Abstract
Urine used as a biomarker was collected and compared between two groups of participants: (1) a groundwater-drinking group and (2) a non-groundwater-drinking group in intensively agricultural areas in Ubon Ratchathani province, Thailand. The statistical relationship with the metal concentration in shallow groundwater wells was established with urine data. According to the groundwater data, the health risk assessment results for four metals appeared to be higher for participants who drank groundwater than for the other group. The carcinogenic risk and non-carcinogenic risk of arsenic (As) were found in 25.86 and 31.03% of participants, respectively. For lead (Pb), 13.79% of the participants had a non-carcinogenic risk. Moreover, 30 of the 58 participants in the groundwater-drinking group had As urine higher than the standard, and 26, 2 and 9 of the 58 participants had above-standard levels for cadmium (Cd), Pb and mercury (Hg) in urine, respectively. Both the risk assessment and biomarker level of groundwater-drinking participants were higher than in the other group. The results showed an average drinking rate of approximately 4.21 ± 2.73 L/day, which is twice as high as the standard. Interestingly, the As levels in the groundwater correlated with those in the urine of the groundwater-drinking participants, but not in the non-groundwater-drinking participants, as well as with the As-related cancer and non-carcinogenic risks. The hazard index (HI) of the 100 participants ranged from 0.00 to 25.86, with an average of 1.51 ± 3.63 higher than the acceptable level, revealing that 28 people appeared to have non-carcinogenic risk levels (24 and 4 people for groundwater-drinking participants and non-groundwater-drinking participants, respectively). Finally, the associated factors of heavy metals in urine were the drinking water source, body weight, smoking, sex and use of personal protective equipment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination is a global concern because of the strong toxicity of these substances. Heavy metals released from anthropogenic activities such as agricultural and industrial activities eventually contaminate the surface water and groundwater. They can be dispersed and accumulated in the food chain. These concentrations tend to be high and accumulate in the human body or animals via food and water, which negatively affect human health (Siriwong 2006; Chotpantarat et al. 2011). Heavy metals are bio-accumulated in the human body by three main routes: inhalation, dermal contact and ingestion. In addition, heavy metals can cause serious health effects with various symptoms depending upon the toxicity and quantity of the metal intake (Adepoju-Bello and Alabi 2005). The following studies were conducted in many parts of the world regarding As and other toxic metals that contaminated groundwater and contributed to urine biomarkers and health risk assessments. A study in Japan reported that the concentration of mercury (Hg) in the urine was related to the fish consumption rate. Their participants found a positive correlation between the Hg intake rate and Hg in humans (Tomoko et al. 2007). Due to influences of As-contaminated groundwater in Cambodia, a linear relationship between As concentrations in human hair and in the local groundwater where As (III) is the dominant type (Suthipong et al. 2010) was revealed. Another study in Cambodia on As contamination in groundwater and a risk assessment in the Kandal Province of Cambodia (Thi et al. 2009) reported that approximately 13 of 15 groundwater samples were not only considerably contaminated with As; the researchers also found barium (Ba), manganese (Mn) and lead (Pb), exceeding the WHO drinking water guidelines. Regarding research in Pakistan, Zafar et al. (2009) reported that the health burden of skin lesions was primarily caused by drinking groundwater contaminated with As in the range of 0.1 to >100 µg/L. The similar result was found in a study in New England, which reported a positively significant correlation between residual bladder cancer mortality rates and private groundwater supply use (Ayotte et al. 2006). Normandin et al. (2014) revealed moderate concentrations of As in groundwater ranging from 0.02 to 140 µg/L and found a significant relationship between biomarkers and As in the drinking water intake. Recently, Middleton et al. (2016) found As concentrations ranging from 0.01 to 233 µg/L and reported that private water supplies were the dominant source of inorganic As exposure for the residents of Cornwall in the UK.
Furthermore, many studies used urine as a biomarker representing daily excretion of Cd, Cr, Mn, Ni and Pb (Gil et al. 2011), As (Nathalie et al. 2012) and Hg (Li et al. 2011). Moreover, Ivanenko et al. (2013) used urine levels as a representative biomarker of doses of Ag, Al, As, Ba, Be, Cd, Co, Cr, Cs, Cu, Fe, Mn, Ni, Pb, Se, Sr, Tl, U, V and Zn, for which a total heavy metal analysis may show the effect from recent daily drinking water (Gil et al. 2011; Nathalie et al. 2012). Urine is the main route of excretion, which is produced by the kidneys and contains the by-products of metabolism: salts, toxins and water that end up in the blood (Nathalie et al. 2012). Urine shows evidence of the risk of ingesting heavy metals contaminated in the groundwater. However, there are a few studies that evaluate the relationship between heavy metals in shallow groundwater and those in urine (Ayotte et al. 2006; Aylward et al. 2014). Moreover, the relationship can be the evidence to support health risk assessment results for people who consumes the groundwater in this area.
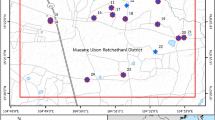
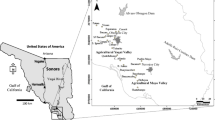
This research focused on locals who had been living and working for a long period of time on one of the largest chili farms in Thailand, where potentially toxic heavy metals contaminated the groundwater, which resulted from long-term intensive agrochemical application (Fig. 1). The locals had been living on the farm, and most had no electricity or tap water in their residences. Groundwater is the main raw water source for both agricultural activities and consumption. A few people used tap water, but most people exploited only groundwater because it is easily accessible and offers a large amount of water. Furthermore, a previous study by Wongsasuluk et al. (2014) in these agricultural areas found adverse human health risks, both non-carcinogenic risks and cancer risks from heavy metals contaminating the locals’ groundwater. In this area, most local farmers had frequently used pesticides and fertilizers on their crops, particularly organophosphate pesticides (OP), which mainly consist of chlorpyrifos and profenofos, and ammonium nitrate fertilizers (NH4NO3) to optimize the product yield (Ooraikul et al. 2011). Moreover, the fertilizers used in the agricultural areas were measured heavy metals, such as Cr, Ni, Cu, Zn, As, Cd, Hg and Pb.
As in earlier studies, the knowledge gap is one in which several previous studies mainly focused on areas located in medium to high levels of As, Cd, Pb and Hg contaminating the groundwater, which particularly exceed the WHO drinking water guidelines, but few studies have mainly focused on low concentrations of As and other toxic metals. The hypothesis in this study was that both non-cancer and cancer risks from groundwater exposure need to be carefully monitored even if heavy metals contaminate the groundwater in low concentrations, particularly in tropical areas, and the main contributing factor to such metals in urine may come from drinking groundwater. To bridge the gap, this research aimed at assessing the health risk and using urine as the indicator of recent exposure to such toxic metals for different participant groups who have been living in intensively agricultural tropical areas. These groups are classified according to their drinking water sources, which were contaminated with low As levels and other toxic heavy metals in the groundwater, most of which do not exceed the Thai groundwater drinking standard. Interestingly, since the main drinking water source is the groundwater and the high drinking intake rate is due to intensive farming (Wongsasuluk et al. 2014), this study can be used as the representative of a health risk assessment in low concentrations of heavy metal contaminating the groundwater in agricultural tropical areas, where most local farmers usually drink groundwater in the field while working. The relationship between heavy metals in urine and these metals in the local drinking groundwater as well as the relationship between urine and the risk of human exposure was then established. Finally, using statistical techniques, which were Kolmogorov-Smirnav test (K-S test), Mann-Whitney U-test (2-tailed), Kendall and Spearman test, and multiple linear regression, the associated factors contributing to the biomarkers were determined. The objectives of this study were (1) to investigate and compare the As and other toxic heavy metals measured in the drinking water (groundwater and tap water) at each site and in the urine of the participants (groundwater-drinking group and non-groundwater-drinking group); (2) to determine the statistical relationship between such metals in groundwater and those in the urine of the two groups; (3) to estimate the risk of human exposure of the two groups and to establish the relationship between urine and the risk of human exposure; and finally (4) to individually determine the major contributing factors on the amount of heavy metals in the urine.
