Abstract
This study assessed the influence of three tropical soil types and soil moisture content on the toxicity and risk of the insecticide fipronil to collembolans Folsomia candida. Chronic toxicity tests were performed in a Tropical Artificial Soil (TAS), an Oxisol and an Entisol spiked with increasing concentrations of fipronil to assess the effects on the reproduction and growth of the species. The soil moisture contents were kept at 60% (standard condition) and 30 or 45% (water restriction) of their water holding capacity (WHC). The toxicity of fipronil on collembolans reproduction was about three times higher in Entisol compared to TAS or Oxisol. Higher toxicities were also found in the drier TAS (EC50 30%WHC = 0.20 vs EC50 60%WHC = 0.70 mg kg−1) and Oxisol (EC50 45%WHC = 0.27 vs EC50 60%WHC = 0.54 mg kg−1), while in Entisol lower impacts were found in the drier samples (EC50 30%WHC = 0.41 vs EC50 60%WHC = 0.24 mg kg−1). For all tested soils, the size of generated collembolans was reduced by the fipronil concentrations, regardless of soil moisture. However, the drier condition increased the effect on the growth in TAS and Entisol for some concentrations. A significant risk of exposure was found in TAS and Oxisol at drier conditions and, for Entisol, regardless of the soil moisture. The toxic effects and risk of fipronil on collembolans were higher in the natural sandy soil. The soil moisture content increase or decrease the toxicity of the insecticide for collembolans, depending on soil type.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Brazilian territory is composed of a wide variety of soil types with different characteristics (EMBRAPA 2006). These particular soil characteristics, such as texture, pH, cation exchange capacity (CEC), clay and OM contents help to regulate the water availability and the pesticide bioavailability/toxicity to collembolans (Amorim et al. 2005; Chelinho et al. 2014; Ogungbemi and Van Gestel 2018; Bandeira et al. 2019). Some studies comparing the toxicity of pesticides in different types of soils indicate that the toxic effects may be enhanced in sandy soils when compared with fine-textured soils (Amorim et al. 2005; Domene et al. 2012; Bandeira et al. 2019).
In addition to the influence of soil types, climatic factors also play an important influence on the behavior and dynamics of pesticides in soils. According to the Intergovernmental Panel on Climate Change (IPCC), an increase in drought events is expected for tropical countries in the next years, as a consequence of climatic changes (IPCC 2014). Although the consequences may vary for Brazilian regions, a 20% reduction in rainfall regimes is expected by the year 2040, 35% between 2041 and 2070, and 50% between 2071 and 2100, for five of the six biomes that constitute the Brazilian territory (PBMC 2014).
Drought events can promote changes in the environmental fate of pesticides, influence the life-cycle of soil fauna, as well as affect their responses to the toxicants (Domene et al. 2012; Bonmatin et al. 2015; Daam et al. 2019). In this way, some studies have reported that reductions in soil moisture increased the toxicity of some pesticides to soil invertebrates (Lima et al. 2011; Bandow et al. 2014b; Bandow et al. 2016; Hennig et al. 2020).
Fipronil (5 - amino - 1 - [2,6 - dichloro - 4 - (trifluoromethyl) phenyl] - 4 - (trifluoromethylsulfinyl) -1H –pyrazole - 3 - carbonitrile) is a broad-spectrum insecticide that belongs to the class of phenylpyrazoles, widely used in chemical seed treatment (Mohapatra et al. 2010; Bonmatin et al. 2015). Negative effects of fipronil on the collembolan’s survival, reproduction and bioaccumulation are reported in the literature. San Miguel et al. (2008) observed that fipronil affected the collembolan’s survival (LC50 = 0.45 mg kg−1) and reproduction (with EC50 varying between 0.335 and 0.500 mg kg−1), also indicating that these non-target organisms are very susceptible to bioaccumulation. Alves et al. (2014) also found that reduction in collembolans reproduction can occur when exposed to increasing concentrations of fipronil (EC20 = 0.12 mg kg−1). Zortéa et al. (2018a, 2018b) found that both survival (with LC50 varying between 0.29 and 0.48 mg kg−1) and reproduction (with EC50 varying between 0.14 and 0.29 mg kg−1) of collembolans can be affected by exposure to fipronil in a veterinary medicine formulation.Collembolans are considered a representative group of soil fauna and are essential to the maintenance of soil quality (Culik and Zeppelini 2003). These organisms are suitable bioindicators due to their high sensitivity to contamination and environmental stress (Culik and Zeppelini 2003). The exposure of these organisms to drought scenarios negatively affect their metabolism, compromise their growth, survival and reproduction (Bursell 1970; Edney 1977; Van Gestel and Van Diepen 1997; Jänsch et al. 2005; Sławski and Sławska 2020). Although several studies have reported the effects of drought on collembolans, no studies were found describing the relationship between lower moisture in tropical soil and fipronil toxicity to these organisms.
In this study, we hypothesize that a) the toxic effects and risk of fipronil via seed dressing formulations vary in contrasting soil types, being higher in natural sandy soil; b) the reduction of soil moisture content increases the fipronil toxicity to collembolans, depending on the tropical soil type. In this way, this study aimed to assess the influence of soil type and soil moisture content on the toxicity and risk of fipronil to collembolans Folsomia candida. For this, the effects of fipronil on the reproduction and growth of F. candida were assessed via chronic toxicity tests in three tropical soils (Tropical Artificial Soil – TAS, Entisol and Oxisol) under a standard (60% WHC) and a water restriction (30 or 45% WHC) conditions.
Material and methods
Test organisms
Collembolans F. candida were cultured in the laboratory according to ISO 11267 (ISO 2014). The organisms were kept in plastic containers containing a substrate composed of plaster of Paris, water, and activated charcoal (10:7:1, respectively). Twice a week, the culture medium received food (dry Saccharomyces cerevisiae) and had the moisture adjusted with a few drops of distilled water. The culture was kept in a room with a controlled temperature (20 ± 2 °C) and photoperiod (12 h). The species sensitivity was confirmed by using a positive control assay with boric acid in TAS (EC50 = 171.55 mg kg−1), according to Niemeyer et al. (2018).
Test soils
The ecotoxicological tests were performed in an artificial tropical soil (TAS) and two contrasting natural tropical soils.
