEs for populations that consume crops and drinking water sourced in close proximity to mine tailing dumps in the Zambian Copperbelt is not well characterised. Therefore, the aim of this study was to: (1) assess the risk of exposure to PHEs by populations living near mine waste dumps, using hair and nails as biomarkers; (2) determine the dietary intake of PHEs from locally produced vegetables and drinking water supplies in Mugala village in close proximity to mine tailings; and (3) determine the exposure to PHEs by comparison between biomarkers of exposure (nails and hair) and dietary intake estimations from the consumption of locally produced foodstuffs and drinking water.
Materials and methods
Ethics
The study protocol was approved by the Directorate of Research, Innovation and Consultancy (DRIC) ethics committee at the Copperbelt University and all the participants consented to the test.
Study location
Mugala village is situated in Kitwe West on the Copperbelt Province of Zambia. It is located near Mopani (TD 15 A) tailings dump site. The populations of Mugala village are predominantly subsistence farmers who grow their crops in the vicinity of TD 15 A. It is a village constantly increasing in population with over 400 households.
The implications of mining on the Zambian communities, especially on the Copperbelt Province, have been known to cause serious environmental impacts, especially where copper mining is concerned. The substantial amounts of sulphur dioxide emitted into the atmosphere have been identified to cause acid rain, soil erosion, crop damage and water pollution. Most of the Copperbelt has 50 times higher concentrations of copper in surface soils than in subsurface samples as reported by Ncube et al. (2012).
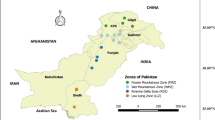
Leaves and roots of cassava and sweet potatoes grown in the contaminated areas of the Copperbelt are known to contain elevated metal concentrations, whilst backyard vegetable gardens are affected by necrosis due to accumulation of heavy metals in the soil and SO2 on plant leaves. The contamination from ongoing mining operations is further aggravated by windborne dust particles (from dry TDs) resulting in accumulation of metals (copper and cobalt and other elements) in the soil (Srivastava and Jokanc 2016). All of these concerns have gradually affected the health of the local inhabitants on the Copperbelt Province, especially in the Mufulira, Chingola and Kitwe municipalities where copper mining operations are done on a large scale (Fig. 1).
The local inhabitants were randomly selected among those who permanently live in the agricultural study area without migrating. Human hair and nails were collected from healthy local inhabitants of the area who consented to the test. The participants were divided into age groups: 11–20 years, 21–30 years and 31–40 years. Personal information was collected by means of face-to-face interviews regarding their sex, age, body weight, medical histories and dietary habits. Consented participants with chemically treated and coloured hair as well as painted nails were excluded from the study. Hair portions closest to the scalp were collected from participants. Both human hair and nails were stored in polyethylene bags at room temperature until analysis. Drinking water and the most commonly consumed vegetables available in the fields (like rape, sweet potato leaves, carrots, pumpkin leaves, beans, bean leaves, cassava leaves and eggplant) were also collected.
Elemental analysis
All PHE measurements were completed on an Agilent 8900 series Inductively Coupled Spectrometry (ICP-QQQ-MS; Agilent Technologies, USA) at the British Geological Survey laboratories. Reagents and standards used were of ultrapure reagent grade, and all solutions were prepared in deionised water as described in Joy et al. (2015).
Hair and nail samples were firstly cleaned by scraping physical materials, rinsing in deionised water and then immersion in acetone, in a sonic bath for 5 min, then rinsed three times with deionised water and the process repeated twice more as reported in Button et al. (2009) and Middleton et al. (2016a). Vegetables were washed three times with deionised water and dried at 40 °C in an oven and then blended to a fine powder using a coffee grinder. The dissolution of hair and nail samples utilised a nitric acid/hydrogen peroxide (4:1 ml) solution and heating using a CEM MARSX microwave (CEM Corporation, USA) as described in Middleton et al. (2016a). The resultant solutions were diluted to 30 ml with deionised water into a 30-ml wide-necked Nalgene bottle for ICP-MS analysis. Vegetable samples followed the same procedure as for nail and hair samples, with the exception of an addition of 1 ml of hydrogen peroxide after one heating cycle and the second heating applied and final dilution to 60 ml with deionised water as described in Joy et al. (2015). Multielemental analyses of hair, nails, vegetables and water by ICP-MS were undertaken using an Agilent 8900 ICP-MS, as described in Middleton et al. (2016a).