Materials and methods
Study area
The agricultural study area has been continuously and intensively farming for a long period of time and remains one of the largest chili farms in Thailand (Norkaew 2009), with other crops including rice, rubber trees and corn. This site located in the Mueang district in the Ubon Ratchathani province in northeast Thailand between a longitude of 1,695,000–1,704,000 UTM and a latitude of 479,000–469,000 UTM (Fig. 1). The average altitude of this study area was 129.76 ± 13.22 m (msl), ranging from 87.00 to 147.00 m (msl). In addition, the direction of groundwater flow was from the upstream reservoir to the south and southern parts. The depth of the groundwater level is fairly shallow and was in the range of 2–4.4 m with an average of 2.85 m throughout the year (Fig. 2a), indicating that this area is located in a discharge area. The soil textures consist of three types: sandy loam, loamy sand and sand with an average hydraulic conductivity of between 3.43 and 49.03 cm/day (Chotpantarat et al. 2011; Masipan et al. 2016). Furthermore, according to our previous study (Wongsasuluk et al. 2014), these agricultural areas had acidity in the shallow groundwater, where the average pH was 4.72 ± 1 (Wongsasuluk 2010; Wongsasuluk et al. 2014). As mentioned, in terms of the hydrogeological characteristics, this intensively farming appears to have the potential for the movement of agrochemicals from the ground surface to shallow groundwater systems.
Sampling and analytical methods
The 100 locals who were the target participants in the study area were randomly selected among those who permanently live in this agricultural study area without migrating and those above the age of 18. Each participant submits urine and general drinking water samples, and they were placed in shallow groundwater or non-groundwater (tap water) drinking water groups. Face-to-face interviews were used to collect personal information such as age, sex, body weight, underlying diseases, occupation, smoking behavior, alcohol use and drinking and washing water sources to investigate the associated factors that may affect heavy metals’ accumulation in humans. The urine was collected in 100-mL glass bottles. The bottles were parafilm-wrapped to prevent leaking or evaporation and were then frozen at −4 °C in sealed containers. Drinking water from both the tap water and groundwater of all participants was also sampled using rinsed polyethylene bottles. The glass bottles used in this research were bottles specifically designed for urine sampling, and the drinking water samples were collected in sterile polyethylene bottles (Aguilera et al. 2010). The water samples were not filtered because they were not crystallized (which was prevented using nitric acid). Urine samples were normally clear; if not, they were filtered using 20–25-µm pore filter paper.
Shallow groundwater samples were collected from local groundwater wells from which participants usually consume. The initial approximately 5–10 min worth of pumped groundwater or 2–3 pore volumes of well water were discarded, and the groundwater samples were collected in rinsed polyethylene bottles. The pH and electrical conductivity (EC) of the freshly collected non-filtered samples were measured in situ using a pH and EC meter (Instrument: pH 3210 set two including SenTix® 41), respectively. Each water sample was acidified with 65% (v/v) HNO3 to pH < 2 to dissolve all heavy metals and to prevent crystallization or precipitation. Approximately 0.3 ml or 5–6 drops per water sample was added in 60-mL bottles (the lowest volume changed not affected concentrations of heavy metals); 0.5% nitric acid was adequate to prevent precipitation (Normandin et al. 2014; UnChalee 2014; Wongsasuluk et al. 2014). All groundwater sample bottles were kept at approximately 4 °C in sealed containers to prevent evaporation and were transported back to the laboratory prior to analyzing the amounts of heavy metals.
For measurement of heavy metals in drinking water, inductively coupled plasma mass spectrometry or ICP-MS from the Milestone Laboratory System ETHOS was used to measure the concentrations of As, Cd, Pb and Hg in all drinking water samples (see Supplementary Information). For the As, Cd and Pb analyses, eight concentration points for standard curves of ICP-MS, ranging from 0.5 to 100 µg/L, were used with their r 2 of 0.9998, 0.9998 and 0.9999, respectively. For Hg, six concentration points ranging from 0.5 to 10 µg/L were used with an r 2 of 1.0000. Deionized water (DI water) was used as a 0.000 µg/L blank for all heavy metal analyses. The atomic absorption spectrophotometer (AAS) (based on the standard of the American Conference of Governmental Industrial Hygienists (ACGIH) process) was used to measure the concentrations of As, Cd, Pb and Hg in urine (see Supplementary Information). The standard curve of AAS for As, five concentration points ranging from 20 to 200 µg/L, was used with an r 2 of 0.9987. The four standard points for Cd ranged from 2 to 20 µg/L with an r 2 of 0.9987. The five standard points for Pb ranged from 5 to 80 µg/L with an r 2 of 0.9993. The four standard points for Hg ranged from 6.25 to 50 µg/L with an r 2 of 0.9989. The diluted 1% nitric acid was used as a 0.00 µg/L standard blank. The detection limits of ICP-MS were 0.001 µg/L for all heavy metals, while the detection limits of AAS were 0.01, 0.01, 0.02 and 0.05 µg/L for As, Cd, Pb and Hg, respectively. The LODs was calculated by SD*3. For the inter-method verification between AAS and ICP-MS, the same urine samples were analyzed for As, Cd, Pb and Hg to assess the different concentrations between the two methods and found that the % of relative error for the four metals was between 3.75 and 5.32% (see Supplementary Information, Table SI.1). The Certified Reference Material data were used as quality assurance for the analytical standard samples (for As, Cd, Pb and Hg) for both the groundwater and urine (see Supplementary Information, Tables SI.2–SI.5). The urine results were trustworthy because the results were measured and certified by the Special Lab Center Clinic of Thailand using the standard method of ACGIH, which is used in medical specialty clinics, especially for blood and urine measurement. The concentration units of Cd, Pb and Hg in urine (µg/g of creatinine) are derived by comparing concentrations of individual heavy metals in urine with creatinine in urine (mg/dL of urine). In addition, the water results of ICP-MS were measured and certified by the Central Laboratory of Thailand, a well-known laboratory in Thailand, following the AWMA (American Water Works Association) in-house method TE-CH-038 that is based on Standard Methods for the Examination of Water and Wastewater by AWWA (22nd edition, 2012, part 3030E). Each sample was tested in triplicate, and the results showed the standard deviation, calculated from the triplicate analysis (see Tables 4, 5, 6, 7).