The TAS (Garcia 2004) consisted of a mixture of fine sand, kaolin and powdered coconut husk (75:20:5, respectively). Calcium carbonate (CaCO3) was used to adjust the pH to 6.0 ± 0.5 (ISO 2014). A clayey soil (Oxisol) was sampled in Palmitos (SC; 27° 04′S 53° 09′W) and a sandy soil (Entisol) was sampled in Araranguá (SC; 29° 00′S 49° 31′W). The soils were taken from the top layer (0–20 cm) of the soil profile in areas with no history of contamination by pesticides. The soil samples were sieved (#2 mm), defaunated through three freezing and thawing cycles, and air-dried (Alves et al. 2019).
For all test soils, the water holding capacity (WHC) and pH (1M-KCl) were determined according to ISO 11267 (2014), and the cation exchange capacity (CEC), soil organic matter (SOM), sand, clay, and silt contents (Table 1) were measured following Tedesco et al. (1995).
Test substance
Soils were spiked with a commercial formulation of an insecticide for seed dressing (Shelter®), which contains 250 g of fipronil L−1 as an active ingredient (a.i.).
Soil samples were spiked with increasing concentrations (Table 2), which were based on literature (Alves et al. 2014; Zortéa et al. 2018a) and preliminary tests (data not shown). A control treatment without contaminant (only with distilled water) was prepared for each soil.
The spiking occurred through an aqueous solution, with volume sufficient to reach 30 and 60% of the WHC in TAS and Entisol, and 45 and 60% of the WHC in Oxisol, since the species did not reproduce in Oxisol at 30% WHC. The soil moisture was checked at the beginning and the end of each assay (Supplementary Information 1).
The time-weighted average Predicted Environmental Concentrations at 28d (PEC) for fipronil were estimated based on the methodology proposed by the European and Mediterranean Plant Protection Organization (EPPO 2003). The software ESCAPE (2013) was used to calculate the PEC based on a soybean crop scenario. It was considered a sowing density of 60 kg of seeds per hectare (ha) (EMBRAPA 1988) at a depth of 0–5 cm (Alves et al. 2013) and soil densities of 1.0 g cm−3 for TAS and Oxisol, and 1.5 g cm−3 for Entisol (Souza et al. 2005; Bandeira et al. 2020). A single Shelter® application dose of 12 g a.i. per 60 kg of seeds (lowest manufacturer’s recommendation), over a single planting cycle, with an interception rate of 5% by the plants (Jackson et al. 2009) and a dissipation half-life of 31 days (EFSA 2006) were assumed.
Chemical analysis
For all tests, soil samples from each treatment were taken at the start of the test for the quantification of the real concentration in soils. The extraction of fipronil was based on the modified QuEChERS acetate, without the cleaning step (Gebrehiwot et al. 2019). For all treatments, 10 g wet soil was used. Then, 10 mL ultrapure water and 10 mL acetonitrile acidified with 1% acetic acid (v/v) were added. The mixture was manually shaken vigorously for 1 min. Afterwards, 6 g anhydrous magnesium sulfate (MgSO4) and 1.5 g anhydrous sodium acetate (CH3COONa) were added to the mixture and, then, it was manually shaken for 1 min. Finally, the tube was centrifuged at 9000 rpm for 5 min. Subsequently, 1 mL supernatant was taken out and diluted in 10 mL methanol. The final diluted extract was filtered through a 0.2 μm polyvinylidene fluoride Millex syringe filter.
The fipronil was analyzed by LC-ESI-MS (LC MS 2020 Shimadzu®, Japan). LCseparation was carried out in C18 ec analytical column. The method was validated (single-laboratory validation; INMETRO 2020), attending linearity, accuracy (in terms of recovery), repeatability and intermediate precision (in terms of relative standard deviation, RSD) and limits of quantification (LOQ = 0.01 mg kg−1). The results of chemical analyzes were converted to mg a.i. per kg of dry soil, based on the soil moisture measured at the beginning of the ecotoxicological test (Table 2).
Test procedures
The chronic toxicity tests were performed according to ISO 11267 (ISO 2014) at the temperature of 25 °C and 12 h-photoperiod.
The experimental units were constituted by glass containers with airtight closure caps that received 30 g of spiked or control soil. Ten organisms with synchronized age (10–12 d) were inserted into each container. On the 1st and 14th day of the test, food was offered to the organisms (≈2 mg of dry Saccharomyces cerevisiae). Weekly, the containers were opened to allow gas exchange and for soil moisture adjustment with distilled water (by weight difference). At the end of the test (28 d), the content of each replicate was transferred to another vessel that received water and a few drops of black ink to allow the flotation and to facilitate the visual contrast of organisms with the water. The surviving adults were visually counted. The tests in each soil occurred individually, however, both moisture treatments were tested simultaneously with the same batch of animals. All treatments were performed in five replicates plus an extra replica, which was used to assess the soil moisture and pH at the end of the bioassays.
To evaluate the reproduction and growth of collembolans, each vessel was photographed from a superior view in high resolution. The photos were analyzed through ImageJ® software (Alves et al. 2014), where all juveniles from each experimental unit were counted through the manual counting tool. In addition, the length (from the front head to the end of the abdomen) of generated collembolans was mensurated on the images by using the same software. For this, at least 30 juveniles of each replicate were randomly selected. For the replicates with <30, all juveniles were measured. In each replicate, the collembolans were classified in three distinct body sizes: small (≤3 mm), medium (>3 and ≤4.5 mm) and large (>4.5 mm). The results were presented in percentage of each size class.
Data analysis
The normality and homoscedasticity of the data from chronic toxicity tests were checked through the Kolmogorov-Smirnov and Bartlett tests, respectively. When necessary, data logarithmic transformations were used to meet the analysis of variance (ANOVA) assumptions. For reproduction and growth data, the differences between treatments were tested by the one-way ANOVA (p < 0.05). When statistical differences were detected in reproduction data, the treatments containing fipronil were compared with the control by using the post-hoc Dunnett’s test (p < 0.05), allowing to determine the non-observed effect concentration (NOEC) and lowest observed effect concentration (LOEC). Differences between the average percentages of small, medium and large juveniles at 30% (or 45%) and 60% WHC, in the same soil type and concentration, were checked via Tukey’s test (p < 0.05).
