Abstract
Purpose
Imidacloprid is a widely used seed dressing insecticide in Brazil. However, the effects of this pesticide on non-target organisms such as soil fauna still present some knowledge gaps in tropical soils. This study aimed to assess the toxicity and risk of imidacloprid to earthworms Eisenia andrei and collembolans Folsomia candida in three contrasting Brazilian tropical soils.
Materials and methods
Acute and chronic toxicity assays were performed in the laboratory with both species in a tropical artificial soil (TAS) and in two natural soils (Oxisol and Entisol), at room temperature of 25 °C. The ecological risk was calculated for each species and soil by using the toxicity exposure ratio (TER) and hazard quotient (HQ) approaches.
Results and discussion
Acute toxicity for collembolans and earthworms was higher in Entisol (LC50 = 4.68 and 0.55 mg kg−1, respectively) when compared with TAS (LC50 = 10.8 and 9.18 mg kg−1, respectively) and Oxisol (LC50collembolans = 25.1 mg kg−1). Chronic toxicity for collembolans was similar in TAS and Oxisol (EC50 TAS = 0.80 mg kg−1; EC50 OXISOL = 0.83 mg kg−1), whereas higher toxicity was observed in Entisol (EC50 = 0.09 mg kg−1). In chronic assays with earthworms, imidacloprid was also more toxic in Entisol (EC50 = 0.21 mg kg−1) when compared to TAS (EC50 = 1.89 mg kg−1). TER and HQ values indicated a significant risk of exposure of the species to imidacloprid in all soils tested, and the risk in Entisol was at least six times higher than in Oxisol or TAS.
Conclusions
The toxicity and risk of imidacloprid varied significantly between tropical soils, being the species exposure to this pesticide particularly hazardous in very sandy natural soils such as Entisol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of plant protection products (PPPs) in agricultural areas of intensive production has been increasing in Brazil, as a result of human population growth and, consequently, due to the increase in food production. In conventional agriculture the use of pesticides in seed dressing is a common practice, because it allows to prevent or to reduce the incidence of pests and diseases in the early stages of the crops (Douglas and Tooker 2015), helping to reach high productivity levels.
Neonicotinoids are a class of insecticides widely used in chemical seed treatment. The choice for this group in agricultural crop areas is mainly due to its high efficiency in the control of insect pests (Jeschke et al. 2011; Goulson 2013). Although imidacloprid (IUPAC: (E)-1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine) has been banned in some countries (EFSA 2018), this is one of the world’s top-selling insecticides for seed dressing and it is also used for foliar applications or via direct application on the soil (Van der Sluijs et al. 2015). This active ingredient (a.i.) blocks the transmission of nerve impulses in insects, preventing the degradation of acetylcholine through the inhibition of acetylcholinesterase (Ihara and Matsuda 2018). In this way, this systemic insecticide can cause paralysis and, eventually, the death of exposed individuals.
Seed treatment with insecticides today commonly is considered an indispensable practice for large-scale food production, but it is also responsible for introducing pollutants into the soil (Wood and Goulson 2017). Due to the persistence and low absorption of imidacloprid by plants (Goulson 2013), residues of this a.i. have been found in the soil (Donnarumma et al. 2011; Ge et al. 2018) and may pose a risk to terrestrial ecosystems, especially for ecological receptors such as soil invertebrates (Bonmatin et al. 2014; Pisa et al. 2015).
Considering that the direct effects of xenobiotics on terrestrial ecosystems are difficult to measure, soil invertebrates are used commonly as indicators of soil quality in ecotoxicological assays (De Silva et al. 2009; Alves et al. 2017). This type of study has increased in tropical regions in the last decade, although most of them have been carried out with tropical artificial soil (TAS) (Niemeyer et al. 2017). Although artificial soils are recommended internationally for terrestrial ecotoxicological assays, their ecological relevance is limited, because the results of the assays based on this type of soil may not accurately represent the toxicity of pesticides in natural soils and, therefore, should not be directly extrapolated to field conditions (Chelinho et al. 2014).
In addition, the bioavailability of contaminants to soil organisms is directly influenced by intrinsic soil characteristics, such as the amount and type of clay minerals and soil organic matter, among others (Amorim et al. 2005; Ogungbemi and van Gestel 2018). Although these soil attributes have influence on the toxicity of pesticides to edaphic species (De Silva et al. 2009; Chelinho et al. 2014), this is neglected frequently in ecotoxicological studies, especially in those using only artificial soils. Particularly in Brazil, where a wide variety of soil textural classes occur (EMBRAPA 2006), the tolerance of species to pesticides may vary according to soil type, especially between soils with distinct textural characteristics (e.g., clayey and sandy soils). Thus, to reduce the uncertainty of the toxicity and risk of pesticides for the regulation of pesticides in tropical regions, several authors have recommended the use of natural tropical soils as test substrates in terrestrial ecotoxicological assays (Niva et al. 2016; Niemeyer et al. 2017).