Data analysis
The data obtained from face-to-face interviews were summarized and were used to calculate the risk assessment and associated risk factors. The results of the health risk assessment estimated the probability of the occurrence of any given probable magnitude of adverse health effects over a specified time period. The health risk assessment of each heavy metal was based on the quantification of parameters in the risk calculation equation. The two principal toxicities are the different toxicities between the carcinogenic effect and non-carcinogenic effect. The first toxicity is the slope factor (SF) for evaluating the carcinogen risk characterization, while the second toxicity is a reference dose (RfD) for evaluating the non-carcinogen risk characterization (Lim et al. 2008). The toxicity indices of each potentially toxic metal are shown in Table 1. The estimations of the concentration, frequency and duration of human exposure to each potentially toxic metal in the environment are measured as the average daily dose (ADD) (USEPA 1992; Siriwong 2006), as shown in Eq. (1);
where ADD is the exposure duration (mg/kg-day), C is the concentration (mg/L), IR is the intake rate (L/day), EF is the exposure frequency (day/year), ED is the exposure duration (year), BW is the body weight (kg), and AT is the average time (day) that the subjects are exposed to heavy metal.
Risk characterization is the last step of the health risk assessment process based on the calculation of ADD. The non-carcinogenic risk was calculated as the hazard quotient (HQ), as shown in Eq. (2);
where the average daily dose (ADD) and reference dose (RfD) are in mg/kg-day.
If the value of HQ exceeds 1, there is an unacceptable risk of adverse non-carcinogenic effects on health, while if the HQ is <1, there is an acceptable level of risk (USEPA 2001). For the risk assessment of many heavy metals, the individual HQs are combined to represent the hazard index (HI), where HI > 1 denotes an unacceptable risk of non-carcinogenic effects on health, while HI < 1 denotes an acceptable level of risk (ECETOC 2001). The carcinogenic risk can be calculated as the product of ADD (mg/kg-day) multiplied by the SF (mg/kg/day). An acceptable level is ≤10−6, which means that, on average, the probability is that approximately one person per 1,000,000 will develop cancer as a consequence of the exposure (Lim et al. 2008), while values ≥10−6 are unacceptable risk levels. According to the cancer risk assessment process, the results were the probability of the occurrence of adverse health effects in participants. As mentioned earlier, in summary, the risk results can be indicated by either the value of the hazard quotient (HQ) in terms of the non-carcinogenic risk or the value of the cancer risk in terms of the carcinogenic risk. The toxicity of heavy metals or the dose responses of each potentially toxic metal are shown in Table 1.
To compare heavy metals’ concentrations in the urine between groundwater-drinking participants and non-groundwater-drinking participants, the Kolmogorov–Smirnov test (K–S test) was used to investigate the normal distribution, and then, the Mann–Whitney U test (two-tailed) was applied to investigate the difference between the two independent groups of continuous data.
Statistical parameters including the range, average and standard deviation were investigated. The independent variables were both category data and continuous data that were obtained from the questionnaire. The dependent variable was the concentration of heavy metals in urine, which was classified as continuous data. The relationship between heavy metals in the urine and heavy metals in the drinking water and the relationship between heavy metals in the urine and the risk assessment were established using Kendall and Spearman tests. Since heavy metals concentrations in urine and the risk assessment (HQ and cancer risk) are both continuous data, Kendall and Spearman tests could be used to investigate their correlations. In addition, a multiple linear regression can be used to identify the associated risk factors using the SPSS 16.0 software (IBM Corporation, New York, NY, USA). The multiple linear regression was used because the independent factors (x) or associated factors included both category data (such as sex, occupations) and continuous data (such as age, weight, height), while the dependent factors (y) were heavy metal concentrations. Both cancer risk and non-cancer risk values were calculated using the cancer risk level and HQ values. Bråtveit et al. (2011) studied heavy metals in urine in Norway and used urine as a biomarker of chronic cadmium exposure in a population residing in the vicinity of a zinc-producing plant and investigated the associated factors affecting metals in the urine using the multiple linear regression technique.
Result and discussion
The characteristic of study area
This study focused on 25 random sampling stations. There are only seven stations where only shallow groundwater (no tap water) was found as follows: stations 3, 4, 9, 12, 18 and 22, with station no. 25 being the reservoir (Fig. 1).
The average of the shallow groundwater well depth was 4.87 ± 3.14 m, ranging from 1.25 to 15.00 m. The average groundwater level was 124.46 ± 14.81 m (msl), ranging from 83.00 to 143.30 m (msl). The average pH of the shallow groundwater was 5.23 ± 1.15, ranging from 3.79 to 7.60, while tap water was 7.24 ± 0.73, ranging from 6.40 to 9.67. The average electric conductivity (EC) of the groundwater was 171.8 ± 183.16 μS/cm, ranging from 25.40 to 790.00 μS/cm, while the tap water was 367.40 ± 410.11 μS/cm, ranging from 22.20 to 1849.00 μS/cm. The highest conductivity was at well no. 24, and the lowest was at well no. 22 (Fig. 2b; Table 2). The results showed that pH of the groundwater indicated acidity, and all pH levels were lower than the tap water, which were mostly normal or slightly alkaline. Figure 2b, c shows the pattern of the characteristics of groundwater; pH and EC showed a similar pattern, which is a relatively lower pH and higher EC in the central area. On the other hand, they appeared to be a higher pH and lower EC in northeastern and southwestern parts of the area.
These results were similar to a study by Srithongdee et al. (2010), which found that the pH values of the shallow groundwater in this area ranged from 3.68 to 4.88. In addition, the result from Srithongdee et al. (2010) reported that pesticides were not found in shallow unconfined aquifer, while nitrate concentrations were found to be negatively correlated with pH, corresponding to the application of nitrate fertilizers in this area for a long period. Likewise, Jeyaruba and Thushyanthy (2009) found that the groundwater appeared to be more acidic due to the fertilizer application used in the agricultural area. In this study area, NH4NO3-based fertilizers have been mostly used, which leads to the groundwater becoming slightly acidic because the ammonium nitrate in water will produce hydronium ions (H3O+) and so lower pH of the soil and groundwater.
The characteristics of the participants
This study involved 100 participants, consisting of 58 people in the groundwater-drinking group and 42 people in the non-groundwater-drinking groups. The participants comprised 28 males and 72 females. Their average weight was 59.9 ± 12.8 kg, ranging from 30.0 to 110.0 kg. Their average height was 157.6 ± 7.31 cm, ranging from 140.0 to 176.0 cm. The average age of the participants, excluding children, was 45.8 ± 13.8, ranging from 18 to 78 years of age. The USEPA normal standard consumption rate of adults ranged from 1.5 to 2.0 L/day/person (USEPA 1980), but the locals in this study area had a drinking intake rate that was twice as high than the standard of 4.21 ± 2.73 L/day, with the minimum and maximum drinking rate of 1.25 L/day and up to 12.5 L/day, respectively. This research found that the causes of health risks were that most were agriculturalists (78%) who had long working hours every day (average 10.02 ± 2.51 h/day; maximum was 14 h/day), implying intensive farm work in the sunshine and in a hot climate. As a result, they consumed a large amount of water while working, which was one of the parameters in risk calculation (average daily dose, ADD), which significantly affected the risk assessment results. In Table 3, this study found a drinking intake rate that was higher than in other studies and differed from other countries, such as 1.5 L/day reported in Canada (Krishnan and Carrier 2008) and 1.8 ± 0.6 L/day reported in France (Marion et al. 2015). Similarly, according to our previous research in 2010 in the Ubon Ratchathani province in Thailand, we found that average groundwater consumption was relatively high at 3.6 ± 2.1 L/day/person for adults and the drinking intake rate for the elderly was 2.6 ± 1.0 L/day/person (Chotpantarat et al. 2014). The interesting part of the study results identified the determinant, which was the relatively high intake rate of drinking water compared to participants in other studies. Thus, this study can be used as a representative of risk of low concentrations of heavy metals contaminating the groundwater in tropical areas, where there should be considerable concern. Moreover, the study used urine as biomarkers to confirm the accumulation of heavy metals in humans who had been consuming groundwater at low concentrations.