Appropriate quality-assurance procedures and precautions were carried out to ensure reliability of the results. Limit of detection was calculated as 3SD of the reagent blanks as follows: Mn, Co, Cu, Pb, Ni, Cd, Cr and Zn were < 0.6, < 0.007, < 0.07, < 0.007, < 0.03, < 0.005, < 0.03 and < 2.00 mg/kg, respectively. Certified reference materials (CRMs) data are summarised in Tables 1, 2 and 3 for CRMs: 1570a spinach leaves (n = 3), 1573a tomato leaves (n = 3), GBW 07601 human hair (n = 3) and BAPS2014 in-house human nails (n = 3) and provided acceptable recoveries.
Statistical analysis
The Pearson’s correlation coefficient was calculated to investigate the relationship of the PHEs in hair to toenails. Linear regression analysis was performed to quantify relationships between levels of PHEs in human hair and toenails. A p value of less than 0.05 was assumed to be statistically significant.
Results and discussion
Potentially harmful elements in human hair and nail samples
The mean concentrations of PHEs for hair and toenail samples are summarised in Tables 4 and 5. The average concentrations of Mn, Co, Cu, Pb, Ni, Cd, Cr and Zn in hair samples were 16.3 ± 2.15 mg/kg, 0.9 ± 0.15 mg/kg, 38 ± 6.88 mg/kg, 4.3 ± 1.95 mg/kg, 1.3 ± 0.23 mg/kg, 0.3 ± 0.02 mg/kg, 1.2 ± 0.21 mg/kg and 137 ± 21.1 mg/kg. Zinc existed with the highest average concentration and cadmium with the least. The average concentrations of heavy metals in toenails in the decreasing order were as follows: Zn > Cu > Mn > Pb > Ni > Co > Cr > Cd having mean concentrations of 172 ± 27.4 mg/kg, 29.6 ± 4.80 mg/kg, 12.0 ± 2.02 mg/kg, 4.8 ± 0.53 mg/kg, 1.7 ± 0.14 mg/kg, 1.0 ± 0.02 mg/kg, 0.6 ± 0.08 mg/kg and 0.1 ± 0.002 mg/kg. Like in hair samples Zn represented the highest PHE concentration and Cd with the least in toenails.
The PHE concentrations in hair samples were compared to normal values, or intervals of concentration of some analysed metals in healthy individuals set by Biolab (2012). The results showed that Mn, Co, Pb, Cd and Zn were above the guideline values of 0.2–2.0 mg/kg for Mn, 0.01–0.2 mg/kg for Co, < 2.0 mg/kg for Pb, < 0.1 mg/kg for Cd and 10–100 mg/kg for Zn where Ni, Cu and Cr concentrations were within the normal range concentrations of < 0.4 mg/kg, 160–240 mg/kg and 0.1–1.5 mg/kg, respectively. The mean concentration of Pb was greatly higher than the concentrations reported from other studies like Poland (Chojnacka et al. 2005) and Beijing (Gang et al. 2017)as given in Table 6, of course this could be due to different dietary habits by individuals and the geographical location of the sites studied.