The effect concentrations causing a reduction in the collembolan’s reproduction or growth in 10 or 50% (only reproduction), in relation to the control (EC10 or EC50, respectively), were estimated by using non-linear regression models (Environment Canada 2007). The EC values were estimated based on measured concentrations (Table 1). Significant differences between EC50 values (reproduction data) from different soil types (at 60% WHC) or moisture contents (within the same soil), were assumed when their 95% confidence intervals did not overlap (Jegede et al. 2017).
A two-way ANOVA was used to evaluate the interaction between the soil moisture and fipronil concentrations, for each soil, on the effects caused on the reproduction and growth of juveniles collembolans. All statistical analyzes were performed using the Statistica® software, version 13.5.0.17.
The risk estimation was performed through the toxicity-exposure ratio (TER) approach (EC 2002), by dividing the EC10 of each soil moisture scenario by the respective PEC value (TER = EC10/PEC). A significant risk for F. candida was considered when TER < 5.
Results
The validity criteria for chronic toxicity tests with F. candida (ISO 2014) were met for all performed tests (Supplementary Information 2).
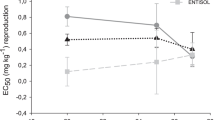
Fipronil caused a reduction in the number of F. candida juveniles in all exposure scenarios, however, the magnitude of the effects varied according to the soil type and soil moisture (Fig. 1).
Mean number (bars ± standard deviation, n = 5) and dose-effect curves (with effect concentrations of 50% - EC50) of juveniles Folsomia candida generated after 28 d of exposure to different concentrations of fipronil in Entisol and Oxisol under regimes of 30 or 45 and 60% of soil water holdong capacity (WHC). Asterisks (*) represent significant reduction in the number of juveniles compared to the respective control (Dunnett’s test, p ≤ 0.05). In dose-effect curves, the EC50 values were plotted as red and yellow circles for the exposures at 30 or 45 and 60%, respectively
At 60% WHC, the fipronil toxicity (EC50-based) did not differ between TAS and Oxisol. On the other hand, higher toxicity was found in Entisol, when compared with the other tested soils. In TAS and Oxisol, the dry condition (30 and 45% WHC respectively) increased the toxicity of fipronil to collembolans, in comparison to 60% WHC (Table 3). For TAS, EC50 values indicated toxicity more than three times higher in the dry soil (30% WHC). For Oxisol, the toxicity at 45% WHC was twice higher than at 60% WHC. On the other hand, in Entisol, the toxicity at 60% WHC was higher when compared with 30% WHC (Table 3). The two-way ANOVA (Supplementary Information 3) showed a significant interaction between the soil moisture content and concentrations of fipronil on the collembolan’s reproduction and growth for all tested soils.
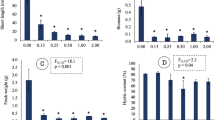
In general, while the percentage of small juveniles increased in all soils with increasing fipronil concentrations, the number of juveniles with large size decreased (in TAS and Entisol) at these conditions. When comparing the different soil moisture contents (Fig. 2) for the same fipronil concentration (i.e., at 1 and 2 mg kg−1), the percentage of small collembolans was significantly higher at 30% WHC than at 60% WHC in TAS and Entisol. On the other hand, no significant influence of soil moisture content on the fipronil effects on juvenile’s growth was observed in Oxisol, except at the concentration of 1 mg kg−1, in which the percentage of small organisms was significantly higher at 45% WHC compared to 60% WHC. For TAS, Entisol and Oxisol, the toxic effects on the juvenile’s growth (based on EC10) were, respectively, about 2.2, 2.4 and 2.7-fold lower at 60% WHC when compared to the respective dry condition (Table 3).
Number of small (≤3 mm), medium (>3 and ≤4.5 mm) and large (>4.5 mm) Folsomia candida juveniles (expressed as the % of the total sample) found after 28 days of exposure to different concentrations of fipronil in TAS, Entisol and Oxisol, under 30 or 45%, and 60% of soil WHC. Asterisks (*) indicate a significant difference between treatments with the same fipronil concentration but distinct soil moisture contents, and ‘ns’ indicate no significant difference (Tukey’s test - p ≤ 0.05)
The estimated PEC was close to EC10 TAS30% WHC and Entisol60% WHC. The TER values indicated a risk for collembolans in TAS and Oxisol only at the dry condition (TER < 5). In Entisol, however, a significant risk was found at both soil moisture contents (TER < 5; Table 4).
Discussion
Fipronil was toxic to F. candida even at lower concentrations, in all soils tested (Fig. 1). A decrease of 50% in the number of generating collembolans occurred at low fipronil concentrations (Table 3), depending on the soil type. The reduction in collembolans reproduction caused by the exposure to fipronil is reported in other studies (San Miguel et al. 2008; Alves et al. 2014; Zortéa et al. 2018a), corroborating our findings.
These effects are probably due to the mode of action of the insecticide on organisms that, in addition to cause the inhibition of GABA neurotransmitters, can affect the reproductive functions of collembolans. Fipronil is able to deregulate the endocrine system linked to sexual maturation and ecdysis in arthropods, causing reduced egg-laying (Cary et al. 2004; Gaertner et al. 2012). Regarding the effects of fipronil on the size of F. candida (Fig. 2), it was probably due to changes in physiological or biochemical processes into organisms (Ribeiro et al. 2001), which are expected because the organisms expend more energy on detoxification mechanisms, thus reducing their resources for growth (Sousa et al. 2000; Ribeiro et al. 2001, Hoffmann et al. 2003, Brodeur et al. 2017).
