Abstract
Arsenic (As) speciation in surface and groundwater from two provinces in Argentina (San Juan and La Pampa) was investigated using solid phase extraction (SPE) cartridge methodology with comparison to total arsenic concentrations. A third province, Río Negro, was used as a control to the study. Strong cation exchange (SCX) and strong anion exchange (SAX) cartridges were utilised in series for the separation and preservation of arsenite (AsIII), arsenate (AsV), monomethylarsonic acid (MAV) and dimethylarsinic acid (DMAV). Samples were collected from a range of water outlets (rivers/streams, wells, untreated domestic taps, well water treatment works) to assess the relationship between total arsenic and arsenic species, water type and water parameters (pH, conductivity and total dissolved solids, TDS). Analysis of the waters for arsenic (total and species) was performed by inductively coupled plasma mass spectrometry (ICP-MS) in collision cell mode. Total arsenic concentrations in the surface and groundwater from Encon and the San José de Jáchal region of San Juan (north-west Argentina within the Cuyo region) ranged from 9 to 357 μg l−1 As. Groundwater from Eduardo Castex (EC) and Ingeniero Luiggi (LU) in La Pampa (central Argentina within the Chaco-Pampean Plain) ranged from 3 to 1326 μg l−1 As. The pH range for the provinces of San Juan (7.2–9.7) and La Pampa (7.0–9.9) are in agreement with other published literature. The highest total arsenic concentrations were found in La Pampa well waters (both rural farms and pre-treated urban sources), particularly where there was high pH (typically > 8.2), conductivity (>2,600 μS cm−1) and TDS (>1,400 mg l−1). Reverse osmosis (RO) treatment of well waters in La Pampa for domestic drinking water in EC and LU significantly reduced total arsenic concentrations from a range of 216–224 μg l−1 As to 0.3–0.8 μg l−1 As. Arsenic species for both provinces were predominantly AsIII and AsV. AsIII and AsV concentrations in San Juan ranged from 4–138 μg l−1 to <0.02–22 μg l−1 for surface waters (in the San José de Jáchal region) and 23–346 μg l−1 and 0.04–76 μg l−1 for groundwater, respectively. This translates to a relative AsIII abundance of 69–100% of the total arsenic in surface waters and 32–100% in groundwater. This is unexpected because it is typically thought that in oxidising conditions (surface waters), the dominant arsenic species is AsV. However, data from the SPE methodology suggests that AsIII is the prevalent species in San Juan, indicating a greater influence from reductive processes. La Pampa groundwater had AsIII and AsV concentrations of 5–1,332 μg l−1 and 0.09–592 μg l−1 for EC and 32–242 μg l−1 and 30–277 μg l−1 As for LU, respectively. Detectable levels of MAV were reported in both provinces up to a concentration of 79 μg l−1 (equating to up to 33% of the total arsenic). Previously published literature has focused primarily on the inorganic arsenic species, however this study highlights the potentially significant concentrations of organoarsenicals present in natural waters. The potential for separating and preserving individual arsenic species in the field to avoid transformation during transport to the laboratory, enabling an accurate assessment of in situ arsenic speciation in water supplies is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1993, the World Health Organisation (WHO) revised its guideline level for arsenic in drinking water from 50 to 10 μg l−1 As (WHO 1993). Argentina still adopts the former WHO limit of 50 μg l−1 As as its national drinking water standard (Frisbie et al. 2005). The new WHO recommended value came in as a direct result of the increased awareness of the toxicity of arsenic, particularly its carcinogenicity (Jong and Parry 2005). Human exposure to arsenic can occur through a variety of pathways, including air, water, soil and food (Mandal and Suzuki 2002). Arsenic exposure through food poses a substantial risk to humans in certain parts of the world, particularly in Asia from the consumption of staple foods such as rice and vegetables, which have been irrigated with As-rich groundwater (Meharg et al. 2009; Mondal and Polya 2008; Kile et al. 2007; Smith et al. 2006). However, drinking water is seen to pose the most significant risk to human health in Argentina, primarily through consumption and cooking, this is due to the various potential sources of arsenic present in the region, such as in groundwater and surface water (Nguyen et al. 2009; Asante et al. 2007; Ning et al. 2007; Ohno et al. 2007).
In recent years various studies have highlighted that countries such as Argentina, Bangladesh and Chile were experiencing high total concentrations of natural and anthropogenic (man-made) arsenic in drinking water from newly identified sources (Halim et al. 2009; Bhattacharya et al. 2006; Oyarzun et al. 2004; Ng et al. 2003; Smedley et al. 2002). The most common sources of arsenic in the natural environment are volcanic rocks (specifically their weathering products and ash), marine sedimentary rocks, hydrothermal ore deposits and associated geothermal waters, and fossil fuels, including coals and petroleum (Wang and Mulligan 2006; Smedley and Kinniburgh 2002). Anthropogenic activity, such as gold mining, has contributed to a sharp rise in natural arsenic concentrations reported for many artesian water supplies, often exceeding 600 μg l−1 As (Duker et al. 2005; Farías et al. 2003; Plant et al. 2003). Local communities use these water supplies for drinking, cooking (Tseng 2009; Roychowdhury et al. 2005), cattle watering (Nriagu et al. 2007) and crop irrigation (Bhattacharya et al. 2007; Hossain 2006; Bundschuh et al. 2004), creating a possible pathway for the arsenic to enter both the animal and human populations.
The chemical species of arsenic, which can exist in the natural environment heavily influence its mobility, adsorptivity and toxicity (Ascar et al. 2008). In solution, arsenic primarily occurs as the inorganic arsenate (AsV) species (H2AsO4 − and HAsO4 2−) and the uncharged arsenite (AsIII) species (H3AsO 03 ), as shown in Fig. 1 (Gault et al. 2005a; Kumaresan and Riyazuddin 2001). The organic species monomethylarsonic acid (MAV), and dimethylarsinic acid (DMAV), have also been measured in surface and groundwater at the sub μg l−1 level (Smedley and Kinniburgh 2002). Variations in redox potential and pH will affect species predominance and the distribution between aqueous and solid phases. The mobility of inorganic arsenic (AsIII and AsV) under reducing conditions in aquifers has been widely reported (Nath et al. 2008; Bhattacharya et al. 2007). Arsenic mobilisation under oxidising conditions is also recognised as an important process, especially in the contamination of water affected by oxidation of sulphide minerals (Schreiber et al. 2000). Very high concentrations of aqueous arsenic are achievable under these conditions, however its mobilisation is heavily restricted due to the strong adsorptive capacity of metal oxides in soils/sediments, especially in the presence of iron (Fe) oxides (Scanlon et al. 2009; Smedley et al. 2002). Although, under oxidising conditions and high pH (~ 8.5–9.5), arsenic is often less strongly bound to Fe oxides than at lower pH ranges, allowing for an enhanced mobility (Verplanck et al. 2008). If these conditions persist in aquifer environments, elevated aqueous arsenic concentrations may be a widespread occurrence. The shallow groundwater aquifers in the Chaco-Pampean Plain of Argentina are a good example (Gomez et al. 2009; Mukherjee et al. 2008; Bundschuh et al. 2004). High concentrations of total arsenic and other trace elements (B, F, Mo, V, U) have been reported to cause water-quality problems in aquifers from the provinces of Córdoba, Santa Fe and Buenos Aires, as well as La Pampa (Gomez et al. 2009; Smedley et al. 2005).
Eh-pH diagram for aqueous arsenic species in the system As–Fe–O–H–S in water at 25°C, 1 bar pressure with total arsenic 10−6 mol l−1 (Daus et al. 2002)
Arsenic has long been recognised as a toxic and carcinogenic element, with widespread health problems from contaminated groundwater reported in South America. Chronic long-term arsenic exposure thought to be a result of contact with inorganic arsenic (iAs) from drinking water sources, typically above 50 μg l−1 As are symptomatic of skin, cardiovascular, renal, haematological and respiratory disorders (Bertolino et al. 2007; Hughes 2002). Documented evidence of illnesses from oral exposure to elevated arsenic is commonly seen in a pattern of skin changes including hyperpigmentation, hypopigmentation and hyperkeratosis lesions of the skin and the appearance of small “corns” or “warts” on the palms, soles, and torso. A small number of corns may develop into skin cancer (Chou and De Rosa 2003). Ingestion of arsenic causes peripheral vascular diseases and is associated with carotid atherosclerosis (Chiou et al. 2005; Wang et al. 2002). Ingesting iAs has also been reported to increase the risk of cancer in the bladder, lung, liver, and kidney (Ferreccio and Sancha 2006). It is becoming increasingly more important to identify arsenic species (particularly iAs) in drinking water sources due to the variability in their metabolic pathway in humans (reduction of AsV to AsIII, followed by oxidative methylation of AsIII) and the potential toxicological effects (Suzuki 2005).
The main objective of this study was to determine the concentration of both total dissolved arsenic and its individual chemical species (AsIII, AsV, MAV and DMAV) using novel solid phase extraction (SPE) technology in relation to different geographical regions of Argentina; water types; and physical parameters (pH, conductivity and TDS). The SPE cartridges utilised in this study (500 mg SCX and SAX Varian Bond Elut® Junior) have been shown to be highly effective in preserving the four arsenic species under investigation in the field, with little influence from external factors such as pH (Watts et al. 2010). This technique allows for greater specificity and accuracy in the determination of arsenic species. Comparison of arsenic concentrations (total and species) from various water sources (surface water, groundwater and tap) provides an insight into characteristic features of arsenic mobility and the potential toxicity in these regions of Argentina.
Materials and methods
Study sites
Surface and groundwater samples were collected from two provinces in Argentina, San Juan and La Pampa (Fig. 2). The sites were selected based on their direct interaction with human exposure pathways, via drinking water, crop production (irrigation) and animal grazing (cattle, goat, horse and sheep). Sites were also selected based on known high total arsenic concentrations in natural waters (Smedley et al. 2002) and evidence of arsenic related health problems within the population cited by local medical practitioners, with symptoms ranging from keratosis and skin lesions to several reported cases of cancer. Additional water samples were collected from the province of Río Negro as a control to the arsenic study (Fig. 2).