PHE concentrations in toenails were compared to the findings done by Ndilila (2009) in Zambia. Elevated environmental concentrations were found in samples collected in mining town residents which nearly show the same results as from this study area in some of the PHEs examined. Toenail concentrations in Co, Pb, Cd and Cu were lower than the concentrations recorded by Ndilila from the mining town residents at 1.39 mg/kg, 21.4 mg/kg, 0.37 and 132 mg/kg. However, Zn and Ni concentrations of 172 mg/kg and 1.7 mg/kg, respectively, in this study were extremely higher than the 113 mg/kg and 0.37 mg/kg recorded from Ndilila’s study (Table 7).
Potentially harmful element concentrations in human hair and nails of different age groups
The mean PHE concentrations varied with different age groups for the selected elements measured in human hair samples and toenails as shown in Fig. 2. Zinc was highest not shown in both figures, copper was second and cadmium was lowest in all age groups for toenails and hair. Metal concentrations in hair samples for all age groups showed different orders among the PHEs in decreasing order as follows: 11–20 years: Zn > Cu > Mn > Pb > Co > Cr > Ni > Cd, 21–30 years: Zn > Cu > Mn > Pb > Ni > Cr > Co > Cd and 31–40 years: Zn > Cu > Mn > Pb > Cr > Ni > Co > Cd. These differences in distribution in the PHE levels among age groups could mainly be due to the metabolic process, environmental exposure and physiological factors (Ashraf et al. 1995; Khalique et al. 2005). Nevertheless, the order of PHE concentrations in human toenails showed the same decreasing order as follows: Zn > Cu > Mn > Pb > Ni > Co > Cr > Cd for all age groups.
Exposure assessment for vegetable consumption
The mean PHE concentrations in consumed vegetables are presented in Table 8 and illustrated in Fig. 3. The concentration values of Co, Pb, Ni and Cd in all vegetables were all above the safe values of 0.1, 0.3, 1.5 and 0.2 mg/kg of foodstuffs recommended by Joint FAO/WHO (1999) Expert Committee. This indicates that vegetables are a potential source to the higher concentrations seen in hair samples for the inhabitants of the local community. The highest contributing vegetables to the PHE intake in Co, Pb, Ni and Cd were green beans, cassava leaves and cabbage. The mean PHE concentrations of Co, Cu and Zn were also above the recommended concentrations of foodstuffs (Joint FAO/WHO 1999) except in green beans and in carrots where the concentrations were observed to be below the guideline values. Cobalt concentrations in all vegetables were above the guideline values reported by Joint FAO/WHO (1999) Expert Committee.
Zinc concentrations in the vegetables were observed to be higher than the guideline value in green beans and cassava leaves as shown in Fig. 3; however, for the rest of the vegetables Zn was below the guideline value. Manganese in all of the vegetables was below the guideline value of 500 mg/kg. Cobalt, Pb and Cd are PHEs whose higher concentrations in vegetables are likely a major contributor to human hair concentrations, for which PHEs were above the normal values set by Biolab (2012). Similarly, the high PHE burden in vegetables was likely a contribution to the higher concentrations of Pb, Co, Cu and Ni in toenails as will be discussed.
Dietary intakes of PHEs from vegetables
The estimated daily intakes and total intakes of each metal through vegetable consumption were calculated for the local community. The calculations of the dietary daily intakes for the selected PHEs were done by multiplying the mean PHE concentration in vegetables by the weight of vegetables consumed by an average individual (Gang et al. 2017). The average daily consumption rate of vegetables was considered to be 0.25 kg/person/day (Qadir et al. (2000)). The average adult body weight for the inhabitants of the local community was assumed to be 60 kg.