Based on the toxicity results, our first work hypothesis could be confirmed, since the effects of fipronil on the reproduction of F. candida (at 60% WHC) in Entisol (natural sandy soil) were greater than those found in TAS and Oxisol (Table 3). The reason for these findings is probably due to the influence of the adsorptive characteristics of tropical soils on the environmental fate of fipronil (Masutti and Mermut 2007; Scorza and Frando 2013). Among the main factors that influence the bioavailability and consequently the ecotoxicity of pesticides in the soil, are the clay and OM contents, the reactivity of the mineral surfaces, and the sorption equilibrium of pesticide between pore water and solid phases of the soil (Smit and Van Gestel 1998; Domene et al. 2010; Domene et al. 2012; Mandal and Singh 2013). TAS contains 1.4% OM (Table 1) based on coconut fiber, which has very rich adsorptive properties such as porous morphology, many carboxylic, hydroxylic, and carbonyl sites (Da Silva et al. 2012), with high WHC and the ability to adsorb lipophilic pesticides, such as fipronil (Tingle et al. 2003; Gunasekara et al. 2007). Oxisol has undergone intense weathering processes, having significant clay contents, high WHC (Table 1), and mineral surfaces with high amounts of iron oxy-hydroxides (Massuti and Mermut 2007), that favor chemical bonds with electronegative atoms of fipronil (F−, Cl−, O2− and N3− - Singh et al. 2014; Singh et al. 2016). Entisol has low WHC, low clay and silt contents (Table 1) and, consequently, fewer binding sites and probably low pesticide adsorption capacity, favoring greater bioavailability of fipronil in soil pores for collembolans uptaking (Van Gestel 2012; Peijnenburg et al. 2012).
Similar to our findings, Zortéa et al. (2018a; 2018b) assessed the effects of a veterinary medicine containing fipronil to collembolans and found high toxicity in Entisol when compared to TAS and Oxisol. Bandeira et al. (2019) found that the toxicity of an imidacloprid-based seed dressing formulation to F. candida was lower in TAS and Oxisol, compared to Entisol. Other studies evaluating the toxicity of swine manure to soil invertebrates also found that the deleterious effects are greater in Entisol compared to Oxisol (Segat et al. 2015; Maccari et al. 2016). Despite testing different contaminants, these studies also corroborated our first hypothesis, that natural sandy soils, like Entisol, enhance the ecotoxicological effects of fipronil to collembolans.
The adsorption of fipronil in the solid matrix depends on the number of adsorptive sites but also on the soil water content. According to Bobé et al. (1997), the adsorption of the fipronil on soil increases as the ratio soil/water decreases. Thus, the increase in soil moisture content may have provided an increase in the adsorption of fipronil to the soil particles (especially to clay and silt), reducing the bioavailability and consequently the toxicity for collembolans. In this way, the decrease in soil moisture content may have provided a reduced adsorption of fipronil to the soil, becoming more bioavailable and toxic for organisms in the soil pores. This could partially explain the increase in the fipronil toxicity on collembolan’s reproduction in TAS and Oxisol with the decrease of soil moisture.
In addition to the relationship between fipronil bioavailability and soil water content, the lowest available water content in TAS and Oxisol may have contributed to a higher species sensitivity, because under drought conditions collembolans may have their metabolic activities reduced, which may affect their growth, compromise the survival and reproductive performance (Bursell 1970; Edney,1977; Van Gestel and Van Diepen 1997; Jänsch et al. 2005; Sławski and Sławska 2020). Some studies have indicated that the optimum soil moisture for metabolic maintenance and guarantee of the existence of the species is about 60% WHC (Van Gestel and Van Diepen 1997; Crouau et al. 1999; Bandow et al. 2014a; Bandow et al. 2014b). This may also be the explanation for the poor reproductive performance in controls at the dryer scenarios, in all tested soils (Fig. 1; Supplementary Information 2).
On the other hand, sandy soils with lower clay and silt contents, such as Entisol, present lower sorption capacities compared to soils with greater contents of these minerals (Spomer and Kamble 2010). In the presence of high available water content, the toxicity of pesticides in sandy soil depends not only on their ability to bind to adsorption sites but also on the molecule’s ability to compete with water for binding sites (Harris 1964; Fishel 2003). In their study, Harris (1964) concluded that in sandy soil the high water content available in the soil pores may compete for the binding sites with the pesticides heptachlor, DDT, diazinon, V-C13 and parathion, increasing their bioavailability and, consequently, their toxicity for insects.
Collembolan size has been considered as a promising toxicity endpoint that can also be used for assessing long-term effects of contaminants (Guimarães et al. 2019; Szabó et al. 2020). Our experiments indicated that the dry condition increased the incidence of small juveniles compared to 60% WHC, in TAS and Entisol at fipronil concentrations of 1 and 2 mg kg−1 (Fig. 2). In addition to the possible metabolic stresses, the drier scenario e may have caused a reduction in the thickness of the water layer that surrounds the soil particles, negatively affecting the habitat of collembolans and the access to food and water, compromising their development (Coleman et al. 2004; Singh et al. 2019). From these inferences, the problem of reduction in soil moisture content becomes evident on an ecological scale for collembolans, since smaller collembolans can be more susceptible to stress and predation, resulting in an additional risk for these organisms (Guimarães et al. 2019).
In Oxisol, soil texture seems to be the main factor that influenced the collembolans growth, given the predominance of small juveniles in the control treatment, regardless of the moisture regime (Fig. 2). This probably occurred because in clayey soil, fewer porous spaces are available to be occupied by collembolans, which can impair the colonization and body development due to limited access to water and food resources (Domene et al. 2011). This assumption can also be demonstrated through the number of juveniles generated and through the proportion of medium and large-size juveniles in the control treatments of each soil (Supplementary Information 2; Fig. 2).
In the same way as our study, Hennig et al. (2020) observed that the toxicity of imidacloprid for F. candida in an Oxisol was greater at 45% WHC when compared to 60% WHC, while in Entisol there was no clear influence of soil moisture content (30 and 60% WHC) on the toxicity. The authors attributed their findings to the adsorptive properties of natural soils and the consequent bioavailability of the contaminant to collembolans. Other studies conducted in artificial soils also agrees with our findings in TAS. Bandow et al. (2014b) observed that the toxicity of pyrimethanil on the reproduction of F. candida and Sinella curviseta in OECD soil (sandy loam soil - 5% OM) was greater at 30% WHC compared to 50% and 70% WHC. Højer et al. (2001) observed that the toxicity of phenols to collembolans increased with the reduction of moisture content in a LUFA 2.2 soil (Loamy Soil - 6% OM). For both studies, the observed effects were related to a synergistic or additive effect of drought stress with contaminant toxicity, as well as to the adsorptive properties of the soil and the consequent bioavailability of the contaminant for collembolans, as reported in the present study.