Therefore, the aim of this study was to assess the toxic potential and risk of imidacloprid for the earthworm E. andrei and the collembolan F. candida in three contrasting Brazilian tropical soils. Laboratory ecotoxicological assays were set up using an artificial soil and two natural soils from southern Brazil.
2 Materials and methods
2.1 Test species
The species E. andrei (Oligochaeta) and F. candida (Collembola) were reared and maintained in the laboratory, in a climate-controlled room with an atmospheric temperature of 20 ± 2 °C, according to the recommendations of ISO guidelines (ISO 2012, 2014). Earthworms were maintained in plastic boxes containing a mixture of horse manure, coconut fiber, distilled water, and fine sand in a proportion of 100:50:330:15 (weight-based), respectively. Weekly, the earthworms were fed with cooked oat flakes, and the moisture of the breeding medium was adjusted with distilled water.
The collembolans F. candida were reared and maintained in a substrate containing activated charcoal (powdered), plaster of Paris (powdered), and distilled water, in a proportion of 1:10:6 (weight-based), respectively. Twice a week, the collembolans were fed with granulated dry yeast (Saccharomyces cerevisiae), and the moisture of the substrate was adjusted with a few drops of distilled water.
2.2 Test soils and substance
A tropical artificial soil (TAS) and two natural tropical soils were the test substrates in this study. TAS is a mixture of fine sand (more than 50% of the particles between 0.05 and 0.2 mm), kaolinite clay and powdered coconut husk, in a proportion of 75:20:5 of dry weight (DW), respectively (Garcia 2004; De Silva and van Gestel 2009). The pH of the TAS was adjusted to 6.0 ± 0.5 with CaCO3, according to ISO 11268-2 (ISO 2012). Two soils with different characteristics were also selected for the study: an Entisol with a higher sand content (above 90%) and an Oxisol with a higher clay content (approximately 59%). The soil samples were taken from the surface layer (0–20 cm) of the soil profile in areas with no history of pesticide use in the municipalities of Araranguá (29° 00′ S 49° 31′ W) and Chapecó (27° 06′ S 52° 42′ W), Brazil, respectively. The natural soils were sieved (# 2.0 mm opening), and all existing soil macrofaunal individuals were killed by a freeze and thaw defaunation process, as described in Alves et al. (2013).
The following physical and chemical properties of the soils (Table 1) were determined according to Tedesco et al. (1995): available contents of Al, Ca, Mg, and Mn (KCl); P and K (Mehlich 1); Cu, Zn and Fe (Mehlich); H+Al (Shoemaker–McLean–Pratt–SMP buffer method); soil organic matter (SOM) by spectroscopy; clay (densitometry); and sand (gravimetry). Cation-exchange capacity (CEC) was calculated by the sum of available K, Ca, Mg, and Al contents. The pH (1 M KCl) and the soil maximum water holding capacity (WHC) were determined following ISO (2012).
The commercial formulation MUCH 600 FS®–containing imidacloprid (600 g of a.i. L−1) was chosen for the study, because of its frequent use in seed treatments in Brazilian agricultural areas.
Ecotoxicological assays were carried out individually in each soil. Immediately before the beginning of the tests, the three different soils were spiked with five increasing concentrations of imidacloprid. For all treatments, the soil moisture was adjusted with distilled water to approximately 60% of their WHC. A control treatment containing only distilled water (also at 60% of the WHC) was run for each test soil. The range of nominal imidacloprid concentrations (in mg of a.i. per kg of dry soil—mg kg−1) used in acute and chronic toxicity assays (Table 2) were based on a range-finding test and literature data (Alves et al. 2013; Alves et al. 2014; de Lima et al. 2017).
2.3 Acute toxicity assays
All ecotoxicological assays with both species were run in a climate-controlled room with a temperature of 25 ± 2 °C and a photoperiod of 12 h, where the earthworms were acclimated in the respective test soil at least 24 h before the beginning of the tests.