To evaluate the health risks derived from the dietary intakes of the selected PHEs Pb, Ni, Cd, Mn, Co, Cu and Zn, the daily intakes (DIs) were calculated and compared to the respective provisional tolerable daily intakes (PTDIs) established by WHO (1993) (Table 9). The estimated DIs through vegetable consumption were 255, 50, 264, 48, 149, 33 and 187 µg/kg bw/day for Mn, Co, Cu, Pb, Ni, Cd and Zn, respectively, following a decreasing order of DI-Cu > DI-Mn > DI-Zn > DI-Ni > DI-Co > DI-Pb > DI-Cd. The calculated daily intakes in Mn, Pb, Ni and Cd were all above the recommended PTDIs, and the rest of the heavy metals were appreciably below the PTDIs (500 µg/kg bw/day for Cu, 40–70 µg/kg bw/day for Mn, 1000 µg/kg bw/day for Zn, 5 µg/kg bw/day for Ni, 100 µg/kg bw/day for Co, 3 µg/kg bw/day for Pb and 1 µg/kg bw/day for Cd). Therefore, from these results there is an indication that these PHEs pose or might pose a health concern for the local people due to the high dietary intakes in Mn, Pb and Cd, also confirmed by the hair analysis results where these three PHEs were extremely higher than the guideline values set by Biolab Medical Unit (2010). Nevertheless, the other PHEs (Co and Zn) recorded to be above the Biolab Medical Unit guidelines in human hair were appreciably below the PTDI posing little to no threat for human health. The levels of Pb and Ni accumulated in toenail corresponded with the high dietary intake levels in vegetables for the two PHEs.
Exposure assessment for water intake
A total of 150 groundwater samples were collected from five wells at different sampling times and analysed for PHEs with measurements for some of them above the guideline values set by WHO (2017) as given in Table 10. Wells labelled 1 up to 5 were all above the guideline values set for Ni. For Mn, only well number 4 was above the guideline value of 0.4 mg/l and well 1 was the only well with Co concentration above its guideline value of 0.05 mg/l.
The results show that all wells used as sources of drinking water in the studied community are highly contaminated with Ni. Some wells constituted higher concentrations of Mn, Co and Cu above the WHO guideline values. The high concentrations of Co, Cu and Ni from the labelled wells were observed in toenails, whereas Mn and Co were observed to be much higher in human hair, indicating that drinking water was rather a minor contributor to the potentially harmful elements accumulation in body tissues.
Dietary intakes of PHEs from water
PHE contribution from drinking water was determined from the daily intakes (DI) per day calculated by multiplying the PHE concentration by 1.7 l/day (Beal et al. 2017) as given in Table 11. The results show that Mn, Cu and Ni were above the tolerable daily intakes (TDI) established by WHO (2017). Mn, Co, Cu, Ni and Zn were all above the guideline values set by WHO. However, Co and Zn concentrations were below the estimated tolerable daily intakes. The high DI from Cu and Ni was confirmed in toenails, where they were highly accumulated and recorded to be extremely higher than a comparative study conducted in the mining towns of Zambia (Ndilila 2009). Mn had a high DI compared to its TDI which can also be seen in hair analysis results where its concentration was above the Biolab (2012) interval values. This shows that Mn was highly accumulated in hair in comparison with toenails.
A total dietary intake from drinking water and vegetables showed that Mn, Cu and Ni were above the total provisional tolerable daily intake (Table 12). Water contributed about 94% of Cu to the total daily intake whose DI was seen to be lower than the provisional tolerable daily intake in vegetables, and this shows that such Cu levels as well as Mn and Ni could cause an effect to human health. Toenails accumulated a high concentration of Cu which could be originated from the uptake of vegetables and groundwater from the community and agricultural fields in the vicinity of a tailing dump.
Relationship between biomarkers and environmental samples
A Pearson’s correlation coefficient was investigated between the studied PHE concentrations in human hair and toenails, and the results showed that there was a significant positive relationship between Cd and Co concentrations (R2 = 0.265, p < 0.05).
Multiple comparisons were applied between hair and vegetables, hair and drinking water, toenails and vegetables as well as toenails and drinking water as given in Tables 13, 14, 15 and 16. Strong correlations were observed (in bold), with correlations been significant at the 0.05 level showing how hair and toenails correlated to vegetables and drinking water and a positive correlation was observed between toenails (Cu) and vegetables (Mn) concentrations with a correlation value of 0.910.