San Juan
San Juan Province is located in western Argentina on the border with Chile within the Cuyo region, approximately 280 km east of the Pacific coastline. Average rainfall in the region is very low ~100 mm per year, creating a semi-arid environment (de Salmuni et al. 2007). The province covers an extensive area of over 89,000 km2 (Dilks 2004). Water samples were principally collected from river/stream systems (surface waters), used for irrigation and cattle watering purposes, groundwater sources as well as untreated domestic tap supplies. Study sites comprised surface waters from the Río Blanco near Angualasto (ANG) [30o3′0S, 69o9′0 W]; the Río Jáchal (RJ) in and around San José de Jáchal [30o14′0S, 68o45′0 W]; the Cuesta del Viento dam (CU) on the Río Jáchal; the Río Colola (CO), a tributary that feeds into CU; Agua Negra (AN), a freshwater spring flowing into the Río Jáchal and the Río Huaco in Huaco (HU) [30o09′17S, 68o28′46 W] as shown in Fig. 3. Surface waters were collected at a range of depths (0–30 cm below the water level). Domestic (untreated) tap supplies were collected from Niquivil (NQ) [30o24′2S, 68o41′47 W] and San Juan (SJ) [31o32′03S, 68o31′34 W] the provincial capital of San Juan Province (Figs. 3, 4). Tap water samples were taken after allowing the water to run for a minimum of 30–60 s through the pipes, in order to flush the pipeline of any potential adsorbed-As deposits. Artesian well and untreated residential tap waters were taken from the community of Encon (EN) [32o12′0S, 67o47′0 W] on the San Juan–Mendoza border (Fig. 4). Water samples (groundwater) collected from the community of Encon originate from two different sources, the main community well supply and a blended combination of this well with a rural farm (finca) well, (which is located approximately 30 km away and the extracted water is piped to Encon where it is blended and stored in tanks for community distribution and usage).
Sampling elevations ranged from 1,635 m above sea level (a.s.l.) at Angualasto in the north to 507 m a.s.l. at Encon in the south. Water collection from San Juan (San José de Jáchal and San Juan areas) amounted to 23 surface water samples and 5 domestic tap supply samples, as well as 15 samples from the community of Encon (5 urban well; 4 finca (rural farm) well; 3 blended untreated tap supplies; 3 school water supplies).
La Pampa
La Pampa Province is located in central Argentina covering an approximate area of 140,000 km2 within the Chaco-Pampean Plain, at a distance of 450 km west of the Atlantic coastline (Nicolli et al. 1989). Rainfall is greatest in the north and north-east of the province, which experiences between 500–700 mm per year (Michelena and Irurtia 1995). The study was focused to the north of the province in the towns of Eduardo Castex [35o53′60S, 64o17′60 W], 80 km north of Santa Rosa, the provincial capital, and Ingeniero Luiggi [35o25′0S, 64o28′60 W], 130 km northwest of Santa Rosa, at elevations of 166–206 m a.s.l. Well waters (groundwater from Quaternary loess aquifers), used predominantly for drinking and irrigation were sampled from individual farmsteads (rural wells), urban wells and from two reverse osmosis water treatment works in Eduardo Castex (EC) and Ingeniero Luiggi (LU) (Fig. 5). Water flow-rates at the private farmsteads (fincas) were dependent on wind conditions, because wind turbines are used to draw up the groundwater into untreated open-air storage tanks. Flow-rates varied from 1 to 19 l min−1 at the time of sampling and between sites. Tap water samples were taken after allowing the water to run for a minimum of 30–60 s through the pipes. A total of 157 water samples (60 urban wells; 52 rural wells; 15 untreated domestic tap supplies; 30 water treatment works) were collected from the province (Fig. 5).
Río Negro
Río Negro Province lies within Patagonia (encompassed in the Monte Desert), extending westward from the Atlantic Ocean to the Cordillera of the Andes to the north of 42°S and the border with Neuquén Province (Abraham et al. 2009) (Fig. 2). The province covers an area of 203,013 km2 (Zárate 2003). Water samples were collected from surface waters (Río Colorado (RC) and Río Negro (RN)), domestic tap supplies from the local school and residences and groundwater well supplies in the province (Fig. 6). Sampling was primarily focused to the north of the province in the town of General Roca (GR) [39o02′S, 67o35′W] approximately 418 km north-west of the provincial capital, Viedma, at typical elevations of 227–362 m a.s.l. Water samples collected from the province consisted of 40 surface waters, 6 groundwater well supplies and 31 domestic tap waters.
Water sampling
Water samples for total arsenic determination were collected in opaque 30 ml Nalgene® bottles (Thermo Fisher Scientific, UK). Prior to collection, each Nalgene® bottle was rinsed three times with the filtered water sample, to minimise potential elemental contamination from the bottle during storage. Filtered/acidified (F/A) water samples for total arsenic analysis were drawn up in clean/rinsed disposable 20 ml BD™ plastic syringes (Becton–Dickinson Ltd, UK), injected into each bottle through a 0.45 μm membrane filter and preserved to an acidity of 1% v/v HNO3 (not accounting for potential neutralisation from carbonates/bicarbonates/phosphates) to prevent arsenic precipitation during transportation (Pandey et al. 2004; Bednar et al. 2002a; Garbarino et al. 2002). A further 30 ml water sample was passed through the SPE cartridge set-up for arsenic speciation. A filtered/unacidified (F/UA) aliquot of water was also taken for arsenic speciation analysis by HPLC-ICP-MS, as an inter-analytical method comparison with the SPE cartridges. All samples were stored at 4°C during field sampling using a Tropicool 14 Litre Thermoelectric Cool Box TC-14 (Waeco©, Dorset, UK) connected to a powered car cigarette lighter socket. All water samples were subsequently transferred to a refrigerator (4°C) on return to the laboratory. The analysis of waters for arsenic species by HPLC-ICP-MS was carried out within 2 weeks of sample collection. It has been shown in the literature that organoarsenicals remain stable in waters for at least 2 weeks when stored at 4°C and in dark conditions (Francesconi and Kuehnelt 2004; Bednar et al. 2002a; Gong et al. 2002; Roig-Navarro et al. 2001).
Physical water parameter measurements (pH; conductivity, μS cm−1; total dissolved solid (TDS), mg l−1) were recorded at the time of sampling (prior to filtration/acidification) using a fully calibrated Hanna HI 98129 Digital Combo Meter (Hanna Instruments Ltd, UK). A GPS reference and elevation was also taken at each sampling site using a Garmin™ Geko 201 (Garmin Ltd., UK).
Field-based arsenic speciation methodology
On-site arsenic speciation was carried out utilising pre-conditioned Varian 500 mg Junior Bond Elut® strong cation exchange (SCX) and strong anion exchange (SAX) cartridges (Varian, UK) as described by Watts et al. (2010). Conditioning of the SCX cartridge was undertaken using 15 ml of 50% methanol (CH3OH; BDH; HPLC Grade, UK) followed by 15 ml of 1 M phosphoric acid (H3PO4; BDH; Aristar®, UK) and 5 ml of de-ionised water. The SAX cartridge was preconditioned using 15 ml of 50% methanol and 5 ml of de-ionised water. Following the conditioning process, the cartridges were connected in series with a 0.45 μm filter (Fig. 7). A known volume of water (typically 30 ml) was then passed through the assembled kit using a clean/rinsed disposable 20 ml BD™ plastic syringe (Becton–Dickinson Ltd, UK). The effluent retained the AsIII species, the SCX cartridge retained DMAV and the SAX cartridge captured the MAV and AsV species. Subsequent elution of the species captured on the cartridges was achieved using 5 ml of 1 M HNO3 for DMAV on the SCX cartridge. MAV was firstly eluted from the SAX cartridge with 5 ml of 80 mM acetic acid (CH3COOH; BDH; Aristar®, UK) into a 15 ml bottle followed by 5 ml of 1 M HNO3 to elute the AsV species also retained on the SAX cartridge, which was eluted into a separate 15 ml bottle (Fig. 7).
Verification of the SPE methodology was achieved using synthetic As solutions (AsIII, AsV, MAV and DMAV each at 10 μg l−1), with recoveries of 94–105% (n = 25). The influence of pH on the recovery of the individual arsenic species was investigated over the pH range 4–10, AsIII and DMAV exhibited recoveries of 95–104%, whilst AsV and MAV showed slightly elevated recoveries of 109–117%. A series of matrix components (Br, F, Fe, Cl, Mn, NO2, NO3, PO4 and SO4) were also tested to investigate the performance of the SPE cartridges, as outlined by Watts et al. (2010). Validation of the SPE field-based arsenic speciation method was undertaken using HPLC-ICP-MS as described in the literature (Button et al. 2009; Watts et al. 2007), to confirm the presence of the individual arsenic species in their respective fractions, as reported by Watts et al. (2010).
Standards and reagents
All chemical reagents were of analytical reagent grade. Aqueous solutions were prepared using de-ionised water (18.2 MΩ; Millipore, UK). Water samples (30 ml) were filtered (0.45 μm; Millex, UK) and preserved on collection with nitric acid (HNO3; BDH; Aristar®, UK) for the analysis of total arsenic.
Instrumentation
Filtered and acidified (F/A) water samples for total arsenic analysis and fractionated water samples from the SPE field-based speciation method were analysed using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500 Series, Agilent Technologies, UK) at the British Geological Survey (BGS), Keyworth, Nottinghamshire. The standard operating conditions were as follows: RF power 1,550 W; reflected power <20 W; coolant gas flow 15 l min−1; auxiliary gas flow 0.8 l min−1; nebuliser gas flow 0.8 l min−1; spray chamber temperature 4°C. The ICP-MS instrument was fitted with a Micromist concentric nebuliser and PTFE Scott-type spray chamber. Arsenic detection was performed in collision cell mode using 4 l min−1 He to minimise polyatomic interferences at m/z 75 such as 40Ar35Cl+.