Impacts of imidacloprid on the survival of E. andrei were assessed through acute toxicity tests, according to ISO 11268-1 (ISO 1993). Ten adult earthworms (with apparent clitellum) weighing between 250 and 600 mg were randomly inserted into cylindrical plastic containers (14.8 cm diameter and 9.8 cm height) containing approximately 600 g of wet soil, spiked with imidacloprid or only with distilled water (control soil). Individuals were fed at the start of the test and after 7 days with 10 g of uncontaminated horse manure and distilled water (5 mL), per experimental unit. The soil moisture was adjusted weekly (weight-based) with distilled water. Four replicates were performed for each treatment. After 14 days, the surviving earthworms were manually removed, gently rinsed with distilled water, weighed, and the survival, and biomass loss of earthworms were evaluated.
Acute toxicity assays with F. candida were based on guideline ISO 11267 (ISO 2014). Thirty grams of wet soil (spiked or control) were added to glass containers (7.5 cm diameter and 6.0 cm height). Then, ten collembolans with synchronized ages between 10 and 12 days were inserted in each experimental unit. Five milligrams of dried granulated yeast were supplied as food for the organisms at the beginning of the test. The containers were sealed hermetically during the test period except for twice a week when they were opened for air renewal and soil moisture adjustment (addition of distilled water based on weight loss). There were five replicates for each treatment. After 14 days, the number of surviving adult collembolans was assessed as described in Alves et al. (2014).
2.4 Chronic toxicity assays
Chronic toxicity assays with E. andrei were performed according to ISO 11268-2 (ISO 2012). Reproduction assays were carried out in a similar way to the acute toxicity assays, except for a longer exposure time (56 days), feeding frequency (worms were fed weekly during the test period), and imidacloprid concentrations (Table 2). Four replicates were used for each treatment, and after 28 days of exposure, the surviving adult earthworms were removed, rinsed with water, counted, and weighed. Only the soil, the eventual juveniles and the generated cocoons remained in the container during the last 28 days of the test. On day 56, the plastic containers were inserted in a water bath (60 ± 5 °C) for 1 h, where juveniles were counted as described by Alves et al. (2013).
The effect of imidacloprid on the reproduction of F. candida was assessed according to ISO 11267 (ISO 2014). The procedures for the assays were identical to those of the acute toxicity tests with F. candida, differing only in the concentrations (Table 2), exposure time, and the counting method. Five replicates were prepared for each treatment. After 28 days, the contents of each experimental unit were submerged in distilled water in similar fashion to the procedure described for the acute toxicity test; however, in this case, the experimental units containing the living floating individuals were photographed in high resolution. The images were analyzed using the computational software ImageJ® to account for the number of juveniles generated during the assay.
2.5 Estimation of PEC and PNEC
The predicted environmental concentrations (PEC) were calculated for each soil based on the procedure described by Alves et al. (2013), simulating a worst-case scenario. The calculated PEC values of imidacloprid were based on a soybean field with a sowing density of 100 kg of seeds ha−1 at a depth of 0–5 cm, with soil densities of 1.0 g cm−3 for TAS and Oxisol and 1.5 g cm−3 for Entisol. According to the commercial recommendation, one needs 200 mL of the insecticide Much 600 FS (600 g a.i. L−1) to treat 100 kg of soybean seeds, resulting in an amount of 120 g of imidacloprid ha−1. The calculated PEC values (Table 2) were checked by a methodology proposed by the European and Mediterranean Plant Protection Organization (EPPO 2003), using the software ESCAPE®, assuming a single application of the pesticide, without interception of the crops, during one planting cycle. The predicted no-effect concentrations (PNEC) were calculated by dividing the lowest EC10 of each soil by an assessment factor of 100 (EC 2003), as described in Renaud et al. (2018).
2.6 Data analysis
The results of the ecotoxicological tests were analyzed using Statistica 7.0® software. The normality and homoscedasticity of the reproduction data were ascertained through the Kolmogorov-Smirnov and Bartlett’s tests, respectively; when necessary, logarithmic transformations were applied in order to comply with the analysis of variance (ANOVA) assumptions. When significant differences (p < 0.05) were detected by ANOVA, the means of the treatments were compared with the control through Dunnet’s post-hoc test, in order to determine the no observed effect concentration (NOEC) and lowest observed effect concentration (LOEC). In addition, concentrations that reduced reproduction by 10% and 50%, in relation to the control treatment (EC10 and EC50, respectively), were estimated using non-linear regression models (the exponential, Gompertz, or logistic model), recommended by Environmental Canada (2007). The lethal concentration values of 50% (LC50) of the populations for the acute toxicity tests were estimated through the probitic model, using PriProbit® software.