Conclusion
This study presented PHE data for drinking water, vegetables, human hair and human toenails at Mugala village in close proximity to a mine tailing dump. Vegetables were shown to accumulate Co, Pb, Ni and Cd above permissible limits of 0.1, 0.3, 0.1 and 0.1 mg/kg, respectively (FAO/WHO 1999), suggesting an environmental influence from the mine tailings. In addition, the evaluation of health risks in vegetables has shown that Mn, Pb, Ni and Cd were above the PTDIs of 40–70, 3, 5 and 1 µg/kg bw/day, respectively and could therefore present PHE exposure to the local inhabitants.
Drinking water at Mugala village contained Mn, Co, Cu and Ni above WHO (2003) guideline values of 0.40, 0.05, 2.00 and 0.02 mg/l, respectively. The daily intakes from drinking water for health risks evaluation were determined, and the results showed that Mn, Cu and Ni were all above the tolerable daily intakes (TDIs) established by WHO (2017), indicating that the local people are at risk of different health effects associated with exposure to PHEs of environmental origin.
Total daily intakes for Mn, Cu and Ni were 2905, 4514 and 4789 µg/kg bw/day, respectively, which were all above total PTDIs for each PHE. The high Cu levels in toenails could originate from the intake of contaminated vegetables and groundwater sourced within community and its agricultural fields in the vicinity of the Mugala mine tailing dump.
The results presented in this paper revealed that the concentration of Co, Pb and Ni was above ‘interval’ concentration ranges for these metals found in hair by the Biolab (2012), indicating that the local inhabitants of the studied area are at potential risk of exposure and require an in-depth study on the implications of exposure and specific exposure pathways. Positive correlations between hair and toenails as biomarkers of exposure and the dietary intake of vegetables and drinking water from Mugala village indicated a link between contamination of the agricultural fields and groundwater supplies either as a natural geogenic input or from the nearby mine tailing. Therefore, regular monitoring of these elements in drinking water, soils and vegetables is essential alongside the identification of the specific source (e.g. wind-blown dust) of the elevated PHE concentrations.
References
Alves, A., Kucharska, A., Erratico, C., Xu, F., Hand, E. D., Koppen, G., et al. (2014). Human biomonitoring of emerging pollutants through non-invasive matrices: State of art and future potential. Anal Bioanalytical Chemistry,406(17), 4063–4088. https://doi.org/10.1007/s00216-014-7748-1.
Ashraf, W., Jaffar, M., Anwer, K., & Ehsan, U. (1995). Age and sex-based comparative distribution of the selected metals in the scalp hair of an urban population from two cities Pakistan. Environmental Pollution,87, 61–64.
Bakri, S. F. Z., Hariri, A., Ma’arop, N. F., & Hussin, N. S. A. (2017). Toenail as non-invasive biomarker in metal toxicity measurement of welding fumes exposure: A review. In International conference on applied sciences (ICAS2016), material science and engineering (Vol. 165). https://doi.org/10.1088/1757-899x/165/1/012019.
Beal, T. Y., Marriott, E., Arsenault, J. E., Smith, M. R., & HIjmano, R. J. (2017). Global trends in dietary micronutrients supplies and estimated prevalence of inadequate intakes. PLoS ONE,12(4), E0175554. https://doi.org/10.1371/journal.pone.0175554.
Biolab. (2012). Nutritional and environmental medicine. London: Hair Mineral Analysis. Retrieved from https://www.biolab.co.uk/docs/Hair_Mineral_Analysis.pdf. Accessed May 10, 2019.
Button, M., Jenkin, G. R. T., Harrington, C. P., & Watts, M. J. (2009). Human toenails as a biomarker of exposure to elevated environmental arsenic. Journal of Environmental Monitoring,11, 610–617. https://doi.org/10.1039/b817097e.