The concentrations of heavy metals in drinking groundwater and tap water
This study found two main drinking water sources: groundwater and tap water. The average concentrations of As in shallow groundwater were 1.584 ± 0.031 and 0.109 ± 0.02 µg/L, 6.902 ± 0.08 µg/L, 0.05 ± 0.006 µg/L for Cd, Pb and Hg, respectively, whereas the average concentrations of As in tap water were 2.185 ± 0.033 and 0.002 ± 0.002 µg/L, 0.11 ± 0.003 µg/L and 0.022 ± 0.005 µg/L for Cd, Pb and Hg in tap water, respectively (Table 4; Fig. 3). Moreover, there were four samples of shallow groundwater in which concentrations of Pb higher than standard were found, which were at stations 4, 8, 9 and 19 (Table 4; Fig. 3c). The concentrations of three heavy metals (i.e., Cd, Pb and Hg) had a similar pattern, which was of relatively high levels in the central area and apparently lower levels in the surrounding areas, while As showed a different distribution pattern, which were fairly low in the central areas and appeared to be higher in the southwestern part of the area, probably affecting the pH of groundwater. Generally, As becomes highly mobilized in high pH or alkaline groundwater and becomes less mobilized in low pH or acidic groundwater. The adsorption of As decreases as the pH increases over the pH 6–9 range (USGS 2016). Therefore, the stations that had a high pH had higher concentrations of As compared to those with a low pH. In addition, the pH was slightly elevated in the groundwater due to the dissolution of carbonates and silicates and from the cation exchange process, promoting the release of As from iron and manganese oxides into the groundwater (Bhattacharya et al. 2006; Smedley et al. 2002). Elementary As is fairly insoluble, whereas arsenic compounds may readily dissolve. As is generally present in groundwater systems as HAsO4 2− (aq) and H2AsO4 − (aq) and most likely partially as H3AsO4 (aq), AsO4 3− (aq) or H2AsO3 − (aq), as shown in the following equation (Lenntech 2016):
In this study area, a slightly basic pH and a relatively high As concentration were found, such as at well no. 24 (pH 7.58 and As 9.081 µg As/L) compared to well no. 23, where the water was found to be acidic and had an As concentration that was 34 times lower (pH 4.41, As 0.27 µg/L) (Table 5).
Comparison between groundwater-drinking group and non-groundwater-drinking group
Urine
As seen in Fig. 4 and Tables 6 and 7, this study found that the average concentrations of all heavy metals in urine from participants in the groundwater-drinking group were higher than those in non-groundwater-drinking group. In addition, the average of As in urine from the participants in groundwater-drinking group was also greater than the ACGIH standard of 35 µg/L (the association advancing occupational and environmental health). The average As concentration in the urine of groundwater-drinking participants was 36.97 µg/L, ranging from 2.80 to 119.60 µg/L, while the non-groundwater-drinking participants’ concentration was 19.30 µg/L, ranging from 2.20 to 34.10 µg/L, since the major factor is the average drinking rate of groundwater-drinking participants of 4.44 L/day, which is relatively higher than the other group at 3.88 L/day. The average Cd concentration in the urine of groundwater-drinking participants was 3.71 µg/g of creatinine, ranging from 0.30 to 8.66 µg/g of creatinine (the standard of Cd in the urine was 4 µg/g of creatinine), while the non-groundwater-drinking participants was 2.38 µg/g of creatinine, ranging from 0.64 to 4.22 µg/g of creatinine. The average concentration of Pb in the urine of groundwater-drinking participants was 21.14 µg/g of creatinine, ranging from 4.99 to 58.82 µg/g of creatinine (the standard of Pb in urine was 50 µg/g of creatinine), while the average was 19.87 µg/g of creatinine, ranging from 6.25 to 45.45 µg/g of creatinine for non-groundwater-drinking participants. The average concentration of Hg in the urine of groundwater-drinking participants was 2.73 µg/g of creatinine, ranging from 0.35 to 9.26 µg/g of creatinine (the standard of Hg in urine was 5 µg/g of creatinine), while the average concentration of non-groundwater-drinking participants was 2.16 µg/g of creatinine, ranging from 0.46 to 5.25 µg/g of creatinine. The urine results of Cd, Hg and Pb were standardized to the creatinine level in the urine because these heavy metals cause adverse effects on the kidney (Gil et al. 2011; Li et al. 2011; Ivanenko et al. 2013), while creatinine is not a suitable correction factor for As in the urine, and so, the As level was, in contrast, reported as µg As/L urine. However, many studies did not use creatinine as a correction factor anymore because of the effect from the hydration status and variable protein intake (Jooste and Strydom 2010; Knudsen et al. 2000; Nermell et al. 2008). Carlos et al. (2014) found that chronic exposure to inorganic As and high urinary As levels had been linked to an increased creatinine concentration in urine. Furthermore, the urine results found that 30 of the 58 participants in the groundwater-drinking group had As in their urine at a higher level than the standard (51.72%), and 26, two and nine participants had higher than standard levels for Cd (44.83%), Pb (3.45%) and Hg (15.52%). On the other hand, only three participants in the non-groundwater-drinking group had Cd levels higher than the standard (7.14%) and only one participant for Hg (2.38%), as shown in Fig. 4.
The Mann–Whitney U test comparison results found a statistically significant difference between As and Cd in the urine of these two groups (p < 0.05, p = 0.002), indicating that the source of As and Cd in the urine mainly come from the As- and Cd- contaminated drinking groundwater. Although there was no such difference for Pb and Hg, the results of the groundwater-drinking group seemed to be higher than those of the non-groundwater-drinking group, referring that these two metals may be contributed from many sources such as water sources, seafood consumption, smoking behavior, and use of pesticides, etc. (Blaurock-Busch et al. 2011).