Our estimated PECs (0.014–0.017 mg kg−1) fitted in the range of fipronil concentrations found in agricultural soils by some authors (Li et al. 2015) in peanut fields. Ying and Kookana (2006) found fipronil concentrations between 0.01 and 2.06 mg kg−1 in field soils after three years of application. Mandal and Singh (2013) reported fipronil concentrations of 1.90 and 5.50 mg kg−1 in two different soils 120 d after the application. Similarly, Silva et al. (2016) found about 3.5 mg kg−1 of fipronil in two soils 40 d after pesticide application. These values led us to suppose that fipronil can be persistent in soils and, in some cases, soil invertebrates may be exposed to environmental levels higher than those estimated in our study.
The TER approach indicated no risk for F. candida exposure to fipronil at the lowest recommended dose of the commercial formulation in TAS and Oxisol under the standard soil moisture condition. However, a significant risk of fipronil was observed in Entisol, revealing that F. candida populations can be affected when exposed to sandy agricultural soils (Table 4). This is also probably due to the soil properties like low clay and OM content as observed to mancozeb (Carniel et al. 2019) and imidacloprid (Bandeira et al. 2019) in Brazilian soils by other authors, which seems to be a relevant finding for Brazil, since the environmental risk assessment of pesticides does not consider the soil type in the risk estimation (IBAMA 1996).
Based on the risk calculated in this study, considering the influence of abiotic factors such as soil type and soil moisture content in prospective risk assessments of pesticides in the soil seems to be essential to avoid underestimation in future scenarios.
Conclusions
Our results showed that the toxic effects of fipronil on F. candida reproduction are dependent on the tropical soil type, and are enhanced in the sandy soil, compared to a sandy loam artificial soil and natural clay soil. The reduction of soil moisture may increase or decrease the toxic effects of fipronil on F. candida reproduction, depending on the soil type. Soils with higher WHC and adsorptive properties tend to increase fipronil toxicity when dry, while those with less WHC and less favorable adsorptive properties can reduce a.i. toxicity. In all tested soils, the percentage of small juveniles increased with fipronil concentration regardless of soil moisture, but more small juveniles were generated at dry scenarios compared to the standard soil moisture in TAS and Entisol. Disregarding the climatic variables, the risk of fipronil seem to depend on the soil texture. When considering the reduction of soil moisture, an increased risk is found in soils with higher WHC.
Data availability
The data that support the findings of this study are available and should be requested by e-mail.
Code availability
ImageJ, RRID:SCR_003070; STATISTICA, RRID:SCR_014213; SCAPE, RRID: Not available.
References
Alves PRL, Cardoso EJBN, Martines AM, Sousa JP, Pasini A (2013) Earthworm ecotoxicological assessments of pesticides used to treat seeds under tropical conditions. Chemosphere 90:2674–2682. https://doi.org/10.1016/j.chemosphere.2012.11.046
Alves PRL, Cardoso EJBN, Martines AM, Sousa JP, Pasini A (2014) Seed dressing pesticides on springtails in two ecotoxicological laboratory tests. Ecotoxicol Environ Saf 105:65–71. https://doi.org/10.1016/j.ecoenv.2014.04.010
Alves PRL, Bandeira FO, Presotto R, Giraldi M, Segat JC, Cardoso EJBN, Baretta D (2019) Ecotoxicological assessment of Fluazuron: effects on Folsomia candida and Eisenia andrei. Environ Sci Pollut Res 26:5842–5850. https://doi.org/10.1007/s11356-018-4022-7
Amorim MJB, Römbke J, Soares AMVM (2005) Avoidance behaviour of Enchytraeus albidus: effects of Benomyl, Carbendazim, phenmedipham and different soil types. Chemosphere 59:501–510. https://doi.org/10.1016/j.chemosphere.2005.01.057
Bandeira FO, Alves PRL, Hennig TB, Schiehl A, Cardoso EJBN, Baretta D (2019) Toxicity of imidacloprid to the earthworm Eisenia andrei and collembolan Folsomia candida in three contrasting tropical soils. J Soils Sediments 20:1997–2007. https://doi.org/10.1007/s11368-019-02538-6
Bandeira FO, Alves PRL, Hennig TB, Toniolo T, Natal-da-Luz T, Baretta D (2020) Effect of temperature on the toxicity of imidacloprid to Eisenia andrei and Folsomia candida in tropical soils. Environ Pollut 267:115565. https://doi.org/10.1016/j.envpol.2020.115565
Bandow C, Coors A, Karau N, Römbke J (2014a) Interactive effects of lambda-cyhalothrin, soil moisture and temperature on Folsomia candida and Sinella curviseta (Collembola). Environ Toxicol Chem 33:654–661. https://doi.org/10.1002/etc.2479
Bandow C, Karau N, Römbke J (2014b) Interactive effects of pyrimethanil, soil moisture and temperature on Folsomia candida and Sinella curviseta (Collembola). Appl Soil Ecol 81:22–29. https://doi.org/10.1016/j.apsoil.2014.04.010
Bandow C, Ng EL, Schmelz RM, Sousa JP, Römbke J (2016) A TME study with the fungicide pyrimethanil combined with different moisture regimes: effects on enchytraeids. Ecotoxicology 25:213–224. https://doi.org/10.1007/s10646-015-1581-y
Bobé A, Coste CM, Cooper JF (1997) Factors Influencing the Adsorption of Fipronil on Soils. J Agric Food Chem 45:4861–4865. https://doi.org/10.1021/jf970362z
Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EA, Noome DA, Simon-Delso N, Tapparo A (2015) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22:35–67. https://doi.org/10.1007/s11356-014-3332-7