Chan, S. M., Wang, W. X., & Ni, L. H. (2003). The uptake of Cd, Cr and Zn by the microalga Enteromorpha crinite and subsequent transfer to the marine herbivorous rabbitfish, Siganus. Archives of Environmental Contamination and Toxicology,44, 298–306.
Chojnacka, K., Gorecka, H., Chojnacka, A., & Gorecki, H. (2005). Inter-element interactions in human hair. Environmental Toxicology and Pharmacology,20, 368–374.
Ciszewski, A., Wasiak, W., & Ciszewski, W. (1997). Hair analysis. Part 2. Differential Pulse anodic stripping volumetric determination of thallium in human hair samples of persons in permanent contact with lead in their workplace. Analytical Chimica Acta,343, 225–229.
Dombovari, J., & Papp, L. (1998). Comparison of sample preparation methods for elemental analysis of human hair. Microchemical Journal,59, 187–193.
Dombovari, J., Papp, L., Uzonyi, L., Barbely-Kiss, I., Elekes, Z., Varga, Z., et al. (1999). Study of cross-sectional and longitudinal distributions of some major and minor elements in the hair samples of haemodialysed patients with micro-PIXE. Journal of Analytical Atomic Spectrometry,14, 553–557.
Gang, L., Ligang, P., & Xinhui, X. (2017). Assessment of typical heavy metals in human hair of different age groups and foodstuffs in Beijing, China. International Journal of Environmental Research and Public Health,14, 914.
Gao, X., Chen, C.-T. A. (2012). Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Research, 46(6), 1901–1911.
Gibson, R. S., Raboy, V., & King, J. C. (2018). Implications of phytate in plant-based foods for iron and zinc bioavailability setting dietary requirements, and formulating programs and policies. Nutrition Review,76(11), 793–804.
Goulle, J. S., Saussereau, E., Mahiem, L., Groenwont, S., Guerbet, M., & Lacroix, C. (2009). Application of inductively coupled plasma mass spectrometry multielement analysis in fingernails and toenail as a biomarker of metal exposure. Journal of Analytical Toxicology,33, 92–98.
Horiguchi, H., Teranishi, H., Niiya, K., Aoshima, K., Katosh, T., Sakuragawa, N., et al. (1994). Hypoproduction of erythropoietin contributes to anaemia in chronic cadmium intoxication: Clinical study on Itai-itai disease in Japan. Archives of Toxicology,68, 632–636.
Ionescu, J., Novotny, J., Stejskal, V., Latsch, A., Blaurock-Busch, E., & Eistenmann-Klein, M. (2006). Increased levels of transition metals in breast cancer tissue. Neuro Endocrinology Letters,27(suppl 1), 36–39.
Jarup, L. (2013). Hazards of heavy metal contamination. British Medical Bulletin,68, 167–182.
Joint FAO/WHO Expert Committee on Food Additives. (1999). Summary and conclusion. In Proceedings of the 53rd meeting Joint FAO/WHO Expert Committee on food additives, Rome, Italy.
Joy, E. J. M., Broadley, M. R., Young, S. D., Black, C. R., Chilimba, A. D. C., Ander, E. L., et al. (2015). Soil type influences crop mineral composition in Malawi. Science of the Total Environment,505, 587–595.
Kaewsarn, P., & Yu, Q. M. (2001). Cadmium(II) removal from aqueous solutions by pre-treated biomass of marine algae Padina sp. Environmental Pollution,112, 209–213.
Kaninga, B., Chishala, B. H., Maseka, K. K., Sakala, G. M., Lark, M. R., Tye, A., et al. (2019). Review: Mine tailings in an African tropical environment—Mechanisms for the bioavailability of heavy metals in soils. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00326-2).
Katz, S. A., & Chatt, A. (1998). Hair analysis: Application in the biomedical and environmental science. New York: VCH Publishers. https://doi.org/10.1002/jat.2550100119.