There were seven stations that caused average As concentrations in the urine of groundwater-drinking group to be higher than the standard (35 µg/g of creatinine) at well as at stations 3, 7, 11–14 and 24, with a range from 36.70 to 80.90 µg/L (see Tables 6, 7). For Cd concentration in the urine of the groundwater-drinking group, there were 10 stations at which the average Cd concentration was greater than the standard (4 µg/g of creatinine) at well nos. 4, 8, 9, 15, 16 and 19–23, with a range from 4.69 to 8.66 µg/g of creatinine (see Tables 6, 7). There was only one station at well no. 19 that showed an average Pb concentration of 53.23 µg/g of creatinine that was higher than the standard (50 µg/g of creatinine). Furthermore, there were three stations that found an average Hg concentration in the urine of groundwater-drinking group that was larger than the standard (5 µg/g of creatinine) at well nos. 8, 18 and 19, with a range from 5.62 to 9.26 µg/g of creatinine (Tables 6, 7). Figure 5 shows the distribution of concentrations of heavy metals in the urine of the groundwater-drinking group that conformed to those found in shallow groundwater, while Fig. 6 shows the distribution of concentrations of heavy metals in the urine of the non-groundwater-drinking group.
a Contour map of the As concentration in the urine of groundwater-drinking participants. b Contour map of the Cd concentration in the urine of groundwater-drinking participants. c Contour map of the Pb concentration in the urine of groundwater-drinking participants. d Contour map of the Hg concentration in the urine of groundwater-drinking participants
a Contour map of the As concentration in the urine of non-groundwater-drinking participants. b Contour map of the Cd concentration in the urine of non-groundwater-drinking participants. c Contour map of the Pb concentration in the urine of non-groundwater-drinking participants. d Contour map of the Hg concentration in the urine of non-groundwater-drinking participants
The groundwater-drinking participants at the well stations were located at the center of the study area and mostly found Cd, Pb and Hg in the urine that was higher than those of non-groundwater-drinking participants, corresponding to the distributions of Cd, Pb and Hg concentrations in the groundwater that was higher in the central area than in the surrounding areas. On the other hand, the concentration of As groundwater in the northeastern and southwestern parts of the study area was relatively higher than those in the central area, conforming to the distribution of As in the urine of groundwater-drinking participants (see Figs. 3, 5).
In general, As has high mobility in shallow aquifer, depending on the oxidation–reduction potential (ORP) where high oxygen levels in shallow groundwater affect high dissolution levels in water. Arsenic adsorbs onto the surfaces of aquifer media, including iron oxides. Then, the oxidation reduction between arsenate and iron-oxide surfaces is very crucial since iron oxides are common in the subsurface environment as coatings on other solids, and the desorption of arsenate from iron-oxide surfaces becomes higher as pH values become alkaline (USGS 2016). As a result, the well sites where high As concentrations in the water were found also found high As in the urine of the participants. A few stations did not find an obvious difference between the heavy metals in the urine of the groundwater-drinking participants and non-groundwater-drinking participant because groundwater is not only one source of heavy metals accumulated in human body, but also other associated factors such as body weight, smoking, sex and using PPE.
Health risk assessment
All heavy metals (As, Cd, Pb and Hg) found in the groundwater-drinking group had higher non-carcinogenic risk (HQ) than those in the non-groundwater-drinking group. To compare the health risk assessment between groundwater-drinking participants and non-groundwater-drinking participants, the Mann–Whitney U test (two-tailed) showed the significant difference of Pb non-carcinogenic risk (p < 0.05, p = 0.000) and Hg non-carcinogenic risk (p < 0.05, p = 0.013) between these two participants, while the As cancer risk, As non-carcinogenic risk and Cd non-carcinogenic risk did not show a statistically significant difference (p > 0.05, p = 0.645, p = 0.511 and p = 0.453, respectively). Although their risks were not significantly different, the results of the groundwater-drinking group were still higher than those of the non-groundwater-drinking group, even for low cancer risk or HQ. In the groundwater-drinking group risk assessment associated with As, 24.14% of the participants had a carcinogenic risk and 27.59% had non-carcinogenic risks and may see adverse health effects from As-contaminated groundwater. Moreover, in the results of the Pb risk assessment, 13.79% of the participants had a non-carcinogenic risk. For the risk assessment of the non-groundwater-drinking group, carcinogenic risk from As was seen in only 11.90% of participants, and only 9.52% had non-carcinogenic risks. Although the non-groundwater-drinking participants did not see adverse health effects from the groundwater, they may be affected by other factors involving accidental exposure (locals unintentionally exposed or unaware of exposure to heavy metals). Similarly, Soma and Abhay (2015) found that the largest chronic risk was contributed by As from the drinking water pathway. Locals can also accumulate toxic metals in their bodies via three exposure pathways: ingestion via the mouth, inhalation via breathing and dermal contact via the skin. For the groundwater-drinking participants, the most important route was ingestion because the locals believe that groundwater tastes good and contains beneficial minerals since their ancestors had long drunk groundwater in the field.
For non-groundwater-drinking participants, the most important route in this study is dermal because most locals used groundwater for washing and cleaning, not for drinking. Moreover, they are generally exposed to agricultural chemicals without proper PPE and most directly touch such chemicals with their hands without gloves due to comfort in the tropical weather.
The human health risk assessment associated with As contaminating the drinking water of groundwater-drinking participants found that the average cancer risk was 8.07E−07, ranging from 7.92E−10 to 8.07E−06 (see Fig. 7a; Table 8), while the non-groundwater-drinking participants revealed that the average cancer risk was 3.45E−07, ranging from 2.77E−16 to 1.54E−06 (see Fig. 7a; Table 9).
For the non-carcinogenic risk, the HQ of As contaminating the drinking water for the groundwater-drinking participants was 0.93, ranging from 0.00 to 8.65 (see Fig. 7b; Table 8), compared to 0.47, ranging from 0.00 to 4.51 for the non-groundwater drinking participants (see Fig. 7b; Table 9). For Cd-contaminated drinking water, the average HQ was 0.0007, ranging from 0.0001 to 0.0028 in the groundwater-drinking participants (see Fig. 7c; Table 8), compared to 0.0005, ranging from 0.0000 to 0.0022 in the non-groundwater-drinking participants (see Fig. 7c; Table 9). For Pb-contaminated drinking water, the average HQ was 1.318, ranging from 0.00 to 25.67 for the groundwater-drinking participants (see Fig. 7d; Table 8), compared to 0.00002, ranging from 0.0000 to 0.0005 for the non-groundwater-drinking participants (see Fig. 7d; Table 9). Finally, for Hg-contaminated drinking water, the average HQ was 0.0029, ranging from 0.000 to 0.057 for the groundwater-drinking participants (see Fig. 7e; Table 8), and 0.0002, ranging from 0.000 to 0.002 for the non-groundwater-drinking participants, as shown in Fig. 7e and Table 9. The main reasons for concomitantly affecting the risk level are much different between groundwater-drinking participants and non-groundwater-drinking participant: the concentration of heavy metals in the drinking water and intake amounts of drinking water consumption. Therefore, the participants who drink groundwater generally have a higher risk than those who drink tap water.