Brodeur JC, Sanchez M, Castro L, Rojas DE, Cristos D, Damonte MJ, Poliserpi MB, D’Andrea MF, Andriulo AE (2017) Accumulation of current-use pesticides, cholinesterase inhibition and reduced body condition in juvenile one-sided livebearer fish (Jenynsia multidentata) from the agricultural Pampa region of Argentina. Chemosphere 185:36–46. https://doi.org/10.1016/j.chemosphere.2017.06.129
Bursell E (1970) An introduction to insect physiology. Academic Press, London, UK, p 276
Carniel LSC, Niemeyer JC, Oliveira Filho LCI, Alexandre D, Gebler L, Klauberg-Filho O (2019) The fungicide mancozeb affects soil invertebrates in two subtropical Brazilian soils. Chemosphere 232:180–185. https://doi.org/10.1016/j.chemosphere.2019.05.179
Cary TL, Chandler GT, Volz DC, Walse SS, Ferry JL (2004) Phenylpyrazole Insecticide Fipronil Induces Male Infertility in the Estuarine Meiobenthic Crustacean Amphiascus tenuiremis. Environ Sci Technol 38:522–528. https://doi.org/10.1021/es034494m
Chelinho S, Domene X, Campana P, Andrés P, Römbke J, Sousa JP (2014) Toxicity of phenmedipham and carbendazim to Enchytraeus crypticus and Eisenia andrei (Oligochaeta) in Mediterranean soils. J Soils Sediments 14:584–599. https://doi.org/10.1007/s11368-013-0818-8
Coleman DC, Crossley DA, Hendrix PF (2004) Fundamentals of Soil Ecology, 2nd ed. Elsevier Inc, Athens, Georgia, p 502
Crouau Y, Chenon P, Gisclard C (1999) The use of Folsomia candida (Collembola, Isotomidae) for the bioassay of xenobiotic substances and soil pollutants. Appl Soil Ecol 12:103–111. https://doi.org/10.1016/S0929-1393(99)00002-5
Culik MP, Zeppelini FD (2003) Diversity and distribution of Collembola (Arthropoda: Hexapoda) of Brazil. Biodivers Conserv 12:1119–1143. https://doi.org/10.1023/A:1023069912619
Daam MA, Chelinho S, Niemeyer JC, Owojori OJ, De Silva PMCS, Sousa JP, van Gestel CAM, Römbke J (2019) Environmental risk assessment of pesticides in tropical terrestrial ecosystems: test procedures, current status and future perspectives. Ecotoxicol Environ Saf 181:534–547. https://doi.org/10.1016/j.ecoenv.2019.06.038
Da Silva KMD, Rezende LCSH, Bergamasco R, Da Silva CA, Gonçalves DS (2012) Caracterização Físico - Química Da Fibra De Coco Verde Para a Adsorção De Metais Pesados Em Efluente De Indústria De Tintas. Engevista 15:43. https://doi.org/10.22409/engevista.v15i1.387
Domene X, Colón J, Uras MV, Izquierdo R, Àvila A, Alcañiz JM (2010) Role of soil properties in sewage sludge toxicity to soil collembolans. Soil Biol Biochem 42:1982–1990. https://doi.org/10.1016/j.soilbio.2010.07.019
Domene X, Chelinho S, Campana P, Natal-da-Luz T, Alcañiz JM, Andrés P, Römbke J, Sousa JP (2011) Influence of soil properties on the performance of Folsomia candida: implications for its use in soil ecotoxicology testing. Environ Toxicol Chem 30:1497–1505. https://doi.org/10.1002/etc.533
Domene X, Römbke J, Chelinho S, Sousa JP, Campana P, Alcañiz JM (2012) Applying a GLM-based approach to model the influence of soil properties on the toxicity of phenmedipham to Folsomia candida. J. Soils Sediments 12:888–899. https://doi.org/10.1007/s11368-012-0502-4
EC - European Commission (2002) Guidance Document on Terrestrial Ecotoxicology under Council Directive 91/414/EEC (SANCO/10329/2002) rev.2 final, 17.10.2002, p. 1 - 39
Edney EB (1977) Water balance in land arthropods. Springer-Verlag, Berlin, Germany, p 282
EFSA - European Food Safety Authority (2006) Conclusion regarding the peer review of the pesticide risk assessment of the active substance metazachlor. EFSA J 6:1–110. https://doi.org/10.2903/j.efsa.2008.145r
EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária (1988) Recomendações técnicas para o cultivo da soja. Circular técnica 16. Unidade de execução de pesquisa de âmbito estadual de Dourados, MS. Dourados
EMBRAPA (2006) Ata da XXVII Reunião de Pesquisa de Soja da Região Central do Brasil. Embrapa Soja, Londrina, Brazil.lSSN 1516-781X: n. 265
Environmental Canada (2007) Guidance Document on Statistical Methods for Environmental Toxicity Test. Environmental Protection Series, EPS 1/RM/46, 2005 with 2007 updates. Environmental Canada, Ottawa
EPPO - European and Mediterranean Plant Protection Organization (2003) Environmental risk assessment scheme for plant protection products. Chapter 4: Soil. OEPP EPP0 Bull 33:151–162
ESCAPE (2013) ESCAPE 2.0v: Estimation of soil concentrations after pesticide applications. Fraunhofer IME, Aachen, Germany
Fishel F (2003) Pesticides and the Environment. Agricultural MU Guide (Insects and Diseases), University of Missouri, Columbia, United States of America
Gaertner K, Chandler GT, Quattro J, Ferguson PL, Sabo-Attwood T (2012) Identification and expression of the ecdysone receptor in the harpacticoid copepod, Amphiascus tenuiremis, in response to fipronil. Ecotoxicol Environ Saf 76:39–45. https://doi.org/10.1016/j.ecoenv.2011.09.008
Garcia MVB (2004) Effects of pesticides on soil fauna: development of ecotoxicological test methods for tropical regions. Ecology and development series, vol. 19. Doctoral thesis, University of Bonn, Bonn, Germany
Gebrehiwot WH, Erkmen C, Uslu B (2019) A novel HPLC-DAD method with dilute-and-shoot sample preparation technique for the determination of buprofezin, dinobuton and chlorothalonil in food, environmental and biological samples. J IAEAC 99:1–15. https://doi.org/10.1080/03067319.2019.1702169
Guimarães B, Maria VL, Römbke J, Amorim MJB (2019) Exposure of Folsomia candida (Willem 1902) to teflubenzuron over three generations – Increase of toxicity in the third generation. Appl Soil Ecol 134:8–14. https://doi.org/10.1016/j.apsoil.2018.10.003