To estimate the ecological risk of imidacloprid for both soil fauna species in the tropical soils, the PEC was compared with the EC10 and PNEC, respectively, through two different approaches: (1) toxicity-exposure ratios (TER), obtained by dividing the EC10 by PEC (TER = EC10/PEC), as described in EC (2002); (2) hazard quotients (HQ), obtained by dividing the PEC by PNEC (HQ = PEC/PNEC), in accordance with the guidelines for the risk assessment of new and existing substances, proposed by the European Commission (EC 2003). Significant risk was considered for the tested species when HQ > 1 or TER < 5.
3 Results
3.1 Suitability of soils for ecotoxicological assays
In the controls of the acute and chronic assays with TAS and Entisol, no mortality of E. andrei was observed. On the other hand, in a preliminary assay with Oxisol, the number of E. andrei juveniles was lower than 30 in all control replicates (data not shown). Therefore, the results for earthworms in Oxisol were not presented in this study. The mean mortality for adult collembolans in the controls of the assays with TAS, Entisol, and Oxisol was respectively 7.5%, 4%, and 12% in the acute toxicity tests, and 12%, 6%, and 30% in the chronic tests. The mean number (± standard deviation) of E. andrei juveniles generated in the controls of TAS and Entisol was 163 ± 36 and 208 ± 15, respectively. In the controls of TAS, Entisol, and Oxisol, the mean number of juvenile collembolans was, respectively, 298 ± 48, 210 ± 46, and 100 ± 28. For all assays, the coefficients of variation in the controls were lower than 30%. The number of adults (7 individuals = 30% mortality) and of juvenile collembolans found in two replicates of the Oxisol control (R4 = 88 and R5 = 72 juveniles) did not meet the validity criteria established by ISO 11267 (ISO 2014) for the chronic toxicity assays. Nevertheless, the results of this assay were considered in this study because an interesting dose-response relationship could be observed using this soil. In general, the validity criteria for the toxicity tests with the three soil types were met for both species (ISO 1993, 2012, 2014), except for the assays with F. candida (chronic) and E. andrei (acute and chronic) in Oxisol.
3.2 Acute toxicity assays
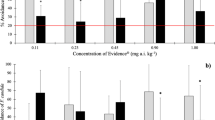
The survival of E. andrei and F. candida was reduced in all spiked soils (Fig. 1), with the intensity of the effect varying according to the concentration tested and soil type. The LOEC values for mortality in the natural sandy soil (Entisol) were, in general, lower than in the other soils (Table 3). Lowest LC50 values for both species were found in Entisol, confirming that the lethal effect of imidacloprid on this species was higher in this soil (Table 3). Significant mortality of collembolans started at 8.0 mg kg−1 for TAS and at 16.0 mg kg−1 for Oxisol. In TAS, acute toxicity was similar for earthworms (LC50 = 9.18 mg kg−1) and collembolans (LC50 = 10.8 mg kg−1), whereas, higher toxicity was found for earthworms (LC50 = 0.55 mg kg−1) when compared with collembolans (LC50 = 4.68 mg kg−1) in Entisol.
Mean number of adult Eisenia andrei (upper line) and Folsomia candida (lower line) survivors found in soils treated with increasing imidacloprid concentration, after 14 days of exposure. Asterisk (*) indicates significant differences (p < 0.05) between the treatment and the control (Dunnet’s test). (┬) Standard deviation (n = 4 for earthworms; n = 5 for collembolans)
3.3 Chronic toxicity assays
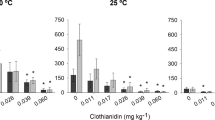
A reduction of the number of juveniles was observed in all tested soils for both species (Fig. 2), and this effect increased with increasing concentrations of imidacloprid. The highest chronic toxicity was observed in Entisol (EC50earthworms = 0.21 mg kg−1; EC50collembolans = 0.09 mg kg−1), where the number of F. candida and E. andrei juveniles were reduced at the lowest tested concentrations (Table 3). For earthworms, the chronic toxicity (EC50-based) in the Entisol was about nine times higher in comparison with the chronic toxicity in TAS (Table 3). Similar EC50 values were found for collembolans in Oxisol (0.83 mg kg−1) and in TAS (0.80 mg kg−1).