The HQ of the As risk in groundwater-drinking participants was higher than the acceptable level (HQ < 1) at well nos. 7, 13, 14 and 24, with HQs of 2.172, 2.148, 1.447 and 5.149, respectively. The cancer risk of As is at a higher than acceptable level: 2.14E−06, 1.62E−06, 1.15E−06 and 4.47E−06 for well nos. 7, 13, 14 and 24 (cancer risk >1 × 10−6 meant concern risk, cancer risk <1 × 10−6 meant acceptable level). Similarly, the HQ of Pb from groundwater-drinking participants found three stations had higher than the acceptable levels: well nos. 4, 9, and 19, with HQs of 1.62, 4.93 and 23.80, respectively, as shown in Tables 8 and 9 and Figs. 8 and 9. As-contaminated water that caused adverse health effects in humans has been found in Thailand and in different countries around the world. For example, Muhammad et al. (2009) reported that in Southeast Asia, more than 100 million people were estimated to be at risk from As-contaminated groundwater and that 700,000 people were affected by As-related diseases. Research in the Baja Peninsula of Mexico reported that urinary samples contained a total arsenic concentration (sum of arsenical species) that ranged from 1.3 to 398.7 µg/L. These areas had reported As measurements in drinking water above the national standard (25 ng As/mL) and that 40.5% of the wells contain 0.35–10 µg As/L, 21.5% contain 10.1–25 µg As/L, 31% contain 25.1–200 µg As/L, and 7% contain 200.1–2270 µg As/L. They showed that the risk from drinking water was seen at an intake rate of 1.6 L/day, which contains a level of 2.5 µg/L or 1 in 1000 persons, and 50 µg/L (1.6 L/day) being 21 in 1000 persons (Carlos et al. 2014).
a Non-carcinogenic risk map of arsenic (As) in groundwater-drinking participants. b Non-carcinogenic risk map of cadmium (Cd) in groundwater-drinking participants. c Non-carcinogenic risk map of lead (Pb) in groundwater-drinking participants. d Non-carcinogenic risk map of mercury (Hg) in groundwater-drinking participants
Interestingly, for the overall risk of 100 participants, the average hazard index (HI) was calculated, and the results found that average HI of 100 participants was higher than the acceptable level. The HI of the 100 participants ranged from 0.00 to 25.86 with an average of 1.51 ± 3.63, revealing that 28 people appeared to have non-carcinogenic risk. To compare the two groups of participants, the HI of the groundwater-drinking participants showed that 24 people had non-carcinogenic risk, with an average HI being higher than the acceptable level at 2.25 ± 4.59 (ranging from 0.01 to 25.86), while the HI of the non-groundwater-drinking participants showed that only four people had non-carcinogenic risk of five times lower than the average HI of 0.47 ± 0.80 (ranging from 0.00 to 4.51).
The statistical analysis: Kendall and Spearman correlation tests
The correlations between the concentrations of these four heavy metals in the groundwater and the average heavy metals in the urine of the participants who drink groundwater from each shallow groundwater well are shown in Fig. 10. The r 2 of the correlation between the As groundwater and As urine was 0.7426, and 0.473, 0.7847, 0.7231, for Cd, Pb and Hg, respectively. The results showed that As in the urine of the groundwater-drinking participants was significantly correlated with As concentrations in drinking groundwater at the 0.05 level (see Fig. 10; Tables SI.7–SI.14). Moreover, the As concentration in the groundwater was significantly correlated with the As cancer risk and As non-carcinogenic risk also at the 0.05 level, while the As urine of non-groundwater-drinking participants was not significantly correlated. This relationship between As urine and As concentrations in groundwater was supported by a study from Normandin et al. (2014), who investigated the As concentration in the urine of participants in a rural region in Canada and found a significant relationship between biomarkers and As in drinking water intake (As in the groundwater ranged from 0.02 to 140 µg/L).
For Cd in the urine of groundwater-drinking participants, the 0.01 level significantly correlated with Cd in the groundwater and the 0.05 level was significant with non-carcinogenic risk, while Cd in the urine of non-groundwater-drinking participants was not significantly correlated at the 0.05 level. Similarly, Pb in the urine of groundwater-drinking participants was significantly correlated at the 0.01 level with Pb concentration in both the groundwater and non-carcinogenic risk, while Pb in the urine of the non-groundwater-drinking participants was not significantly correlated. In addition, Hg in the urine of the groundwater-drinking participants had a 0.01 level that was significantly correlated with Hg non-carcinogenic risk, while Hg in the urine of non-groundwater-drinking participants was not significantly correlated. The correlation results showed that groundwater and biomarkers have a strong correlation, indicating that groundwater contamination results in the bio-accumulation of heavy metals in human. Moreover, there were no correlations between heavy metals and biomarkers in the non-groundwater-drinking group, supporting the fact that the groundwater considerably affected the locals who consumed it. According to a study in the USA regarding the effect of As in populations exposed to As in water and the diet, they found that 76% of total As in the urine was affected by drinking groundwater (Steven et al. 2012). Similarly, a study on urinary heavy metal levels and the relevant factors among exposed people reported that urinary heavy metal levels were related to the human health risk (Hongmei et al. 2011). The relationship between heavy metals in the urine and the drinking water in the population of West Bengal showed linear regressions with very good correlations between As concentrations in the water (ranging from 0.01 to 0.25 mg/L) and urine. This study in India found a moderately high concentration of As in the urine, approximately 83 and 68% of the urine samples (n = 250) containing As above 100 and 200 µg/L, respectively (Tarit 2010).
The associated factors related to heavy metals in urine
The associated factors affecting the concentrations of heavy metals in the urine were investigated using a multiple linear regression analysis. The outputs of As concentrations in the urine showed that the associated factors were water drinking sources (groundwater or tap water) and body weight (p < 0.05), confirming the findings as mentioned in the previous section that As in the urine of groundwater-drinking participants was significantly correlated with As concentrations in groundwater. The other factors that may affect As in the urine were seafood. Consequently, As concentrations in urine were significantly correlated with As cancer risk and As non-carcinogenic risk also at the 0.05 level. Aylward et al. (2014) reported that urinary dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA) were commonly used as biomarkers for inorganic arsenic (iAs) exposure but may also arise from seafood consumption. The National Health and Nutrition Examination Survey (NHANES) assessed potential correlations among urinary DMA, MMA and arsenobetaine (organic arsenic species) and reported that urinary DMA and MMA were positively associated with urinary arsenobetaine, suggesting these levels had directly resulted from seafood consumption (Aylward et al. 2014). Based on the face-to-face interviews in this study, none of participants had been exposed to seafood. However, they may have accidentally been exposed via instant food or conventional food without realizing it.
For Cd in the urine of all participants, the results showed that the associated factors were the body weight of the participants and their smoking behavior (smoking or non-smoking) (p < 0.05, p = 0.022 and 0.011, respectively). In addition, the statistical results showed that the associated factors related to Pb were sex (male and female) and the use of personal protective equipment by participants (p < 0.05, p = 0.001 and 0.027, respectively). This study used a cut point at four pieces, which is half of the amount of total PPE generally using eight pieces. The associated factors related to Hg in the urine of all participants were sex (male and female) and the use of personal protective equipment by the participants (p < 0.05, p = 0.048 and 0.009, respectively). From these results, the As in groundwater was significantly correlated with As in the urine, confirming that As in humans was caused by the arsenic-contaminated drinking groundwater. This finding is in line with the study in West Bengal, India (Tarit 2010). The research study on As contamination in groundwater and the health effects from As poisoning found linear regressions with very good correlations between As concentrations in groundwater and As in urine. Likewise, Gautam et al. (2004) reported that drinking water and food were the primary sources of environmental exposure in a study about biological and behavioral factors of biomarkers for As exposure in a US population. They reported that the concentrations of As in the environment had affected the concentrations of As in the urine, mainly depending on the direct consumption of water (Rebecca et al. 2013). Similarly, a study about urinary levels of As and heavy metals in children and adolescents living in the industrialized area in Ria of Huelva, Spain, reported that the effects of associated factors of the increase of heavy metal levels in urine were age, sex, area of residence and frequency of intake (Aguilera et al. 2010).