Optimisation of the ICP-MS was performed daily for arsenic and tuned using a 100 μg l−1 dilution of Claritas PPT® multi-element tune solution 1 (SPEX CertiPrep®, UK). A 50 μg l−1 solution of tellurium (SPEX CertiPrep®, UK) in 1% v/v HNO3 was used as an internal standard for arsenic to correct for signal drift (<±1% signal change) through addition to the sample solution via a T-piece. Calibration standards were prepared from a multi-element standard (SPEX CertiPrep®, UK) over the calibration range 0.1–100 μg l−1 As. The instrumental limit of detection (LOD) for arsenic by the Agilent ICP-MS represented as the mean blank (n > 10) signal + 3 × standard deviation (SD) was 0.1 μg l−1 As. The instrumental LOD (mean blank (n > 10) signal + 3 × SD) for each of the arsenic species by the field-based SPE method (using ICP-MS detection) was AsIII: 0.12 μg l−1, AsV: 0.02 μg l−1, MAV: 0.02 μg l−1 and DMAV: 0.03 μg l−1. A preconcentration factor of ×6 (30 ml water sample passed through cartridge set-up, followed by arsenic species removal with 5 ml eluting solution) was applied to the eluted fractions of AsV, MAV and DMAV from the SPE cartridges. A subsequent ×2 dilution was performed prior to ICP-MS analysis, resulting in a final preconcentration factor for AsV, MAV and DMAV of ×3.
Validation of the ICP-MS for arsenic was achieved using certified reference waters TMDA-54.4 (National Water Research Institute, Ontario, Canada) and SRM® 1643e (National Institute of Standards and Technology, Maryland, USA). Mean total arsenic concentrations (± SD) over a series of 10 analytical runs (n = 36) for TMDA-54.4 was 45.3 ± 2.3 μg l−1 As (certified: 43.6 ± 0.8 μg l−1 As) and 58.3 ± 3.3 μg l−1 As for SRM® 1643e (certified: 60.5 ± 0.7 μg l−1 As). The HPLC-ICP-MS procedure was monitored using the certified reference material CRM No. 18 Human Urine (National Institute for Environmental Studies, NIES, Ibaraki, Japan). A mean total arsenic value of 131.1 ± 1.2 μg l−1 As (n = 8) was achieved (certified: 137 ± 11 μg l−1 As).
A comparison of the mean arsenic species concentrations from the SPE field-based method and the HPLC-ICP-MS laboratory based method showed no statistically significant difference between the two techniques at P < 0.01 (Watts et al. 2010). The relationship between the sum of the arsenic species (AsIII, AsV, MAV and DMAV) by the SPE method and the total arsenic concentration in the F/A water samples reported a strong positive correlation (R2 = 0.9874).
Results
Water parameter results
Physical water parameter (pH, conductivity, total dissolved solids (TDS)) measurements for surface, ground and domestic tap waters show clear variations both within and between the provinces of San Juan and La Pampa, Argentina (Table 1).
Different pH ranges were observed between the surface and domestic tap waters from the San Juan (SJ) sampling locations (Figs. 3, 4), namely 7.8–8.9 and 7.2–7.7, respectively (Table 1). Conductivity levels ranged from 1,295–2,506 μS cm−1 in the surface waters and 1,126–1,837 μS cm−1 in the domestic tap supplies. Conversely, TDS levels in surface waters ranged from 527–1,245 mg l−1 and 922 to >2,000 mg l−1 in domestic tap supplies (Table 1).
The two different water sources in Encon, as well as their blended supply demonstrated similar parameter measurements. The pH conditions ranged from 9.3 to 9.7 for the urban well, 9.4–9.7 for the rural well and 9.1–9.4 for the blended tap supply (Table 1). Conductivity levels ranged from 972 to 1,603 μS cm−1 for the urban well, 1,387–1,552 μS cm−1 for the rural well and 1,047–1,534 μS cm−1 for the combined tap water supply (Table 1). Similar levels were also reported for TDS, 526–1,346 mg l−1 for the urban well, 690–1,405 mg l−1 for the rural well and the combined water supply exhibited 997–1,267 mg l−1 (Table 1). Treatment of the drinking water by ion-exchange in the local school—Escuela Albergue Dr Juan Carlos Navarro, reduced pH levels from 9.6 (untreated) to 8.0 (treated), conductivity levels from 1,387 μS cm−1 (untreated) to 743 μS cm−1 (treated) and TDS levels from 1,107 mg l−1 (untreated) to 561 mg l−1 (treated). Conductivity measurements showed little variability between water types in Encon, with the urban well displaying the greatest range (Table 1). TDS results for Encon were comparable with those exhibited in the surface waters from the province, ranging from 526–1,405 mg l−1 to 527–1,245 mg l−1, respectively (Table 1).
Water parameter measurements for La Pampa demonstrate a much wider range of pH, conductivity and TDS than those from San Juan (Table 1). Urban well samples from Eduardo Castex (EC) reported higher pH values compared to similar waters taken in Ingeniero Luiggi (LU), with ranges of 7.9–9.3 and 7.4–8.7, respectively (Table 1); somewhat lower than San Juan. Rural well supplies, showed pH ranges of 7.4–9.9 and 7.5–9.0 for EC and LU, respectively. Tap water pH values from EC were 7.7–8.8. Water samples collected from the two reverse osmosis water treatment works (WTWs) in EC and LU exhibited the lowest pH levels with 7.0–8.6 in EC and 7.4–8.7 in LU (Table 1). Both EC and LU displayed similar conductivity levels for the urban and rural well water samples with ranges of 399 to >3,999 μS cm−1 and 940 to >3,999 μS cm−1, respectively (Table 1). Conductivity measurements reported in the tap waters in EC displayed levels of 1,446 to >3,999 μS cm−1 (Table 1). The introduction of the water treatment works in both towns notably lowered all water parameter values, with conductivities as low as 79 μS cm−1 in EC and 9 μS cm−1 in LU (Table 1). This trend was also seen in TDS measurements, with EC displaying a reduction in the low end TDS range from 87 mg l−1 to 39 mg l−1 and for LU from 242 mg l−1 to 4 mg l−1 (Table 1). With the exception of the water treatment works in LU, all water types, namely ground (rural and urban wells) and tap, collected from La Pampa reported conductivity and TDS ranges to maximum recordable values of 3,999 μS cm−1 and 2,000 mg l−1, respectively. No statistically significant relationship was observed between pH and either conductivity or TDS in San Juan (conductivity: R 2 = −0.0321; TDS: R 2 = −0.0333) or La Pampa (conductivity: R 2 = 0.0523; TDS: R 2 = 0.0181).
Water parameter measurements for the control province of Río Negro (RN) exhibited a pH range of 6.9–9.2 for Río Colorado and Río Negro surface waters; 7.1–8.0 for well waters and 7.0–8.8 for domestic tap supplies (Table 1). Conductivity and TDS measurements for surface waters ranged from 987–3,149 μS cm−1 and 219–1,994 mg l−1, respectively. Well waters had conductivity levels of 1,109–2,129 μS cm−1 and TDS levels of 1,258–1,853 mg l−1, whereas domestic tap supplies showed lower levels, reporting 908–1,574 μS cm−1 and 470–1,620 mg l−1 for conductivity and TDS, respectively (Table 1).
Total arsenic
Total arsenic concentrations in all surface, ground and some tap waters from San Juan and La Pampa (Tables 2, 3) determined by ICP-MS were elevated in comparison to the control province of Río Negro (Table 4) in almost all study locations. In San Juan, surface waters, primarily used for irrigation and livestock, exhibited the lowest concentrations of arsenic (mean: 67; median: 74; range: 11–133 μg l−1 As) measured in natural waters in this study (Figs. 8, 9). The highest concentrations of arsenic in these surface waters were found in the Río Blanco near Angualasto (47–133 μg l−1 As), the uppermost sampling location at 1,635 m a.s.l. and the closest to the Andes and sites of local gold mining activities. The lowest total arsenic concentrations were found in the Río Huaco - a separate river system north of San José de Jáchal (Fig. 3). Arsenic in the man-made lake of the Río Blanco/Río Jáchal, namely, Cuesta del Viento dam (CU1) was 78 μg l−1 As (Fig. 9), compared to the Río Colola inflow (52 μg l−1 As). A raised arsenic concentration was measured at the outflow of the Pachimoco dam (RJ1) of the Río Jáchal with a value of 116 μg l−1 As (Fig. 9), which is in agreement with a previously reported value (Hill 2009).
Total dissolved arsenic remained relatively constant along the Río Jáchal (mean: 78; median: 74; range: 55–116 μg l−1 As), with minimal addition of arsenic from the freshwater spring at Agua Negra (Fig. 9). Arsenic concentrations in untreated domestic tap water samples from the same area ranged from 9 to 100 μg l−1 As, with a mean of 38 μg l−1 As (Tables 1, 2), almost all of which exceed the WHO drinking water limit for arsenic with a maximum measured concentration of 10× that value.
The different sources of water in the community of Encon (in the south of San Juan Province) displayed significantly different total arsenic concentrations in comparison to the main provincial sampling sites in the north. Encon (EN) rural well waters (n = 4) had a more limited range of arsenic concentrations (mean: 59; range: 25–76 μg l−1 As) as shown in Fig. 8. However, the EN urban well waters (mean: 254; range: 31–357 μg l−1 As) (Tables 1, 2), are all well above the WHO recommended limit of 10 μg l−1 for arsenic in drinking water (WHO 1993). Untreated domestic tap waters composed of the two different well supplies blended together is the main drinking water source for the community; these had a mean total arsenic concentration of 253 μg l−1 As. A reduction in total arsenic was seen after treatment by ion exchange at the local school from 66 to 21 μg l−1 As (Table 1). The distribution of total arsenic in the rural well and surface waters were comparable, with median values of 68 μg l−1 As and 74 μg l−1 As, respectively (Fig. 8). The Encon urban well and domestic tap supply waters showed a similar arsenic concentration range, however the distribution of the results were different with median arsenic concentrations of 324 μg l−1 As and 270 μg l−1 As, respectively (Fig. 8).