Khalique, A., Ahmad, S., Anjum, T., Jaffar, M., Munir-Shah, M. H., Shaheen, N., et al. (2005). Comparative study based on gender and age dependence of selected metals in scalp hair. Environmental Monitoring and Assessment,104(1–3), 45–57.
Liu, G., Yu, Y., Hou, J., Xue, W., Liu, X., Liu, Y., et al. (2014). An ecological risk assessment of heavy metal pollution of the agricultural ecosystem near a lead-acid battery factory. Ecological Indicators,47, 210–218.
McBride, M. B. (1994). Environmental chemistry in soils. Oxford: Oxford University Press. ISBN-10: 0195070119.
Middleton, D. R. S., McCormack, V. A., Munishi, M. O., Mwenya, D., Marriott, A. L., Hamilton, E. M., et al. (2019a). Intra-household agreement of urinary elemental concentrations in Tanzania and Kenya: Potential surrogates in case–control studies. Journal of Exposure Science & Environmental Epidemiology,29, 335–343. https://doi.org/10.1038/s41370-018-0071-8.
Middleton, D. R. S., McCormack, V. A., Watts, M. J., & Schuz, J. (2019b). Environmental geochemistry and cancer: A pertinent global health problem requiring interdisciplinary collaboration. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00303-9.
Middleton, D. R. S., Watts, M. J., Hamilton, E. M., Ander, E. L., Close, R. M., Exley, K. S., et al. (2016a). Urinary arsenic profiles reveal exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK. Scientific Reports,6, 25656. https://doi.org/10.1038/srep25656.
Middleton, D. R. S., Watts, M. J., Hamilton, E. M., Fretcher, T., Leonardi, G. S., Close, R. M., et al. (2016b). Prolonged exposure to arsenic in UK private water supplies: Toenail, hair and drinking water concentrations. Environmental Science: Processes & Impacts,2016(18), 562–574.
Middleton, D. R. S., Watts, M. J., Lark, M. R., Milne, C. J., & Polya, D. A. (2016c). Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environmental Health,15, 68–81.
Ncube, E., Banda, C., & Mundike, J. (2012). Air pollution on the Copperbelt Province of Zambia: Effects of sulphur dioxide on vegetation and humans. Journal of Natural and Environmental Sciences,3(1), 34–41.
Ndilila, W. (2009). Investigating metal exposure on the general populace of the copper mining town of Kitwe, Zambia. Retrieved from https://www.ro.ecu.edu.cuu/thesis/1844. Accessed 18 Jan 2019.
Nies, D. H. (1999). Microbial heavy-metal resistance. Applied Microbiology and Biotechnology,51, 730–750.
O’Reilly, J., Watts, M. J., Shaw, R. A., & Ward, N. L. (2010). Arsenic contamination of natural waters in San Suan and La Pampa, Argentina. Environmental Geochemistry and Health,32(6), 491–515. https://doi.org/10.1007/s10653-010-9317-7.
Oyoo-Okoth, E., Admiraal, W., Osano, O., & Kraak, M. H. S. (2012). Element profiles in hair and nails of children reflect the uptake from food and the environment. Environmental Toxicology and Chemistry,31, 2012.
Oyoo-Okoth, E., Admiraal, W., Osano, O., Manguya-Lusega, D., Ngure, V., Kraak, M. H. S., et al. (2013). Contribution of soil, water and food consumption to metal exposure of children from geological enriched environments in the coastal zone of Lake Victoria, Kenya. International Journal of Hygiene and Environmental Health, 216, 8–16.
Qadir, M., Ghafoor, A., & Murtaza, G. (2000). Cadmium concentration in vegetables grown on urban soils irrigated with untreated municipal sewage. Environment, Development and Sustainability,2(1), 13–21.