Conclusion
This research studied 58 groundwater-drinking participants and 42 non-groundwater-drinking participants. Their average drinking intake rate was twice that of the standard at 4.21 ± 2.73 L/day. First, as per the drinking groundwater investigation, there were four samples of shallow groundwater in which Pb concentrations were found to be higher than the standard level at well nos. 4, 8, 9 and 19. Second, all risk assessment results found that all heavy metals in groundwater-drinking participants caused a higher risk than in non-groundwater-drinking participants. In the groundwater-drinking group risk assessment associated with As, 24.14% of participants were found to have a carcinogenic risk, and 27.59% had a non-carcinogenic risk and may see adverse health effects from As-contaminated groundwater. Similarly, the urine results showed that 30 of the 58 participants in the groundwater-drinking group had As in the urine that was higher than the standard, as well as 26, 2 and 9 participants for Cd, Pb and Hg, respectively. Third, the urine results showed that As in groundwater-drinking participants had significantly correlated with As cancer risk, As non-carcinogenic risk, and arsenic concentration in drinking groundwater at the 0.01 level. The Cd and Pb in urine of groundwater-drinking participants were significantly correlated with Cd and Pb in groundwater and their non-carcinogenic risk, while Hg in urine was significantly correlated with Hg non-carcinogenic risk. Finally, this study concluded that the associated factors related to heavy metal exposure were the water source, weight, body weight, smoking behavior, sex and using PPE.
This study could serve as a database for using urine as biomarker even at low concentrations of heavy metal contamination in sites such as agricultural areas. The results could serve as an informative database for groundwater drinking standards, especially for tropical zones or agricultural countries that were affected by high water intake rates. Furthermore, there is a greater need for risk awareness and communication with locals who live in farming area and use groundwater as a main water supply or with the village head about risks from heavy metal contamination in the groundwater, even at low concentrations.
References
Adepoju-Bello, A. A., & Alabi, O. M. (2005). Heavy metals: A review. Nigerian Journal of Pharmaceutical Sciences, 37, 41–45.
Aguilera, I., Daponte, A., Gil, F., Hernandez, A. F., Godoy, P., Pla, A., et al. (2010). Urinary levels of arsenic and heavy metals in children and adolescents living in the industrialised area of Ria of Huelva (SW Spain). Environment International, 36(2010), 563–569.
Aylward, L. L., Ramasamy, S., Hays, S. M., Schoeny, R., & Kirman, C. R. (2014). Evaluation of urinary speciated arsenic in NHANES: Issues in interpretation in the context of potential inorganic arsenic exposure. Regulatory Toxicology and Pharmacology, 69(2014), 49–54.
Ayotte, J. D., Baris, D., Cantor, K. P., Colt, J., Robinson, G. R., Jr., Lubin, J. H., et al. (2006). Bladder cancer mortality and private well use in New England: An ecological study. Journal of Epidemiology and Community Health, 60, 168–172.
Bhattacharya, P., Claesson, M., Bundschuh, J., Sracek, O., Fagerberg, J., Jacks, G., et al. (2006). Distribution and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Science of the Total Environment, 358, 97–120.
Blaurock-Busch, E., Amin, O. R., & Rabah, T. (2011). Heavy metals and trace elements in hair and urine of a sample of Arab children with autistic spectrum disorder. Maedica, 6(2011), 247–257.
Bråtveit, M., Magerøy, N., Gundersen, H., Vahter, M., & Moen, B. E. (2011). Biomarker of chronic cadmium exposure in a population residing in the vicinity of a zinc producing plant. Science of the Total Environment, 409, 4222–4228.
Carlos, G. C., Janette, M., Luz, M. D., Luz, C. S., Oscar, F. B., & Ana, J. M. (2014). Urinary arsenic levels influenced by abandoned mine tailings in the Southernmost Baja California Peninsula, Mexico. Environmental Geochemistry and Health, 36, 845–854.
Chotpantarat, S., Limpakanwech, C., & Sutthirat, C. (2011). Effects of soil water characteristic curves on simulation of nitrate vertical transport in a Thai agricultural soil. Sustainable Environment Research, 21(3), 187–193.
Chotpantarat, S., Wongsasuluk, P., Siriwong, W., Borjan, M., & Robson, M. (2014). Non-carcinogenic hazard maps of heavy metal contamination in shallow groundwater for adult and aging populations at an agricultural area in northeastern Thailand. Human and Ecological Risk Assessment: An International Journal, 20(3), 689–703.
ECETOC (European Centre for Ecotoxicology of Chemicals). (2001). Aquatic toxicity of mixtures. Technical report 80, Brussels, Belgium.
Gautam, S., Ramesh, S., Tarit, R., & Dipankar, C. (2004). Arsenic and other elements in hair, nails, and skin-scales of arsenic victims in West Bengal, India. Science of the Total Environment, 326, 33–47.
Gil, F., Hernández, A. F., Márquez, C., Femia, P., Olmedo, P., López-Guarnido, O., et al. (2011). Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Science of the Total Environment, 409, 1172–1180.
Hongmei, W., Mei, H., Suwen, Y., Yanqing, C., Qian, L., & Shen, K. (2011). Urinary heavy metal levels and relevant factors among people exposed to e-waste dismantling. Environment International, 37, 80–85.
Ivanenko, N. B., Ivanenko, A. A., Solovyev, N. D., Zeimal, A. E., Navolotskii, D. V., & Drobyshev, E. J. (2013). Biomonitoring of 20 trace elements in blood and urine of occupationally exposed workers by sector field inductively coupled plasma mass spectrometry. Talanta, 116, 764–769.
Jeyaruba, T., & Thushyanthy, M. (2009). The effect of agriculture on quality of groundwater. Environment International, 4, 110–114.
Jooste, P. L., & Strydom, E. S. (2010). Methods for determination of iodine in urine and salt. Best Practice and Research Clinical Endocrinology and Metabolism, 24(2010), 77–88.
Knudsen, N., Christiansen, E., Brandt-Christensen, M., Nygaard, B., & Perrild, H. (2000). Age- and sex-adjusted iodine = creatinine ratio. A new standard in epidemiological surveys? Evaluation of three different estimates of iodine excretion based on casual urine samples and comparison to 24 h values. European Journal of Clinical Nutrition, 54, 361–363.
Krishnan, K., & Carrier, R. (2008). Approaches for evaluating the relevance of multiroute exposures in establishing guideline values for drinking water contaminants. Journal of Environmental Science and Health Part C: Environmental Carcinogenesis and Ecotoxicology Review, 26(3), 300–316.
Lenntech. (2016). Arsenic and water: Reaction mechanisms, environmental impact and health effects. http://www.lenntech.com/periodic/water/arsenic/arsenic-and-water.htm. Accessed July 06, 2016.
Li, P., Feng, X., Qiu, G., & Wan, Q. (2011). Hair can be a good biomarker of occupational exposure to mercury vapor: Simulated experiments and field data analysis. Science of the Total Environment, 409, 4484–4488.
Lim, H. S., Lee, J. S., Chon, H. T., & Sager, M. (2008). Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. Journal of Geochemical Exploration, 96, 223–230.
Marion, A., Antoine, D., Claire, G., Sylvie, S., Fabrice, P., Sylvie, R., et al. (2015). Estimating drinking-water ingestion and dermal contact with water in a French population of pregnant women: The EDDS cohort study. Journal of Exposure Science and Environmental Epidemiology, 25, 308–316.