Total arsenic in La Pampa for EC and LU displayed distinct variability and overall had a greater range in arsenic concentrations compared to San Juan. Urban well waters in EC had total arsenic concentrations of 39–290 μg l−1 As, whilst LU were 115–327 μg l−1 As (Table 1). The rural well waters from both towns had the highest concentrations of total arsenic found in this study, with EC (n = 29) having a maximum of 1,128 μg l−1 As and LU (n = 23) 1,326 μg l−1 As (Table 1 and Fig. 8); with overall means of 484 μg l−1 As (EC) and 212 μg l−1 As (LU). Tap water samples from EC were also above the WHO guideline level, with a range of 41–747 μg l−1 As (mean: 379 μg l−1 As) (Table 1). Groundwater values reported in the literature for the town of Eduardo Castex ranged from <4 to 5,300 μg l−1 As (Smedley et al. 2002). The rural well waters displayed the broadest concentration range in La Pampa with arsenic values of 3–1,326 μg l−1 As (Fig. 8). The urban well and domestic tap supply samples yielded narrower ranges of 39–327 μg l−1 As and 41–747 μg l−1 As, respectively (Fig. 8). Little similarity was seen in the range of total arsenic concentrations for each water type from the provinces of La Pampa and San Juan.
Total arsenic in the control province of Río Negro displayed concentrations well below the WHO drinking water limit of 10 μg l−1 As in the majority of samples (surface waters: 0.8–16.4; well waters: 1.5–5.2; tap supplies: 0.5–2.5 μg l−1 As) (Table 1). Surface water arsenic concentrations in Río Negro were on average tenfold lower than those reported in San Juan; well waters were approximately 100-fold lower than in Encon and as much as 500-fold lower than corresponding samples in La Pampa. Tap water samples from Río Negro displayed a 150-fold reduction in total arsenic concentrations in comparison to the two impact areas of San Juan and La Pampa.
Arsenic species
The individual arsenic species (AsIII, AsV, MAV and DMAV), separated in the field using the SPE cartridge methodology, displayed varying concentrations and percentage contributions between the two main study provinces in Argentina.
San Juan
Dissolved arsenic in waters (surface and tap) collected from the main San Juan study sites (San José de Jáchal region) was largely composed of AsIII. Surface waters had an AsIII concentration range of 4 –138 μg l−1 (n = 19), contributing 69–100% of the total arsenic (Table 2). The highest AsIII concentrations in surface waters were found near Angualasto (ANG) (mean: 98; range: 51–138 μg l−1), with little contribution from AsV, MAV or DMAV. This trend was observed in the Río Colola (CO1), with values of 45 (AsIII) > 3.7 (MAV) > 2.4 (AsV) > 0.03 (DMAV) μg l−1 (Table 2). A similar relative abundance at lower arsenic concentrations was found in the Cuesta del Viento dam and Río Jáchal samples. The freshwater stream at Agua Negra and the Río Huaco had some of the lowest concentrations of arsenic species in the surface waters with mean values for the Río Huaco of 7.6 μg l−1 (AsIII), 0.3 μg l−1 (AsV), 0.6 μg l−1 (MAV) and 0.2 μg l−1 (DMAV). Tap water samples from San Juan city and Niquivil (n = 5) showed an 85% mean contribution of AsIII (mean: 31; range: 6–90 μg l−1), as highlighted in Table 2.
Tap water samples from Encon exhibited a high concentration of AsIII (mean: 153; range: 68–201 μg l−1), equating to a 53% average contribution with the remainder principally composed of AsV (25%) and MAV (22%). Encon urban well waters contained 80% AsIII (mean: 219 μg l−1) and 8% AsV (mean: 16 μg l−1). However, the arsenic species composition in the rural well waters from Encon (n = 4), consisted of 99% AsIII (mean: 54; range: 23–78 μg l−1), with the remaining 1% made up of the three other species (Table 2). These findings show a substantial increase in AsIII concentrations in Encon well waters (urban and rural) in comparison to the untreated domestic tap waters.
Low concentrations of organoarsenicals were found in the main San Juan (surface waters) region, with values of <0.02–8.4 μg l−1 for MAV and <0.03–0.7 μg l−1 for DMAV (Table 2). These low concentrations are indicative of low microbial activity, such as algae growth, which has been noted to metabolise arsenic into these organoarsenicals in surface waters during summer months (Smedley and Kinniburgh 2002). The lack of farming activity around the sampling locations also leads us to believe there is no input from agrochemicals by surface run-off. In contrast, the high concentrations of the organoarsenic species MAV, in Encon tap supplies (mean: 57; range: 23–79 μg l−1) and urban well water (mean: 26; range: 10–45 μg l−1) suggests possible microbial activity leading to methylation of iAs, which is worthy of follow-up in future studies.
La Pampa
The loess groundwater of EC exhibited a very high proportion of AsIII, as was evident in the community of Encon in San Juan Province. Urban well waters in EC had a mean (n = 3) AsIII concentration of 158 μg l−1 (range: 46–226 μg l−1), equating to 93% of the total arsenic. The highest contribution of iAs species in rural well waters was in EC, with AsIII accounting for 68% of the total arsenic (mean: 466; range: 5–1,332 μg l−1), compared with 26% (mean: 132; range: 3–592 μg l−1) for AsV, as reported in Table 3. Tap waters from EC had mean iAs concentrations of 141 μg l−1 (range: 131–151 μg l−1) and 13 μg l−1 (range: 2–23 μg l−1) for AsIII and AsV, respectively (Table 3). Urban well waters in LU had a mean AsIII concentration of 100 μg l−1 (range: 32–163 μg l−1), accounting for 48% of total arsenic (Table 3). A large proportion of the arsenic recorded in the individual LU urban well waters consisted of AsV (18–60% of total arsenic), with a mean concentration of 88 μg l−1 (42% of total arsenic), which was not observed in the EC samples. Inorganic arsenic concentrations in the LU rural well waters paralleled those measured in the urban wells, consisting predominantly of AsIII (56%) and AsV (39%) at mean concentrations of 130 μg l−1 and 109 μg l−1, respectively. Comparison of the arsenic species within the province of La Pampa shows a slight variability between inorganic and organic species.
Organoarsenicals were present throughout the province within a range of 1.2–59 μg l−1 and <0.03–11.4 μg l−1 for MAV and DMAV, respectively (Table 3). The dominant species was that of MAV, with the highest concentrations in the EC rural well waters (mean: 26; range: 1.2–57 μg l−1). This pattern was not found in the LU waters, which saw the highest MAV concentration (10%) in urban well waters (mean: 21; range: 5–59 μg l−1), compared with a 5% mean contribution in rural well waters (mean: 12; range: 6–23 μg l−1) (Table 3). The lowest MAV concentrations were found in the domestic tap supplies (mean: 6; range: <0.02–11 μg l−1) and urban well waters (mean: 6; range: 4–8 μg l−1) in EC.
Río Negro
The control province of Río Negro is characterised by sandy plains of Quaternary fluvial, lacustrine and aeolian origin (Abraham et al. 2009). Mean iAs species concentrations for the surface waters (n = 8) were 4.3 μg l−1 for AsIII (range: 0.1–8.6 μg l−1) and 7.5 μg l−1 for AsV (range: 3.6–13.3 μg l−1) (Table 4). Average concentrations of AsIII and AsV in the urban well waters (n = 2) were 1.0 and 0.7 μg l−1, respectively and for the tap supplies (n = 2) 3.4 and 0.1 μg l−1, respectively (Table 4). Detectable levels of organoarsenicals were seen in all water types with the highest concentrations in the surface waters, 3.3 μg l−1 (MAV) and 1.2 μg l−1 (DMAV).
Water Treatment Works
Two water treatment works in La Pampa, namely Eduardo Castex (EC) and Ingeniero Luiggi (LU), were also investigated. Both treatment plants were built in the 1980s, as a potential remediation method for the high concentrations of arsenic in the domestic drinking water. At both locations the raw water originates from a mixture of underground urban well supplies, which are extracted at various depths (20–90 m). Firstly, raw well water undergoes a filtration step, which removes any mud/solid particulates (Fig. 10). The filtered water then enters a two-stage reverse osmosis (RO) process using iron (Fe) oxide. Residual (waste) water is removed from the system at two outlets during the RO phase, and is subsequently pumped back into the underground urban well supply. The addition of sodium carbonate to the system is required to help stabilise the pH (Fig. 10). The final ‘cleaning’ process uses UV radiation and ozone (O3) or chlorination to sterilise the water. Constant monitoring of the water is then undertaken to ensure a minimal bacterial presence. The final drinking water is housed in a water tower (approximate capacity 10,000 litres) or in 10 litre individual containers.
Total arsenic concentrations from water samples taken at 8 different stages along the water treatment process in EC and LU (Fig. 10) show a substantial decrease in arsenic concentrations at the outflow. Initial total arsenic concentrations reported from the mixed raw well water sources in EC and LU displayed concentrations of 216 μg l−1 As and 224 μg l−1 As, respectively (Table 5). From Stage 5 (outflow after RO) onwards there was a significant reduction in arsenic with the concentrations in the samples from the final drinking water supplies of 0.8 μg l−1 As and 0.3 μg l−1 As for EC and LU, respectively (Table 5). These total arsenic concentrations, following treatment of the drinking water are below the WHO limit of 10 μg l−1 As (WHO 1993). A difference was noted in total arsenic between both water treatment works at Stage 6 (RO + Na2CO3), with EC reporting 30.1 μg l−1 As and LU 0.1 μg l−1 As. This difference in total arsenic concentration may be due to dissimilarity in construction or operation between the two treatment plants. A higher concentration of total Fe was also seen at Stage 6 in the EC water treatment works (387 μg l−1) compared to an undetectable level at LU (<7 μg l−1), which may support this theory.