Gunasekara A, Truong T, Goh KS, Spurlock F, Tjeerdema RR (2007) Environmental fate and toxicology of fipronil. J Pestic Sci 32(3):189–199. https://doi.org/10.1584/jpestics.R07-02
Harris CR (1964) Influence of soil type and soil moisture on the toxicity of insecticides in soils to insects. Nature 202:724
Hennig TB, Bandeira FO, Dalpasquale AJ, Cardoso EJBN, Baretta D, Alves PRL (2020) Toxicity of imidacloprid to collembolans in two tropical soils under different soil moisture. J Environ Qual 49:1491-1501. https://doi.org/10.1002/jeq2.20143
Hoffmann AA, Sørensen JG, Loeschcke V (2003) Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol 28:175–216
Højer R, Bayley M, Damgaard CF, Holmstrup M (2001) Stress synergy between drought and a common environmental contaminant: Studies with the collembolan Folsomia candida. Glob Chang Biol 7:485–494. https://doi.org/10.1046/j.1365-2486.2001.00417.x
IBAMA - Instituto brasileiro do meio ambiente e dos recursos naturais renováveis - IBAMA (1996) Normative Ordinance No. 84, October 15th, 1996. Establish Procedures to Be Adopted in the Brazilian Institute of Environment and Renewable Natural Resources e IBAMA, for Registration Purposes and for Assessment of the Potential Environmental Hazard (PPA) of Pesticides, vol. 203. Union Official Journal No, p. 21358, 1996. Brasília, Brazil (in Portuguese)
INMETRO - Instituto Nacional de Metrologia, Qualidade e Tecnologia (2020) Orientações sobre Validação de Métodos Analíticos: DOQ-CGCRE-008. Rev. 09 de jun 2020. Brasília, Brazil, p. 1 - 30. Available at: https://www.inmetro.gov.br/Sidoq/pesquisa_link.asp?seq_tipo_documento=5&cod_uo_numeracao=00774&num_documento=008. Accessed 06 August 2021.
IPCC - Intergovernmental Panel on Climate Change (2014) In: Pachauri, R.K., Meyer, L.A. (eds), Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team]. IPCC, Geneva, Switzerland
ISO - International Organization for Standardization (2014) Soil quality: Inhibition of reproduction of Collembola (Folsomiacandida) by soil contaminants. ISO, Geneva, Switzerland
Jackson D, Cornell CB, Luukinen B, Buhl K, Stone D (2009) Fipronil Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services, Oregon, United States of America
Jänsch S, Amorim MJ, Römbke J (2005) Identification of the ecological requirements of important terrestrial ecotoxicological test species. Environ Rev 13:51–83. https://doi.org/10.1139/a05-007
Jegede OO, Owojori OJ, Römbke J (2017) Temperature influences the toxicity of deltamethrin, chlorpyrifos and dimethoate to the predatory mite Hypoaspis aculeifer (Acari) and the springtail Folsomia candida (Collembola). Ecotoxicol Environ Saf 140:214–221. https://doi.org/10.1016/j.ecoenv.2017.02.046
Li M, Li P, Wang L, Feng M, Han L (2015) Determination and dissipation of fipronil and its metabolites in peanut and soil. J Agric Food Chem 63:4435–4443. https://doi.org/10.1021/jf5054589
Lima MPR, Soares AMVM, Loureiro S (2011) Combined effects of soil moisture and carbaryl to earthworms and plants: Simulation of flood and drought scenarios. Environ Pollut 159:1844–1851. https://doi.org/10.1016/j.envpol.2011.03.029
Maccari AP, Baretta D, Paiano D, Leston S, Freitas A, Ramos F, Sousa JP, Klauberg-Filho O (2016) Ecotoxicological effects of pig manure on Folsomia candida in subtropical Brazilian soils. J Hazard Mater 314:113–120. https://doi.org/10.1016/j.jhazmat.2016.04.013
Mandal K, Singh B (2013) Persistence of fipronil and its metabolites in sandy loam and clay loam soils under laboratory conditions. Chemosphere 91:1596–1603. https://doi.org/10.1016/j.chemosphere.2012.12.054
Masutti CSM, Mermut AR (2007) Degradation of fipronil under laboratory conditions in a tropical soil from sirinhaém Pernambuco, Brazil. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes 42:33–43. https://doi.org/10.1080/03601230601017981
Mohapatra S, Deepa M, Rashmi N, Jagdish GK, Kumar S, Prakash GS (2010) Fate of Fipronil and its Metabolites in/on Grape Leaves, Berries and Soil Under Semi Arid Tropical Climatic Conditions. Bull Environ Contam Toxicol 84:587–591. https://doi.org/10.1007/s00128-010-9965-4
Niemeyer JC, Carniel LSC, de Santo FB, Silva M, Klauberg-Filho O (2018) Boric acid as reference substance for ecotoxicity tests in tropical artificial soil. Ecotoxicology 27:395–401. https://doi.org/10.1007/s10646-018-1915-7
Ogungbemi AO, Van Gestel CAM (2018) Extrapolation of imidacloprid toxicity between soils by exposing Folsomia candida in soil pore water. Ecotoxicology 27:1107–1115. https://doi.org/10.1007/s10646-018-1965-x
PBMC (2014) Base científica das mudanças climáticas. Contribuição do Grupo de Trabalho 1 do Painel Brasileiro de Mudanças Climáticas ao Primeiro Relatório da Avaliação Nacional sobre Mudanças Climáticas [Ambrizzi, T., Araujo, M. (eds.)]. COPPE. Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brasil, p 464
Peijnenburg W, Capri E, Kula C, Liess M, Luttik R, Montforts M (2012) Technology Evaluation of Exposure Metrics for Effect Assessment of Soil Invertebrates. Crit Rev Env Sci Tec 42:17, 1862-1893. https://doi.org/10.1080/10643389.2011.574100
Ribeiro S, Sousa JP, Nogueira AJA (2001) Effect of endosulfan and parathion on energy reserves and physiological parameters of the terrestrial isopod Porcellio dilatatus. Ecotoxicol Environ Saf 49:131–138
San Miguel A, Raveton M, Lempérière G, Ravanel P (2008) Phenylpyrazoles impact on Folsomia candida (Collembola). Soil Biol Biochem 40:2351–2357. https://doi.org/10.1016/j.soilbio.2008.05.014