Mean number of Eisenia andrei (upper line) and Folsomia candida (lower line) juveniles found in soils treated with increasing imidacloprid concentration, after 56 and 28 days of exposure, respectively. Asterisk (*) indicates significant differences (p < 0.05) between the treatment and the control (Dunnet’s test). (┬) Standard deviation (n = 4 for earthworms; n = 5 for collembolans)
3.4 Ecological risk assessment
Both approaches used to calculate the potential risk of imidacloprid for the tested species (TER and HQ) indicated a significant risk of exposure for soil fauna to the expected levels of the a.i. in the environment (PEC), in all tested soils (Table 4). Considering the values of TER, the risk for collembolans is similar when exposed to the a.i. in TAS or Oxisol. The lowest TER values for F. candida and E. andrei were determined in Entisol, revealing the highest risk in this soil. HQ values confirm these results, indicating that the risk is at least six times higher in Entisol than in the other soils (Table 4).
4 Discussion
The lower survival and reproduction of E. andrei in Oxisol indicates that this soil was not able to maintain this population during the test period (data not shown). In the same way, the mean number of F. candida juveniles was lower than the minimum for validation of the test (ISO 2014) in this soil, indicating that both species presented lower reproductive performance in Oxisol, when compared with the other soils. This may be due to the pedogenetic characteristics of this soil, such as its higher clay content (Table 1). Similar effects were observed for the collembolans F. candida (Domene et al. 2011) and the oligochaetes E. andrei and E. crypticus (Chelinho et al. 2011) in fine-textured soils, possibly due to the greater difficulty of colonization and occupation of porous space by these organisms in such heavy clayey soils. In addition, according to Amorim et al. (2005), there may be a positive influence of the porous structure in sandy soils (e.g., Entisol and TAS) on the reproduction of soft-bodied organisms that allows for better mobility and reproductive performance of these soil invertebrates.
In our study, the negative effects of imidacloprid on the biological parameters of both species were detected in all assays. Adverse effects of this a.i. on F. candida and E. andrei were also seen in other studies (Alves et al. 2013; Alves et al. 2014; Chevillot et al. 2017), and they have been attributed to the mode of action of imidacloprid on soil organisms. This molecule acts by blocking the acetylcholine receptors in the nervous system of the organisms, through irreversible agonist connections, leading to the accumulation of the neurotransmitter acetylcholine (Catae et al. 2018). The systemic action of this a.i. may trigger a number of negative effects on exposed individuals, such as reduced fecundity, disorientation, paralysis, changes in DNA and in detoxifying enzymes, and even death (Laycock et al. 2012; Ge et al. 2018; Simon-Delso et al. 2015; Wang et al. 2016; de Lima et al. 2017; Sillapawattana and Schäffer 2017).
Although the mode of action of imidacloprid seems to be similar for the two tested species, the toxicity values were different (Table 3). In general, collembolans were more sensitive to imidacloprid than earthworms in the chronic toxicity assays, since lower EC50 values were found for F. candida in all soils. The higher sensitivity of F. candida to imidacloprid compared with E. andrei was identified equally in other studies (Alves et al. 2013; Alves et al. 2014; de Lima et al. 2017) and may be explained by their different intrinsic sensitivity to this class of pesticide, as well as to different exposure routes of the species. When acting on the nervous system of the exposed species, the neonicotinoids are generally more selective for the nicotinic acetylcholine receptors (nAChRs) of arthropods when compared with oligochaetes (Akeju 2014). A higher selectivity of the imidacloprid by the collembolan’s receptor sites also may be expected due to the phylogenetic proximity of this species to the arthropods, which are the main target of the action of imidacloprid.
On the other hand, it is well known that earthworms absorb the contaminants mainly by passive diffusion of pore water through the dermal layer, in addition to the intestinal absorption, which occurs together with the ingestion of soil particles (Belfroid et al. 1994). Collembolans, however, are exposed mainly through the absorption of pore water by specific organs, such as the ventral tube (Fountain and Hopkin 2005). When earthworms are exposed to high concentrations of imidacloprid in the soil, probably the absorption of the a.i. from the soil solution directly through the skin may be favored. This could be an explanation for the higher mortality of E. andrei compared with F. candida in the acute toxicity assays in Entisol (Table 3), especially because earthworms are in a compulsory exposure to high concentrations of the contaminant (dermal contact + feeding). For collembolans, the short-time exposure at high concentrations (as in the case of acute toxicity assays) seems to be less harmful, because they can avoid the consumption of pore water and also stay protected from the direct contact with contaminated soil by an exoskeleton.