Masipan, T., Chotpantarat, S., & Boonkaewwan, S. (2016). Experimental and modelling investigations of tracer transport in variably saturated agricultural soil of Thailand: Column study. Sustainable Environmental Research, 26, 97–101.
Middleton, D. R. S., Watts, M. J., Hamilton, E. M., Ander, E. L., Close, R. M., Exley, K. S., et al. (2016). Urinary arsenic profiles reveal exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK. Scientific Reports, 6, 25656.
Muhammad, M. R., Maidu, R., & Prosun, B. (2009). Arsenic contamination in groundwater in the Southeast Asia region. Environmental Geochemistry and Health, 31, 9–21.
Nathalie, M., Chantal, S., & Marie-Pierre, S. (2012). What is the best biomarker to assess arsenic exposure via drinking water? Environment International, 39, 150–171.
Nermell, B., Lindberg, A., Rahman, M., Berglund, M., Persson, L. A., Arifeen, S. E., et al. (2008). Urinary arsenic concentration adjustment factors and malnutrition. Environmental Research, 106, 212–218.
Norkaew, S. (2009). Knowledge, attitude and practice (Kap) of using personal protective equipment (Ppe) for chilli-growing farmers in Huarua Sub-District, Mueang District, Ubon Ratchathani province, Thailand. Degree of Master of Public Health Program in Public Health College of Public Health Sciences, Graduate School Chulalongkorn University.
Normandin, L., Ayotte, P., Levallois, P., Ibanez, Y., Courteau, M., Kennedy, G., et al. (2014). Biomarkers of arsenic exposure and effects in a Canadian rural population exposed through groundwater consumption. Journal of Exposure Science and Environmental Epidemiology, 24, 127–134.
Ooraikul, S., Siriwong, W., Siripattanakul, S., Chotpantarat, S., & Robson, M. (2011). Risk assessment of organophosphate pesticides for chili consumption from chili farm area, Ubon Ratchathani province, Thailand. Journal of Health Research, 25(3), 141–146.
Rebecca, L. C., Edward, E. H., Cara, C. A., Bin, H., ChrisLe, X., John, R., et al. (2013). Biological and behavioral factors modify biomarkers of arsenic exposure in a U.S. population. Environmental Research, 126, 134–144.
Siriwong, W. (2006). Organophosphate pesticide residues in aquatic ecosystem and health risk assessment of local agriculture community. Doctor dissertation Program in Environmental Management (Interdisciplinary Programs), Graduate School Chulalongkorn University, Thailand.
Smedley, P. L., Nicolli, H. B., Macdonald, D. M. J., Barros, A. J., & Tullio, J. O. (2002). Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Applied Geochemistry, 17, 259–284.
Soma, G., & Abhay, K. S. (2015). Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environmental Monitoring and Assessment, 187, 63.
Srithongdee, T., Chotpantarat, S., Siriwong W., & Siripattanakul, S. (2010). Nitrate and pesticide contamination of shallow groundwater under chilli field of Hua Rua, Ubon Ratchathani province. In Proceedings of the APRC-2010, Ubon Ratchathani, Thailand.
Steven, E. S., Robson, M. G., Ruimin, X., & Brian, T. B. (2012). Importance of arsenic speciation in population exposed to arsenic in drinking water. Human and Ecological Risk Assessment, 18, 1271–1291.
Suthipong, S., Kyoung-Woong, K., Kyung, H. C., Kitirote, W., Sieng, S., Choup, S., et al. (2010). Arsenic levels in human hair, Kandal province, Cambodia: The influences of groundwater arsenic, consumption period, age and gender. Applied Geochemistry, 25, 81–90.
Tarit, R. (2010). Groundwater arsenic contamination in one of the 107 arsenic-affected blocks in West Bengal, India: Status, distribution, health effects and factors responsible for arsenic poisoning. International Journal of Hygiene and Environmental Health, 213, 414–427.
Thi, T. G. L., Suthipong, S., & Kyoung-Woong, K. (2009). Arsenic and other trace elements contamination in groundwater and a risk assessment study for the residents in the Kandal province of Cambodia. Environment International, 35, 455–460.
Tomoko, O., Mineshi, S., Tomoko, K., Miwako, D., Toyoto, I., & Katsuyuki, M. (2007). Total mercury levels in hair, toenail, and urine among women free from occupational exposure and heir relations to renal tubular function. Environmental Research, 103, 191–197.
UnChalee, H. (2014). Nitric acid. www.siamchemi.com. Accessed November 3, 2016.
USEPA (US Environmental Protection Agency). (1980). Water quality criteria documents; availability. 45 Federal Register 45(231), 79318–79379.
USEPA (US Environmental Protection Agency). (1992). Definitions and general principles for exposure assessment. Guidelines for exposure assessment. Washington, DC: Office of Pesticide Programs, USA.
USEPA (US Environmental Protection Agency). (2001). Baseline human health risk assessment Vasquez Boulevard and I-70 superfund site, Denver CO. http://www.epa.gov/region8/superfund/sites/VB-170-Risk.pdf. Accessed January 20, 2011.
USEPA IRIS (US Environmental Protection Agency’s Integrated Risk Information System). (2015). http://www.epa.gov/iris/. Accessed September 17, 2015.
USGS (United State Geological Survey). (2016). Geochemistry of arsenic, review of geochemical processes controlling arsenic mobility. http://or.water.usgs.gov/pubs_dir/Html/WRIR98-4205/as_report6.html. Accessed June 07, 2016.
Wongsasuluk, P. (2010). Human health risk assessment associated with heavy metals in drinking shallow groundwater wells at Ubon Ratchathani province, Thailand. Master Thesis, Chulalongkorn University, Thailand.
Wongsasuluk, P., Chotpantarat, S., Siriwong, W., & Robson, M. G. (2014). Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environmental Geochemistry and Health, 36, 169–182.
Zafar, F., Iqbal, A., Faiza, A., Ambreen, K., Albert, B. G., Muhmmad, M. K., et al. (2009). Health burden of skin lesions at low arsenic exposure through groundwater in Pakistan. Is river the source? Environmental Research, 109, 575–581.
Acknowledgements
The authors acknowledge the support of the Center of Excellence on Hazardous Substance Management (HSM) and the International Postgraduate Programs in Environmental Management, Graduate School, Chulalongkorn University, for their invaluable supports in terms of facilities and scientific equipment. We would like to express our sincere thanks to the 90th Anniversary of the Chulalongkorn University Fund, Ratchadaphisek Somphot Endowment Fund, the Grant for International Research Integration: Chula Research Scholar, Ratchadaphisek Somphot Endowment Fund (GCURS-59-06-79-01), the Office of Higher Education Commission (OHEC) and the S&T Postgraduate Education and Research Development Office (PERDO) for the financial support of the Research Program and thank the Ratchadaphisek Somphot Endowment Fund, Chulalongkorn University for the Research Unit.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wongsasuluk, P., Chotpantarat, S., Siriwong, W. et al. Using urine as a biomarker in human exposure risk associated with arsenic and other heavy metals contaminating drinking groundwater in intensively agricultural areas of Thailand. Environ Geochem Health 40, 323–348 (2018). https://doi.org/10.1007/s10653-017-9910-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-9910-0