Rashed, M., & Hossam, F. (2007). Heavy metals in fingernails and scalp hair of children, adults and workers from environmentally exposed areas and Aswan, Egypt. Environmental Bioindicators,2, 131–145.
Reason, D. A., Watts, M. J., Devez, A., & Broadley, M. R. (2015). Quantification of phytic acid in grains. British Geological Survey Open Report, OR/15/070. http://nora.nerc.ac.uk/id/eprint/512947. Accessed March 2019.
Romanowicz-Makoska, H., Forma, E., Brys, M., Krajewska, W., & Smolarz, B. (2011). Concentration of cadmium, nickel and aluminium in female breast cancer. Polish Journal of Pathology,62(4), 257–261.
Srivastava, S., & Jokanc, M. (2016). Mining and environmental remediation and implications project. Zambia. http://documents.Worldbank.org/curated/en/813951469077929423/Environmental-and-social-management-framework. Accessed 18 Jan 2019.
Stewart, A. G. (2019). Mining is bad for health. Env: Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00367-7.
Stihi, C., Radulescu, C., Busuioc, G., Popescu, I.V., Gheboianu, A., & Ene, A. (2011). Studies on accumulation of heavy metals from substrate to edible wild mushrooms. Romanian Journal of Physics, 56(1–2), 257–264.
Vogel, A. I. (1989). Vogel’s textbook of quantitative chemical analyses (5th ed.). Harlow: Longman Scientific and Technical. ISBN 0-582-44693-7.
WHO. (2003). The world health report 2003 - shaping the future, https://www.who.int/whr/2003/en/. Accessed 16 July 2019.
WHO. (2011). Evaluation of certain food additives and contaminants, Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives, https://apps.who.int/iris/bitstream/handle/10665/44515/WHO_TRS_960_eng.pdf;jsessionid=4F53FFEF556B5813D551A61BF138E197?sequence=1. Accessed July 16 2019.
WHO. (2017). Guidelines for drinking water quality. First Addendum to the Fourth Edition. ISBN 978-92-4-155001-7 https://apps.who.int/iris/bitstream/handle/10665/254636/9789241550017-eng.pdf;jsessionid=6C94668688C1D8E87609E6EA3F17B783?sequence=1. Accessed March 2019.
Wongsasuluk, P., Chotpantarat, S., Siriwong, W., & Robson, M. (2018). Using urine as a biomarker in human exposure risk associated with arsenic and other heavy metals contaminating drinking groundwater in intensively agricultural areas of Thailand. Environmental Geochemistry and Health,40(1), 323–348. https://doi.org/10.1007/s10653-017-9910-0.
Zhuang, P., McBride, M. B., Xia, H., Li, N., & Li, Z. (2009). Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Science of the Total Environment,407, 1551–1561.
Zhunk, L. I., & Kist, A. A. (1995). Human hair instrumental neutron activation analysis and medicine. Journal of Radioanalytical and Nuclear Chemistry, 195, 75–81. https://doi.org/10.1007/BF02036475.
Zia, M. H., Watts, M. J., Niaz, A., Middleton, D. R. S., & Kim, A. W. (2017). Health risk assessment of PHEs and dietary minerals from vegetables irrigated with untreated wastewater, Pakistan. Environmental Geochemistry and Health,39(4), 707–728. https://doi.org/10.1007/s10653-016-9841-1.
Acknowledgements
Authors acknowledge funding from the Royal Society International Exchange Scheme (IES_RS_170206) through the project ‘Risk of exposure to mine tailings’, in addition to the Zambia Agricultural Research Institute, the British Geological Survey and the Copperbelt University for funding and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakaona, L., Maseka, K.K., Hamilton, E.M. et al. Using human hair and nails as biomarkers to assess exposure of potentially harmful elements to populations living near mine waste dumps. Environ Geochem Health 42, 1197–1209 (2020). https://doi.org/10.1007/s10653-019-00376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00376-6