Some studies have evaluated the chronic toxicity of imidacloprid to soil fauna species using representative soils from temperate regions. Wang et al. (2019) and Ge et al. (2018) found EC50 values of 0.70 and 0.87 mg kg−1, respectively, for hatchability and number of juveniles when E. fetida was exposed to pure imidacloprid in an OECD artificial soil. Ogungbemi and van Gestel (2018) found EC50 of 0.63 and 2.07 mg kg−1 when F. candida was exposed to the pure a.i. in OECD artificial soils containing 5% and 10% of peat, respectively. Similarly, Mabubu et al. (2017) reported an EC50 of 0.82 mg kg−1 for F. candida reproduction when the pure compound was tested in OECD artificial soil (5% peat). On the other hand, a relatively lower toxicity was found by Alves et al. (2013, 2014) when soil invertebrates were exposed to the commercial formulation Gaucho (600 mg a.i. L−1) in a tropical artificial soil with 10% coconut husk. They found EC50 values of 4.07 mg kg−1 for E. andrei (Alves et al. 2013) and > 1 mg kg−1 for F. candida (Alves et al. 2014). The toxicity of imidacloprid (pure a.i.) also was tested in a natural LUFA 2.2 soil, and an EC50 of 0.39 mg kg−1 for E. andrei was found (de Lima et al. 2017). The EC50 values ranged from 0.1 to 0.3 mg a.i. kg−1 in studies with F. candida (de Lima et al. 2017; van Gestel et al. 2017; Ogungbemi and van Gestel 2018).
Despite the methodological differences between the reported studies, a relatively similar imidacloprid toxicity for collembolans is common between artificial substrates with resembling compositions, especially with regard to the organic matter content. For example, the EC50 values reported in the assays with OECD soils containing 5% peat (Mabubu et al. 2017; Ogungbemi and van Gestel 2018) are similar to our EC50 in TAS, which contains 5% coconut fiber. A lower imidacloprid toxicity could be identified in assays where artificial substrates containing 10% of organic material were used (Alves et al. 2013, 2014; Ogungbemi and van Gestel 2018), suggesting that, when the soil texture is quite similar, the SOM has an important influence on imidacloprid bioavailability. On the other hand, different toxicities can be seen between natural soils, which have similar SOM contents but present distinct textures. The EC50 values for F. candida found in natural LUFA 2.2 soil, containing about 4% SOM and less than 20% clay + silt (de Lima et al. 2017; van Gestel et al. 2017), were at least two times lower than those found for Oxisol (Table 3), which has 3.7% SOM but high levels of silt + clay (> 90%).
Some authors also suggest that the adsorption of insecticides such as imidacloprid on soils may decrease with increasing pH (Sheng et al. 2005; Ping et al. 2010), indicating that lower bioavailability may occur in soils with low pH. However, in this study, the imidacloprid bioavailability seems to have been mainly modulated by silt and clay contents, since the toxicity values (Table 3) found in the natural soils with similar pH (3.9 vs 4.2), but with clearly different textures (Table 1), varied almost by an order of magnitude. Furthermore, similar toxicities were found in TAS (EC50 = 0.80) and Oxisol (EC50 = 0.83), which presented the largest difference of pH (5.9 vs 3.9, respectively), but closer silt + clay contents (Table 1). Similarly, in soils from temperate regions, the pH does not appear to be the main modulating factor for imidacloprid toxicity to soil fauna (de Lima et al. 2017; van Gestel et al. 2017; Ogungbemi and van Gestel 2018).
The abovementioned results suggest that the toxicity of imidacloprid is not exclusively driven by the organic material or pH, but it is also regulated by their interactions with the mineral soil fraction, which probably had a stronger influence on the sorption of the imidacloprid molecules than SOM and pH in our assays.
Some studies have identified a key role of the fine-textured soil mineral fraction in pesticide toxicity (Rutherford et al. 1992; Ogungbemi and van Gestel 2018). Mineral particles with high specific surface area are able to adsorb the imidacloprid molecules and therefore decrease their bioavailability to soil invertebrates (Ogungbemi and van Gestel 2018). Thus, the higher amounts of clay and silt in the Oxisol used in our study could partially explain the relatively lower toxicity of imidacloprid, when compared with that reported by de Lima et al. (2017) and van Gestel et al. (2017) in the natural LUFA 2.2 soil, as well as the lower acute toxicity for collembolans in this soil when compared with TAS (Table 3). Likewise, the highest toxicity found in Entisol in this study could be due to its low contents of clay and silt, which probably led to a greater fraction of the contaminant available for uptake by the organisms in the soil solution (van Gestel 2012; Peijnenburg et al. 2012). For animal wastes such as swine and pig manure (Segat et al. 2015; Maccari et al. 2016), deleterious effects on soil invertebrates were also higher in Entisol, when compared with Oxisol. There are some differences between the toxicity values reported in the literature and our results, obtained in this present study. They should not be attributed exclusively to the differences in soil properties, because most of the abovementioned studies were performed at 20 °C instead of 25 °C, as ours, and were based on the effects of the pure substance instead that of commercial formulations of imidacloprid. However, the use of commercial formulations in ecotoxicological assessments is more ecologically relevant than the use of pure active ingredients, since pesticides are used via formulations in agricultural areas (Renaud et al. 2018).