The arsenic speciation studies at both of these treatment works found that the inflow water had a relative abundance of AsIII > AsV > MAV > DMAV (Table 6). Treated drinking water from EC (Stage 8) showed the only measurable alteration to this trend with the greatest contribution (0.6 μg l−1) coming from AsV. Due to the effective nature of the water treatment process, speciation analysis was undetectable at most post-treatment stages, with species concentrations reported <0.12 μg l−1 for AsIII, <0.02 μg l−1 for AsV and MAV, and <0.03 μg l−1 for DMAV (Table 6).
Discussion
Total arsenic concentrations reported from San Juan and La Pampa (Argentina) were shown to be elevated well above the control province of Río Negro, the WHO recommended drinking water limit of 10 μg l−1 As and in some cases above the 100 μg l−1 As limit set by the FAO for irrigational waters (FAO 1994). Total arsenic concentrations for surface waters and groundwater (San Juan) are in good agreement with previously reported values, namely, 140–260 μg l−1 As (Cáceres et al. 2005; WHO 2001; Williams 2001). Total arsenic concentrations for La Pampa groundwater (median: 164; range: 3–1,326 μg l−1 As) are notably lower than those reported by others, namely, as high as 5,300 μg l−1 As (median: 150 μg l−1 As) (Smedley et al. 2002; WHO 2001).
The elevated total arsenic concentrations in San Juan surface waters (11–133 μg l−1 As) could be classified from either natural or anthropogenic sources and will be discussed further. The main river system in the north of the province is the Río Blanco, a third or fourth order river (Rawlins et al. 1997), which feeds the subsequent river systems that were sampled, with the exception of the Río Huaco (Fig. 3). The source of the Río Blanco comes from the periphery of the Andes mountain range. The geology of the area is dominated by marine sedimentation from the Carboniferous, which also experienced periods of localised volcanism in the Permian (Limarino et al. 2006). The input from natural processes (leaching/weathering) and anthropogenic activity (base metal and gold mining) in the area may both contribute to the measured arsenic in these river systems (Williams 2001; Rawlins et al. 1997). The semi-arid nature of San Juan would tend to suggest a limited interaction between As-rich mine waste and drainage water. However, Rawlins et al. (1997) reported 150 μg l−1 dissolved arsenic in the Río Blanco system, which if used as potable water could provide a regional source of arsenic that could potentially have health implications for the local population. It was noted, by Rawlins et al. (1997) that water samples collected further downstream did display smaller arsenic concentrations, suggesting the possibility of a dilution effect or a higher presence of Fe oxides acting as arsenic binders preventing mobilisation. This trend was also seen in the current study. The total arsenic concentration at the uppermost sampling site (1,635 m a.s.l.) along the Río Blanco/Río Jachal system (ANG1a) was 133 μg l−1 As compared with the lowest sampling location (1,001 m a.s.l.) along the same river system (RJ6) at 55 μg l−1 As (Table 2). These results suggest the effect of downstream arsenic dilution, consistent with increasing distance from mining activity and run-off from the Andes. Low concentrations of Al, Fe and Mn in the surface waters downstream indicates a lack of arsenic binding to metal oxides (Table 2).
The lower concentrations of arsenic (11–16 μg l−1 As) reported from the Río Huaco (HU) system may be attributed to a distinct difference in the geology of the river basin. The Río Huaco is primarily located within Tertiary continental marine deposits. There is also little evidence to suggest the recent involvement of volcanic or mining activity in the area (Jenchen and Rosenfeld 2002). The Río Huaco is also not as strongly influenced by seasonal climatic effects that are more apparent in the Río Blanco, such as snowmelt from the Andes, as it is a smaller more localised system. This would tend to suggest that the input of arsenic into the Río Huaco from natural and anthropogenic sources is on a much smaller scale to that seen in the Río Blanco and Río Jáchal. High concentrations of boron (3.92 mg l−1) and sodium (1,020 mg l−1) were also found in the Río Jáchal, which have been associated with the rivers high salinity (Adamo and Crews-Meyer 2006). High levels of salinity have been shown to correlate well with high arsenic concentrations (Smedley and Kinniburgh 2002).
Concentrations of arsenic in domestic tap water supplies from the San José de Jáchal region of San Juan ranged from 9 to 100 μg l−1 As within a pH range of 7.2–7.7 (Table 1). This range in pH is indicative of conditions that enable the mobility of arsenic under both oxidising and reducing environments (Smedley and Kinniburgh 2002). It is thought that in oxidising conditions, the dominant arsenic species in surface waters is that of AsV, as shown in Fig. 1 (Impellitteri and Scheckel 2006). However, SPE methodology from this study suggests that AsIII was the prevalent species at the time of collection in these San Juan samples (relative abundance: 69–100%) (Figs. 11, 12). This is potentially due to a greater influence from reductive processes and also, with a pH lower than 9.2, the predominant species is likely to be the uncharged AsIII species H3AsO 03 (Fig. 1), aiding desorption of arsenic from metal oxides such as Al, Fe and Mn (Smedley and Kinniburgh 2002). However, redox potentials (Eh) for San Juan have reported ranges of 197–230 mV (Rawlins et al. 1997), suggesting the presence of AsV in the form HAsO2 2− (Fig. 1). This suggests that the prevalence of AsIII at the time of collection was generated within the soil-aquifer strata and could essentially be a relic feature of the surface water environment.
Concentrations of total arsenic (159–331 μg l−1 As) in untreated water samples from Encon (southern San Juan) which originate from a different water source to the San José de Jáchal region highlights the potential increase in arsenic concentrations due to variation in physical and geochemical conditions. Encon has a different geology to the north of the province. The community is predominantly set upon Quaternary continental deposits of sand and loess (Lloret and Suvires 2006; DNGM 1964). The geological composition of the sampling area may lead us to expect similar arsenic concentrations to those found in La Pampa (3–1,326 μg l−1 As), due to the environmental development of both regions during the Quaternary age (Smedley et al. 2005; Bundschuh et al. 2004; DNGM 1964). However, the apparent variation in total arsenic between the two regions may be ascribed to differences in the natural environment. Encon displays a greater expanse/diversity of vegetation in comparison to La Pampa, which may indicate a difference in the geochemistry of the two areas or potentially a greater uptake of arsenic by plants in Encon leading to lower total arsenic concentrations.
Arsenic speciation suggests the presence of the organoarsenical, MAV, in both San Juan (<0.02–79 μg l−1) and La Pampa (<0.02–59 μg l−1), which was measured in up to 97% of water samples from both San Juan and La Pampa. Organoarsenicals can arise through anthropogenic activity (Cowen et al. 2008; Vaclavikova et al. 2008; McSheehy et al. 2003). The community of Encon is principally an arid semi-desert rural community with local goat farming being the main agricultural activity. Surface and groundwater collected from isolated farm areas have been shown to contain methylated arsenicals, such as MAV, due to the use of arsenic-containing herbicides (Bednar et al. 2004; Bednar et al. 2002b) and pesticides (Choong et al. 2007). However, agrochemicals containing high levels of NO3 and PO4 in waters have been shown to enhance/suppress MAV levels from the SPE methodology utilised in this study by as much as 13–21% (Watts et al. 2010). Previous studies on the levels of arsenic in waters have mainly reported only inorganic species (AsIII and AsV), in which AsV was deduced from the subtraction of AsIII from the total arsenic level (Sigrist and Beldoménico 2004; Smedley et al. 2002; Edwards et al. 1998). This subtraction method does not account for organoarsenicals and may provide apparently elevated concentrations for AsV. The use of SPE cartridges that have the ability to separate and retain the individual aqueous arsenic species (inorganic and organic) in the field affords an opportunity to detect and measure arsenic in environmental samples.
The concentrations of organoarsenicals (MAV and DMAV) in both San Juan and La Pampa were greater in groundwater (<0.02–79 μg l−1) than surface waters (<0.02–8 μg l−1). This suggests that due to the lack of farming activity around surface water sampling locations, little input of organoarsenicals is seen from agrochemical use (herbicides and pesticides), therefore the main source of the organoarsenicals must be attributed to algae formation. In contrast, high concentrations of organoarsenicals in groundwater from Encon (farming community) in San Juan and La Pampa (rural farmsteads) may have arisen as a result of anthropogenic input from agrochemical use, for example nitrate fertilisers (Tables 2, 3). The presence of phosphate (potentially from herbicides and pesticides) has been shown to substantially suppress arsenic adsorption by soils (Smith et al. 2002). However, arsenic methylation to MAV and DMAV may increase arsenic mobility, as these organoarsenicals are less strongly adsorbed than iAs, leading to highly measurable concentrations (Deutsch 1997).
High AsIII species concentrations were also measured in the groundwater (aquifer) systems within Encon from the community and rural farm well supplies. The difference in AsIII concentrations between the two water sources may be due to variability in redox conditions at different well depths impacting on the geological influence, which would require further investigation. The community or urban well was built in 1963 and is in continuous use, it is approximately 15 m deep, the rural well however (called the Manantial de Cuyo well), was built in the 1950s and is 300-m deep. It is assumed that both wells are open for much of their length, as no reported screening processes have been undertaken. As there is no significant variation in the pH of both water types, it may be considered that a difference in the sedimentation of the aquifer systems or the residence time of the groundwater as a consequence of well depths, may influence the mobility of the arsenic (Gault et al. 2005b; Polizzotto et al. 2005; Kinniburgh and Kosmus 2002).