Scorza Jr RP, Frando AA (2013) A temperatura e umidade na degradação de fi pronil em dois solos de Mato Grosso do Sul. Ciência Rural 43:1203–1209
Segat JC, Alves PRL, Baretta D, Cardoso EJBN (2015) Ecotoxicological evaluation of swine manure disposal on tropical soils in Brazil. Ecotoxicol Environ Saf 122:91–97. https://doi.org/10.1016/j.ecoenv.2015.07.017
Silva RO, Scorza Jr RP, Bonfá MRL, Campanari MFZ, Mendes I, de C (2016) Degradation and sorption of fipronil and atrazine in Latossols with organic residues from sugarcane crop. Ciência Rural 46:1172–1177. https://doi.org/10.1590/0103-8478cr20150696
Singh A, Srivastava A, Srivastava PC (2014) Sorption kinetics of fipronil on soils. Bull Environ Contam Toxicol 93:758–763. https://doi.org/10.1007/s00128-014-1391-6
Singh A, Srivastava A, Srivastava PC (2016) Sorption-desorption of fipronil in some soils, as influenced by ionic strength, pH and temperature. Pest Manag Sci 72:1491–1499. https://doi.org/10.1002/ps.4173
Singh J, Schälder M, Demetrio W, et al. (2019) Climate change effects on earthworms—a review. Soil Organisms 3:113–137. (ISSN 2509-9523 (online)). https://doi.org/10.25674/so91iss3pp114
Sławski M, Sławska M (2020) Collembolan Assemblages Response to Wild Boars (Sus scrofa L.) Rooting in Pine Forest Soil. Forests 11(11):1123. https://doi.org/10.3390/f11111123
Smit CE, Van Gestel CAM (1998) Effects of soil type, prepercolation, and ageing on bioaccumulation and toxicity of zinc for the springtail Folsomia candida. Environ Toxicol Chem 17:1132–1141. https://doi.org/10.1002/etc.5620170621
Sousa JP, Loureiro S, Pieper S, Frost M, Kratz W, Nogueira AJA, Soares AMVM (2000) Toxicokinetics of hydrophobic compounds in isopods. The importance of exposure route on the uptake of Lindane. Environ Toxicol Chem 19:2563–2577
Souza ED, Carneiro MAC, Paulino HB (2005) Physical attributes of a Typic Quartzipisamment and a Rhodic Hapludox under different management systems. Pesquisa Agropecuaria Brasileira 40:1135–1139. https://doi.org/10.1590/S0100-204X2005001100012
Spomer NA, Kamble ST (2010) Sorption and Desorption of Fipronil in Midwestern Soils. Bull Environ Contam Toxicol 84:264–268. https://doi.org/10.1007/s00128-009-9915-1
Szabó B, Seres A, Bakonyi G (2020) Distinct changes in the life-history strategies of Folsomia candida Willem (Collembola: Isotomidae) due to multi- and transgenerational treatments with an insecticide. App Soil Ecol 152:103563. https://doi.org/10.1016/j.apsoil.2020.103563
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais, 2nd edn. Universidade Federal do Rio Grande do Sul, Porto Alegre, p 147 (Boletim Técnico, 5)
Tingle CC, Rother JA, Dewhust CF, Lauer S, King WJ (2003) Fipronil: Environmental Fate, Ecotoxicology, and Human Health Concerns. Rev Environ Contam Toxicol 176:1–66
Van Gestel CAM, Van Diepen AMF (1997) The influence of soil moisture content on the bioavailability and toxicity of cadmium for Folsomia candida Willem (Collembola: Isotomidae). Ecotoxicol Environ Saf 36:123–132. https://doi.org/10.1006/eesa.1996.1493
Van Gestel CAM (2012) Soil ecotoxicology: state of the art and future directions. Zookeys 176:275–296. https://doi.org/10.3897/zookeys.176.2275
Ying GG, Kookana RS (2006) Persistence and movement of fipronil termiticide with under-slab and trenching treatments. Environ Toxicol Chem 25:2045–2050. https://doi.org/10.1897/05-652R.1
Zortéa T, dos Reis TR, Serafini S, de Sousa JP, da Silva AS, Baretta D (2018a) Ecotoxicological effect of fipronil and its metabolites on Folsomia candida in tropical soils. Environ Toxicol Pharmacol 62:203–209. https://doi.org/10.1016/j.etap.2018.07.011
Zortéa T, da Silva AS, dos Reis TR, Segat JC, Paulino AT, Sousa JP, Baretta D (2018b) Ecotoxicological effects of fipronil, neem cake and neem extract in edaphic organisms from tropical soil. Ecotoxicol Environ Saf 166:207–214. https://doi.org/10.1016/j.ecoenv.2018.09.061
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (CNPq) for the research grant (Project 407170/2016-2). DB thanks the CNPq for the Research Productivity Grant (CNPq 305939/2018-1). TT and WES thanks the CNPq for a Grant of Scientific Initiation (process numbers 160246/2019-9 and 103524/2020-7, respectively). FOB and TBH thanks the Coordination for the Improvement of Higher Education Personnel (CAPES) for their master grant (process numbers 1735590 and 88882.447285/2019-01, respectively).
Author contributions
TBH: Conceptualization, Data curation, Formal analysis, Investigation, Writing—original draft. PRLA: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing—review & editing. TT: Investigation, Formal analysis. FOB: Investigation, Data curation, Formal analysis. WES: Investigation. LCC: Investigation, Data curation, Formal analysis. IKG: Investigation. DB: Resources, Supervision, Writing—review & editing.
Funding
This study was supported by the National Council for Scientific and Technological Development (CNPq) for the research grant (Project 407170/2016-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Brazilian regimentation for the scientific use of animals - Law no. 11.794).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hennig, T.B., Alves, P.R.L., Toniolo, T. et al. Toxicity of fipronil to Folsomia candida in contrasting tropical soils and soil moisture contents: effects on the reproduction and growth. Ecotoxicology 31, 64–74 (2022). https://doi.org/10.1007/s10646-021-02490-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02490-7