Although our PEC values (Table 2) are estimated based on a conservative approach by considering the worst-case scenario, similar imidacloprid concentrations have been found in agricultural soils. In soils from cocoa plantations, residues of imidacloprid ranged between 0.005 and 0.25 mg kg−1 (Dankyi et al. 2014). Donnarumma et al. (2011) identified residues of imidacloprid of 0.65 mg kg−1, 30 days after sowing of seeds treated with the commercial formulation Gaucho 350 FS. When considering direct applications of imidacloprid on the crops, residues in soil may reach concentrations above 4 mg kg−1 (Sharma and Singh 2014), and even higher levels of this a.i. may occur in the environment after successive applications (Ge et al. 2018). The detected imidacloprid concentrations in the reported studies are closer or even higher than our LOEC values for chronic assays with earthworms (0.12–2 mg kg−1) and collembolans (0.25–1.0 mg kg−1), indicating that species reproduction is easily affected by the expected levels of imidacloprid in tropical regions.
In addition, this study found lethal effects of imidacloprid in Entisol at concentrations 2.5 and 25 times higher than the PECs for earthworms and collembolans (Tables 2 and 3), respectively. In TAS and Oxisol, the species survival was affected only at concentrations higher than those causing mortality in Entisol. Although in the European Union the acute toxicity tests with earthworms are no longer required for the registration of Plant Protect Products (EU 2013), in Brazil, this is the unique assay with soil organisms required for pesticide registration (Brazil 1996). Because of this, our results also highlight the need to include more sensitive and ecologically relevant endpoints (such as reproduction) as a requirement for pesticide registration into the national regulations (Niva et al. 2016).
5 Conclusions
Imidacloprid caused mortality and reduced the reproduction of E. andrei and F. candida in all performed assays, and the toxic effects were modulated by the soil type. Our results revealed a higher toxicity and risk of imidacloprid for edaphic species in natural sandy soil and highlighted the importance of using different soil types to predict the effects of pesticides in tropical regions.
References
Akeju TO (2014) Assessment of the effects of the neonicotinoids thiacloprid and acetamiprid on soil fauna. Master’s thesis, University of Coimbra
Alves PRL, Cardoso EJBN, Martines AM, Sousa JP, Pasini A (2013) Earthworm ecotoxicological assessments of pesticides used to treat seeds under tropical conditions. Chemosphere 90:2674–2682
Alves PRL, Cardoso EJBN, Martines AM, Sousa JP, Pasini A (2014) Seed dressing pesticides on springtails in two ecotoxicological laboratory tests. Ecotoxicol Environ Saf 105:65–71
Alves PRL, Niemeyer JC, Cardoso EJBN (2017) Section I: terrestrial invertebrates as experimental models chapter 1. The use of non-standardized invertebrates in soil ecotoxicology. Issues in toxicology, 1-30, Royal Society of Chemistry
Amorim MJB, Römbke J, Scheffczyk A, Soares AMVM (2005) Effect of different soil types on the enchytraeids Enchytraeus albidus and Enchytraeus luxuriosus using the herbicide Phenmedipham. Chemosphere 61:1102–1114
Belfroid A, Meiling J, Sijm D, Hermens J, Seinen W, van Gestel K (1994) Uptake of hydrophobic halogenated aromatic hydrocarbons from food by earthworms (Eisenia andrei). Arch Environ Contam Toxicol 27:260–265
Bonmatin J-M, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EAD, Noome DA, Simon-Delso N, Tapparo A (2014) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22:35–67
Brazil (1996) IBAMA Normative Ordinance No. 84, October 15th, 1996. Establish procedures to be adopted in the Brazilian Institute of Environment and Renewable Natural Resources – IBAMA, for registration purposes and for assessment of the potential environmental hazard (PPA) of pesticides. DOU No. 203, Section 1, p. 21358 (in Portuguese)