Groundwater arsenic concentrations from the province of La Pampa have been reported in the literature to be elevated due to weathering processes/volcanic activity (Smedley et al. 2002). These natural processes have subsequently created a soil strata composed of Quaternary deposits of loess sedimentation (Smedley et al. 2005). The pH conditions within the province (7.0–9.9) demonstrate ideal conditions in which arsenic can become mobilised. Elevated concentrations of total arsenic (>1,300 μg l−1) have been observed, with the most contribution (7–97%) coming from AsIII (Table 3 and Fig. 11). This implies reducing conditions in the aquifer; however, with a high probability of mobilisation, the groundwater seems to exhibit both reducing and oxidising characteristics, which is demonstrated by the presence of AsV in the region, 0.09–592 μg l−1 (<1–82%) (Figs. 11, 12). Typically the highest concentrations of AsIII were reported from groundwater aquifer systems in Eduardo Castex (EC), with a range of 5–1,332 μg l−1 (Table 3), demonstrating the potential stability of these arsenic species in the environment prior to extraction and use by humans. Since the reporting of data from previous studies in Argentina, it has come to light that AsIII species in water are unstable in the presence of high concentrations of FeIII, causing oxidation of AsIII to AsV (Gault et al. 2005a; Gong et al. 2002; Le et al. 2000). This process can also occur from the photolytic reduction of nitrate to nitrite, which has been shown to convert up to 50% of the AsIII to AsV within 3 h of collection (Hug et al. 2001; Sharpless and Linden 2001). The use of opaque polyethylene bottles for water collection is one method to eliminate the effect of photochemical oxidation by omitting light exposure (Garbarino et al. 2002). This provides a more representative sample, in terms of arsenic species composition, in order to verify the SPE method. Local populations that rely solely on As-rich groundwater supplies exhibit symptoms that parallel those of arsenic exposure, such as skin lesions and pigmentation changes (Choong et al. 2007; Bhattacharya et al. 2006; Hopenhayn-Rich et al. 1998).
The implementation of a water treatment works in two towns in La Pampa has addressed the issue of reducing the arsenic concentrations in raw well water supplies. Treatment of the raw water with a series of RO, UV radiation and ozone/chlorination processes reduces overall arsenic levels by almost 100%, from the initial concentrations of 216 μg l−1 As (EC) and 224 μg l−1 As (LU) to 0.8 μg l−1 As and 0.3 μg l−1 As, respectively (Table 5). Reverse osmosis filters containing Fe oxide reduces the mobility of the arsenic, adhering it to the surface of the solid packed material. The resultant drinking water achieves arsenic concentrations well below the WHO recommended limit of 10 μg l−1 As, and predominantly in the less toxic AsV form.
The main limitation to the use of SPE methodology for the assessment of arsenic species in the field is the effect on the ion exchange efficiency of the cartridges from high matrix components and competing ions in the waters. The presence of fluoride, chloride (25 mg l−1) and phosphate (1 mg l−1) has been shown to inhibit MAV retention on the SAX cartridge, with recoveries of 75, 68 and 79%, respectively (Watts et al. 2010). However, enhancements were seen in the subsequent AsV fraction eluted from the SAX cartridge effluent. Conversely, for sulphate (25 mg l−1), enhancement was seen in the MAV fraction (113%) and a reduction in AsV (79%), as reported by Watts et al. (2010). Measurement of water conductivity is one way to address this problem. A ceiling value of 1,500 μS cm−1 was established at which conductivity remained relatively constant with varying percent differences between total arsenic measurements in filtered/acidified (F/A) water and the sum of arsenic species measured using the SPE method (Watts et al. 2010). Therefore, in order to overcome high matrix effects, a dilution factor of ×2 or higher should be applied to a water sample that exceeded the 1,500 μS cm−1 limit, using de-ionised water (pH 6.8) immediately prior to speciation through the SPE cartridges. This reduces the potential for saturating the ion-exchange cartridges, as reported in other studies (Bednar et al. 2004; Garbarino et al. 2002).
Conclusions
Water samples collected from San Juan and La Pampa Provinces in Argentina showed elevated total dissolved arsenic compared to Río Negro (control) and natural background concentration ranges in uncontaminated water of 1–10 μg l−1 As (Smedley and Kinniburgh 2002; Williams 2001). Surface waters exhibited arsenic concentrations of 11–133 μg l−1 As in San Juan with tap, rural well and urban well levels in the range 9–357 μg l−1 As. Arsenic levels in groundwater from La Pampa ranged from 39 to 327 μg l−1 As in urban wells; 3–1,326 μg l−1 As in rural wells; and 41–747 μg l−1 As in domestic untreated tap water supplies. Drinking water concentrations greatly exceed the WHO limit of 10 μg l−1 As, recommended as a maximum allowable level in potable waters (WHO 1993). Additionally, many surface water samples were shown to surpass the FAO irrigational limit of 100 μg l−1 As (FAO 1994). Not only do both provinces experience higher arsenic concentrations in the waters compared to typical background levels, but the majority of the dissolved arsenic resides as the more toxic inorganic species (percent contributions: San Juan, AsIII: 32–100% and AsV: <1–35%; La Pampa AsIII: 7–97% and AsV: <1–82%). However, a high presence of organoarsenicals were also found in both provinces. Monomethylarsonic acid (MAV) was seen in the range <0.02–79 μg l−1 in San Juan and <0.02–59 μg l−1 in La Pampa (Tables 2, 3). The presence of organoarsenicals has rarely been reported in the literature due to the limitations in certain analytical techniques, such as the lack of hydride formation from MAV and DMAV (Sigrist and Beldoménico 2004).
Higher concentrations of AsIII were apparent in high alkaline and high TDS environments, particularly in groundwater (aquifer) systems (Figs. 11, 12). Concentrations of the individual arsenic species in these waters were generally of the order:
which also represents the order of arsenic species toxicity (Francesconi and Kuehnelt 2004). Individual arsenic species concentrations reported in the literature for La Pampa have reported higher levels of AsV (Smedley et al. 2005; Smedley et al. 2002), based on a subtraction method (AsV = Total As–AsIII). However, based on the high levels of organoarsenicals reported in some samples in this study by SPE methodology, the subtraction method may inadvertently provide a more elevated concentration for AsV than is actually the case.
The identification of individual arsenic species provides increased understanding of the likely toxicity of arsenic in the environment. Total arsenic concentrations in San Juan were in good agreement with other literature sources (Cáceres et al. 2005; Williams 2001). However, total arsenic reported in La Pampa showed lower maximum concentrations than other reported cases (Smedley et al. 2002). Smedley et al. (2002) reported a maximum of 5,300 μg l−1 As in groundwater supplies throughout the province, compared to a maximum of 1,326 μg l−1 As seen in this study (Table 3). Differences in reported concentrations could be based on the geology of the province, the time of sampling and the different wells visited. This may also explain the difference in arsenic species contribution between the two studies. The Quaternary loess that predominates in La Pampa is not uniformly spread throughout the soil strata. Therefore large variations in arsenic concentrations can be seen at sampling sites only a few metres apart, due to differences in depth and the open-interval of individual wells. However, this study primarily focused on a small sampling radius and did not reflect the same scale as that undertaken by Smedley et al. (2002). Although sampling was localised to the main towns of Eduardo Castex and Ingeniero Luiggi, the study does highlight the need for arsenic mitigation strategies to be employed for the wider community allowing for greater accessibility to clean drinking water sources.
Arsenic exposure from these water sources is highly achievable via numerous direct (drinking) and indirect pathways (crops, meat, washing, cooking). Consequences of arsenic exposure to humans are evident when levels surpass 50 μg l−1 As (the current Argentine drinking water limit) (Bhattacharya et al. 2006; Frisbie et al. 2005). Health problems observed in the Argentine population are symptomatic of arsenic exposure (Steinmaus et al. 2006; Tchounwou et al. 2003). The most common manifestation is from skin disorders (lesions, keratosis, hyperpigmentation), but other more chronic conditions have been reported such as cancers (Steinmaus et al. 2006; Bates et al. 2004; Farías et al. 2003; Hopenhayn-Rich et al. 1998). Remediation and mitigation strategies have been adopted in the provinces. The introduction of water treatment works in La Pampa highlighted the significant contribution they provide in relieving the arsenic contamination problem, providing the local population with clean uncontaminated drinking water. Full-scale known remediation methods for arsenic are not viable options in the more rural areas of the provinces due to economic constraints, therefore cheaper, more sustainable alternatives are needed to lessen the effects of arsenic contamination.
This research consolidates work undertaken by Smedley et al. (2005, 2002) to highlight the extent of the arsenic contamination problem in Argentina, with particular emphasis on the provinces of San Juan and La Pampa. The SPE method utilised for the determination and identification of arsenic species in water samples in this study provides a safe, simple and robust field-based technique, allowing for greater specificity and accuracy. It allows for a prolonged time period between sampling and analysis due to the stable preservation of the arsenic species on the SPE cartridges. The method also enables a greater flexibility in the transportation of samples, requiring a minimal volume (typically 30 ml) and no addition of chemicals. It provided a method for arsenic species identification, to aid the understanding of arsenic exposure through drinking water and the need for biomonitoring in the regions due to the possible exposure pathways (particularly drinking water), which is being investigated by the authors.
References
Abraham, E., del Valle, H. F., Roig, F., Torres, L., Ares, J. O., Coronato, F., et al. (2009). Overview of the geography of the Monte Desert biome (Argentina). Journal of the Arid Environments, 73, 144–153.
Adamo, S. B., & Crews-Meyer, K. A. (2006). Aridity and desertification: exploring environmental hazards in Jáchal, Argentina. Applied Geography, 26, 61–85.
Asante, K. A., Agusa, T., Subramanian, A., Ansa-Asare, O. D., Biney, C. A., & Tanabe, S. (2007). Contamination status of arsenic and other trace elements in drinking water and residents from Tarkwa, a historic mining township in Ghana. Chemosphere, 66, 1513–1522.
Ascar, L., Ahumada, I., & Richter, P. (2008). Influence of redox potential (Eh) on the availability of arsenic species in soils and soils amended with biosolid. Chemosphere, 72, 1548–1552.
Bates, M. N., Rey, O. A., Biggs, M. L., Hopenhayn, C., Moore, L. E., Kalman, D., et al. (2004). Case-control study of bladder cancer and exposure to arsenic in Argentina. American Journal of Epidemiology, 159, 381–389.
Bednar, A. J., Garbarino, J. R., Burkhardt, M. R., Ranville, J. F., & Wildeman, T. R. (2004). Field and laboratory arsenic speciation methods and their application to natural-water analysis. Water Research, 38, 355–364.
Bednar, A. J., Garbarino, J. R., Ranville, J. F., & Wildeman, T. R. (2002a). Preserving the distribution of inorganic arsenic species in groundwater and acid mine drainage samples. Environmental Science and Technology, 36, 2213–2218.
Bednar, A. J., Garbarino, J. R., Ranville, J. F., & Wildeman, T. R. (2002b). Presence of organoarsenicals used in cotton production in agricultural water and soil of the Southern United States. Journal of Agricultural and Food Chemistry, 50, 7340–7344.
Bertolino, S. R. A., Zimmermann, U., & Sattler, F. J. (2007). Mineralogy and geochemistry of bottom sediments from water reservoirs in the vicinity of Córdoba, Argentina: Environmental and health constraints. Applied Clay Science, 36, 206–220.
Bhattacharya, P., Claesson, M., Bundschuh, J., Sracek, O., Fagerberg, J., Jacks, G., et al. (2006). Distribution and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Science of the Total Environment, 358, 97–120.
Bhattacharya, P., Welch, A. H., Stollenwerk, K. G., McLaughlin, M. J., Bundschuh, J., & Panaullah, G. (2007). Arsenic in the environment: biology and chemistry. Science of the Total Environment, 379, 109–120.
Bundschuh, J., Farias, B., Martin, R., Storniolo, A., Bhattacharya, P., Cortes, J., et al. (2004). Groundwater arsenic in the Chaco-Pampean Plain, Argentina: Case study from Robles County, Santiago del Estero Province. Applied Geochemistry, 19, 231–243.
Button, M., Jenkin, G. R. T., Harrington, C. F., & Watts, M. J. (2009). Human toenails as a biomarker of exposure to elevated environmental arsenic. Journal of Environmental Monitoring, 11, 610–617.
Cáceres, R. E., Aguirre, L. O., Rosas, E. O., & Segovia, R. F. (2005). Arsenic removal from water through pitting corrosion of a metallic iron fixed bed. In 2nd Mercosur congress on chemical engineering. 4th Mercosur congress on process systems engineering, pp. 1–9.
Chiou, J.-M., Wang, S.-L., Chen, C.-J., Deng, C.-R., Lin, W., & Tai, T.-Y. (2005). Arsenic ingestion and increase microvascular disease risk: Observations from the south-western arseniasis-endemic arsenic in Taiwan. International Journal of Epidemiology, 34, 936–943.
Choong, T. S. Y., Chuah, T. G., Robiah, Y., Koay, F. L. G., & Azni, I. (2007). Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination, 217, 139–166.
Chou, C.-H., & De Rosa, C. T. (2003). Case studies–arsenic. International Journal of Hygiene and Environmental Health, 206, 381–386.
Cowen, S., Megha, D., Tuan, H., & Al-Abadleh, H. A. (2008). Vibrational spectroscopic characterization of some environmentally important organoarsenicals—a guide for understanding the nature of their surface complexes. Canadian Journal of Chemistry, 86(10), 942–950.
Daus, B., Mattusch, J., Wennrich, R., & Weiss, H. (2002). Investigation on stability and preservation of arsenic species in iron rich water samples. Talanta, 58, 57–65.
de Salmuni, G., Valasco, I., Fresina, M., & Flores, A. L. (2007). Irrigated area determination: A case study in the Province of San Juan, Argentina. GeoJournal, 70, 273–279.
Deutsch, W. J. (1997). Practical applications: Metal contamination—arsenic. In W. J. Deutsch (Ed.), Groundwater geochemistry: Fundamentals and applications to contamination (p. 175). Florida: CRC Press.
Dilks, C. (2004). San Juan Province. In C. Dilks (Ed.), Argentina (3rd ed., p. 208). Bath: Footprint Handbooks.
DNGM (Direccion Nacional de Geologia y Mineria) (1964). Map geologica de la Republica Argentina, H8740 majari (1), ISBN X780636456.
Duker, A. A., Carranza, E. J. M., & Hale, M. (2005). Arsenic geochemistry and health. Environment International, 31, 631–641.
Edwards, M., Patel, S., McNeill, L., Chen, H.-W., Frey, M., Waton, A. D., et al. (1998). Considerations in as analysis and speciation. Journal—American Work Works Association, 90, 103–113.
FAO. (1994). Water quality for agriculture, water quality for livestock and poultry, No. 6.
Farías, S. S., Casa, V. A., Vázquez, C., Ferpozzi, L., Pucci, G. N., & Cohen, I. M. (2003). Natural contamination with arsenic and other trace elements in ground waters of Argentine Pampean Plains. Science of the Total Environment, 309, 187–199.
Ferreccio, C., & Sancha, A. M. (2006). Arsenic exposure and its impact on health in Chile. Journal of Health, Population and Nutrition, 24, 164–175.
Francesconi, K. A., & Kuehnelt, D. (2004). Determination of arsenic species: A critical review of methods and applications. Analyst, 129, 373–395.
Frisbie, S. H., Mitchell, E. J., Yusuf, A. Z., Siddiq, M. Y., Sanchez, R. E., Ortega, R., et al. (2005). The development and use of an innovative laboratory method for measuring arsenic in drinking water from Western Bangladesh. Environmental Health Perspectives, 113, 1196–1204.
Garbarino, J. R., Bednar, A. J., & Burkhardt, M. R. (2002). Methods of analysis by the US geological survey national water quality laboratory—arsenic speciation in natural-water samples using laboratory and field methods. US Geological Survey Water-Resources Investigations Report, 02–4144, 1–47.
Gault, A. G., Islam, F. S., Polya, D. A., Charnock, J. M., Boothman, C., Chatterjee, D., et al. (2005a). Microcosm depth profiles of arsenic release in a shallow aquifer, West Bengal. Mineralogical Magazine, 69, 855–863.
Gault, A. G., Jana, J., Chakraborty, S., Mukherjee, P., Sarkar, M., Nath, B., et al. (2005b). Preservation of inorganic arsenic species in high iron, low-Eh groundwater from West Bengal, India. Analytical and Bioanalytical Chemistry, 381, 347–353.
Gomez, M. L., Blarasin, M. T., & Martínez, D. E. (2009). Arsenic and fluoride in a loess aquifer in the central area of Argentina. Environmental Geology, 57, 143–155.
Gong, Z., Lu, X., Ma, M., Watt, C., & Le, X. C. (2002). Arsenic speciation analysis. Talanta, 58, 77–96.
Halim, M. A., Majumder, R. K., Nessa, S. A., Hiroshiro, Y., Uddin, M. J., Shimada, J., et al. (2009). Hydrogeochemistry and arsenic contamination of groundwater in the Ganges Delta Plain, Bangladesh. Journal of Hazardous Materials, 164, 1335–1345.
Hill, S. (2009). Boron toxicity in surface waters, soils and crop plants in an arid region of San Juan, Argentina: The relationship to human levels and health status. Ph.D. Thesis, Chemical Sciences, Faculty of Health and Medical Sciences, University of Surrey, Guildford, Surrey, England.
Hopenhayn-Rich, C., Biggs, M. L., & Smith, A. H. (1998). Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. International Journal of Epidemiology, 27, 561–569.
Hossain, M. F. (2006). Arsenic contamination in Bangladesh—An overview. Agriculture, Ecosystems & Environment, 113, 1–16.
Hug, S. J., Canonica, L., Wegelin, M., Gechter, D., & Von Gunten, U. (2001). Solar oxidation and removal of arsenic and circum neutral pH in iron containing waters. Environmental Science and Technology, 35, 2114–2121.
Hughes, M. F. (2002). Arsenic toxicity and potential mechanisms of action. Toxicology Letters, 133, 1–16.
Impellitteri, C. A., & Scheckel, K. G. (2006). The distribution, solid-phase speciation, and desorption/dissolution of As in waste iron-based drinking water treatment residuals. Chemosphere, 64, 875–880.
Jenchen, U., & Rosenfeld, U. (2002). Continental triassic in Argentina: Response to tectonic activity. Journal of South American Earth Sciences, 15, 461–479.
Jong, T., & Parry, D. L. (2005). Evaluation of the stability of arsenic immobilised by microbial sulfate reduction using TCLP extractions and long-term leaching techniques. Chemosphere, 60, 254–265.
Kile, M. L., Houseman, A., Breton, C. V., Smith, T., Quamruzzaman, Q., Rahman, M., et al. (2007). Dietary arsenic exposure in Bangladesh. Environmental Health Perspectives, 115, 889–893.
Kinniburgh, D. G., & Kosmus, W. (2002). Arsenic contamination in groundwater: Some analytical considerations. Talanta, 58, 165–180.
Kumaresan, M., & Riyazuddin, P. (2001). Overview of speciation chemistry of arsenic. Current Science, 80, 837–846.
Le, X. C., Yalçin, S., & Ma, M. S. (2000). Speciation of submicrogram per litre levels of arsenic in water—on-site species separation integrated with sample collection. Environmental Science and Technology, 34, 2342–2347.
Limarino, C., Tripaldi, A., Marenssi, S., & Fauqué, L. (2006). Tectonic, sea-level, and climatic controls on Late Paleozoic sedimentation in the western basins of Argentina. Journal of South American Earth Sciences, 22, 205–226.
Lloret, G., & Suvires, G. M. (2006). Groundwater basin of the Tulum Valley, San Juan, Argentina: A morphohydrogeologic analysis of its central sector. Journal of South American Earth Sciences, 21, 267–275.
Mandal, B. K., & Suzuki, K. T. (2002). Arsenic round the world: A review. Talanta, 58, 201–235.
McSheehy, S., Szpunar, J., Morabito, R., & Quevauviller, P. (2003). The speciation of arsenic in biological tissues and the certification of reference materials for quality control. Trends in Analytical Chemistry, 22(4), 191–209.
Meharg, A. A., Williams, P. N., Adomako, E., Lawgali, Y. Y., Deacon, C., Villada, A., et al. (2009). Geographical variation in total and inorganic arsenic content of polished (white) rice. Environmental Science and Technology, 43, 1612–1617.
Michelena, R. O., & Irurtia, C. B. (1995). Susceptibility of soil to wind erosion in La Pampa Province, Argentina. Arid Soil Research and Rehabilitation, 9, 227–234.
Mondal, D., & Polya, D. A. (2008). Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: A probabilistic risk assessment. Applied Geochemistry, 23, 2987–2998.
Mukherjee, A., Bhattacharya, P., Savage, K., Foster, A., & Bundschuh, J. (2008). Distribution of geogenic arsenic in hydrologic systems: Controls and challenges. Journal of Contaminant Hydrology, 99, 1–7.
Nath, B., Berner, Z., Chatterjee, D., Mallik, S. B., & Stüben, D. (2008). Mobility of arsenic in West Bengal aquifers conducting low and high groundwater arsenic. Part II: Comparative geochemical profile and leaching study. Applied Geochemistry, 23, 996–1011.
Ng, J. C., Wang, J., & Shraim, A. (2003). A global health problem caused by arsenic from natural sources. Chemosphere, 52, 1353–1359.
Nguyen, V. A., Bang, S., Viet, P. H., & Kim, K.-W. (2009). Contamination of groundwater and risk assessment for arsenic exposure in Ha Nam province, Vietnam. Environment International, 35, 466–472.
Nicolli, H. B., Suriano, J. M., Gómez Peral, M. A., Ferpozzi, L. H., & Baleani, O. H. (1989). Groundwater contamination with arsenic and other trace-elements in an area of the Pampa, province of Córdoba, Argentina. Environmental Geology and Water Sciences, 14, 3–16.
Ning, Z., Lobdell, D. T., Kwok, R. K., Liu, Z., Zhang, S., Ma, C., et al. (2007). Residential exposure to drinking water arsenic in Inner Mongolia, China. Toxicology and Applied Pharmacology, 222, 351–356.
Nriagu, J. O., Bhattacharya, P., Mukherjee, A. B., Bundschuh, J., Zevenhoven, R., & Loeppert, R. H. (2007). Arsenic in soil and groundwater: An overview. Trace Metals and other Contaminants in the Environment, 9, 3–60.
Ohno, K., Yanase, T., Matsuo, Y., Kimura, T., Rahman, M. H., Magara, Y., et al. (2007). Arsenic intake via water and food by a population living in an arsenic-affected area of Bangladesh. Science of the Total Environment, 381, 68–76.
Oyarzun, R., Lillo, J., Higueras, P., Oyarzún, J., & Maturana, H. (2004). Strong arsenic enrichment in sediments from the Elqui watershed, Northern Chile: Industrial (gold mining at El Indio-Tambo district) vs. geologic processes. Journal of Geochemical Exploration, 84, 53–64.
Pandey, P. K., Yadav, S., Nair, S., & Pandey, M. (2004). Sampling and preservation artifacts in arsenic analysis: Implications for public health issues in developing countries. Current Science, 86, 1426–1432.
Plant, J. A., Kinniburgh, D. G., Smedley, P. L., Fordyce, F. M., & Klinck, B. A. (2003). Arsenic and selenium. Treatise on Geochemistry, 9. In H. D. Holland, K. K. Turekian, & B. S. Lollar (Eds.), Environmental geochemistry (pp. 17–66). Amsterdam: Elsevier.
Polizzotto, M. L., Harvey, C. F., Sutton, S. R., & Fendorf, S. (2005). Processes conductive to the release and transport of arsenic into aquifers of Bangladesh. Proceedings of the National Academy of Sciences, 102, 18819–18823.
Rawlins, B. G., Williams, T. M., Breward, N., Ferpozzi, L., Figueiredo, B., & Borba, R. (1997). Preliminary investigation of mining-related arsenic contamination in the provinces of Mendoza and San Juan (Argentina) and Minas Gerais State (Brazil). British Geological Survey Technical Report WC/97/60.
Roig-Navarro, A. F., Martinez-Bravo, Y., López, F. J., & Hernández, F. (2001). Simultaneous determination of arsenic species and chromium(VI) by high-performance liquid chromatography-inductively coupled plasma-mass spectrometry. Journal of Chromatography, 912, 319–327.
Roychowdhury, T., Tokunaga, H., Uchino, T., & Ando, M. (2005). Effect of arsenic-contaminated irrigation water on agricultural land soil and plants in West Bengal, India. Chemosphere, 58, 799–810.
Scanlon, B. R., Nicot, J. P., Reedy, R. C., Kurtzman, D., Mukherjee, A., & Nordstrom, D. K. (2009). Elevated naturally occurring arsenic in a semiarid oxidising system, Southern High Plains aquifer, Texas, USA. Applied Geochemistry, 24, 2061–2071.
Schreiber, M. E., Simo, J. A., & Freiberg, P. G. (2000). Stratigraphic and geochemical controls on naturally occurring arsenic in groundwater, eastern Wisconsin, USA. Journal of Hydrogeology, 8, 161–176.
Sharpless, C. M., & Linden, K. G. (2001). UV photolysis of nitrate—effects of natural matter and dissolved inorganic carbon and implications for UV water disinfection. Environmental Science and Technology, 35, 2949–2955.
Sigrist, M. E., & Beldoménico, H. R. (2004). Determination of inorganic arsenic species by flow injection hydride generation atomic absorption spectrometry with variable sodium tetrahydroborate concentrations. Spectrochimica Acta Part B, 59, 1041–1045.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Smedley, P. L., Kinniburgh, D. G., Macdonald, D. M. J., Nicolli, H. B., Barros, A. J., Tullio, J. O., et al. (2005). Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Applied Geochemistry, 20, 989–1016.
Smedley, P. L., Nicolli, H. B., Macdonald, D. M. J., Barros, A. J., & Tullio, J. O. (2002). Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Applied Geochemistry, 17, 259–284.
Smith, N. M., Lee, R., Heitkemper, D. T., Denicola Cafferky, K., Haque, A., & Henderson, A. K. (2006). Inorganic arsenic in cooked rice and vegetables from Bangladeshi households. Science of the Total Environment, 370, 294–301.
Smith, E., Naidu, R., & Alston, A. M. (2002). Heavy metals in the environment. Chemistry of inorganic arsenic in soils: II. Effects of phosphorus, sodium, and calcium on arsenic sorption. Journal of Environmental Quality, 31, 557–563.
Steinmaus, C., Bates, M. N., Yuan, Y., Kalman, D., Atallah, R., Rey, O. A., et al. (2006). Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. Journal of Occupational and Environmental Medicine, 48, 478–488.
Suzuki, K. T. (2005). Speciation of arsenic in biological samples. Analytica Chimica Acta, 540, 71–76.
Tchounwou, P. B., Patlolla, A. K., & Centeno, J. A. (2003). Carcinogenic and systemic health effects associated with arsenic exposure—A critical review. Toxicologic Pathology, 31, 575–588.
Tseng, C.-H. (2009). A review on environmental factors regulating arsenic methylation in humans. Toxicology and Applied Pharmacology, 235, 338–350.
Vaclavikova, M., Gallios, G. P., Hredzak, S., & Jakabsky, S. (2008). Removal of arsenic from water streams: An overview of available techniques. Clean Technologies and Environmental Policy, 10(1), 89–95.
Verplanck, P. L., Mueller, S. H., Goldfarb, R. J., Nordstrom, D. K., & Youcha, E. K. (2008). Geochemical controls of elevated arsenic concentrations in groundwater, Ester Dome, Fairbanks district, Alaska. Chemical Geology, 255, 160–172.
Wang, C.-H., Jeng, J.-S., Yip, P.-K., Chen, C.-L., Hsu, L.-I., Hsueh, Y.-M., et al. (2002). Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation, 105, 1804–1809.
Wang, S., & Mulligan, C. N. (2006). Occurrence of arsenic contamination in Canada: Sources, behaviour and distribution. Science of the Total Environment, 366, 701–721.
Watts, M. J., O’Reilly, J., Marcilla, A., Shaw, R. A., & Ward, N. I. (2010). Field based speciation of arsenic in UK and Argentinean water samples. Environmental Geochemistry and Health (this issue). doi:10.1007/s10653-010-9321-y.
Watts, M. J., O’Reilly, J., Smiles, C. A., & Cook, J. M. (2007). Measurement of arsenic compounds in water by HPLC-ICP-MS. British Geological Survey Internal Report, OR/07/021.
WHO. (1993). Guidelines for drinking—Water quality, volume 1, recommendations (2nd ed.). Geneva: World Health Organisation.
WHO. (2001). Environmental health criteria 224: Arsenic and arsenic compounds (2nd ed.). Geneva: World Health Organisation.
Williams, M. (2001). Arsenic in mine waters: An international study. Environmental Geology, 40, 267–278.
Zárate, M. A. (2003). Loess of southern South America. Quaternary Science Reviews, 22, 1987–2006.
Acknowledgments
The authors wish to thank the British Geological Survey University Funding Initiative (BUFI) and the EPRSC for providing funding for this research as part of a PhD studentship. We are also grateful to the contribution from Mr Adrian Brizio (Cospec, Eduardo Castex) and Mr Adrian Fenocchio (Copeospil Ltda, Ingeniero Luiggi) for their invaluable assistance in the collection of water samples from La Pampa. Thanks must also go to Dr Louise Ander for reviewing the manuscript and Mr Paul Lappage for creating the illustrated map of Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Reilly, J., Watts, M.J., Shaw, R.A. et al. Arsenic contamination of natural waters in San Juan and La Pampa, Argentina. Environ Geochem Health 32, 491–515 (2010). https://doi.org/10.1007/s10653-010-9317-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-010-9317-7



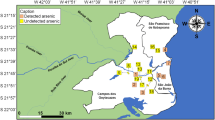









 ; Río Colola (CO1)
; Río Colola (CO1)  ; Río Jáchal (CU1, RJ1–RJ6)
; Río Jáchal (CU1, RJ1–RJ6)  ; Agua Negra (AN1)
; Agua Negra (AN1)  ; Río Huaco (HU1–HU3)
; Río Huaco (HU1–HU3) 