Catae AF, Roat TC, Pratavieira M, Menegasso AR, Palma MS, Malaspina O (2018) Exposure to a sublethal concentration of imidacloprid and the side effects on target and nontarget organs of Apis mellifera (Hymenoptera, Apidae). Ecotoxicology 27:109–121
Chelinho S, Domene X, Campana P, Natal-da-Luz T, Scheffczyk A, Römbke J, Andrés P, Sousa JP (2011) Improving ecological risk assessment in the Mediterranean area: selection of reference soils and evaluating the influence of soil properties on avoidance and reproduction of two oligochaete species. Environ Toxicol Chem 30:1050–1058
Chelinho S, Domene X, Campana P, Andrés P, Römbke J, Sousa JP (2014) Toxicity of phenmedipham and carbendazim to Enchytraeus crypticus and Eisenia andrei (Oligochaeta) in Mediterranean soils. J Soils Sediments 14:584–599
Chevillot F, Convert Y, Desrosiers M, Cadoret N, Veilleux É, Cabana H, Bellenger J (2017) Selective bioaccumulation of neonicotinoids and sub-lethal effects in the earthworm Eisenia andrei exposed to environmental concentrations in an artificial soil. Chemosphere 186:839–847
Dankyi E, Gordon C, Carboo D, Fomsgaard IS (2014) Quantification of neonicotinoid insecticide residues in soils from cocoa plantations using a QuEChERS extraction procedure and LC-MS/MS. Sci Total Environ 499:276–283
de Lima ESC, Brennan N, Brouwer JM, Commandeur D, Verweij RA, van Gestel CAM (2017) Comparative toxicity of imidacloprid and thiacloprid to different species of soil invertebrates. Ecotoxicology 26:555–564
De Silva PMCS, van Gestel CAM (2009) Development of an alternative artificial soil for earthworm toxicity testing in tropical countries. Appl Soil Ecol 43:170–174
De Silva PMCS, Pathiratne A, van Gestel CAM (2009) Influence of temperature and soil type on the toxicity of three pesticides to Eisenia andrei. Chemosphere 76:1410–1415
Domene X, Chelinho S, Campana P, Natal-da-Luz T, Alcañiz JM, Andrés P, Römbke J, Sousa JP (2011) Influence of soil properties on the performance of Folsomia candida: implications for its use in soil ecotoxicology testing. Environ Toxicol Chem 30:1497–1505
Donnarumma L, Pulcini P, Pochi D, Rosati S, Lusco L, Conte E (2011) Preliminary study on persistence in soil and residues in maize of imidacloprid. J Environ Sci Health B 46:469–472
Douglas MR, Tooker JF (2015) Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environ Sci Technol 49:5088–5097
EC (2002) SANCO/10329/2002 - Final Guidance Document on Terrestrial Ecotoxicology under Council Directive 91/414/EEC. European Commission Health & Consumer Protection Directorate-General. E1-Plant Health
EC (2003) Technical Guidance Document on Risk Assessment. In Support of Commission Directive 93/67/EEC. Commission Regulation (EC) No 1488/94 and Directive 98/8/EC. European Commission Joint Research Center
EFSA (2018) European food safety authority. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid considering the uses as seed treatments and granules. EFSA J 16(2):5178–5113
EMBRAPA (2006) Centro Nacional de Pesquisa de Solos (Rio de Janeiro, RJ). Sistema brasileiro de classificação de solos, 2nd edn. EMBRAPA-SPI, Rio de Janeiro
Environmental Canada (2007) Guidance Document on Statistical Methods for Environmental Toxicity Test. Environmental Protection Series, EPS 1/RM/46, 2005 with 2007 updates. Environmental Canada, Ottawa
EPPO (2003) Environmental risk assessment scheme for plant protection products. Chapter 4: Soil. OEPP EPP0 Bull 33:151–162
EU (2013) COMMISSION REGULATION (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. Off J Eur Union L 93:1–84
Fountain MT, Hopkin SP (2005) Folsomia candida (COLLEMBOLA): a “standard” soil arthropod. Annu Rev Entomol 50:201–222
Garcia MVB (2004) Effects of pesticides on soil fauna: development of ecotoxicological test methods for tropical regions. Ecology and development series, vol 19. Doctoral thesis, University of Bonn
Ge J, Xiao Y, Chai Y, Yan H, Wu R, Xin X, Wang D, Yu X (2018) Sub-lethal effects of six neonicotinoids on avoidance behavior and reproduction of earthworms (Eisenia fetida). Ecotoxicol Environ Saf 162:423–429
Goulson D (2013) REVIEW: an overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987
Ihara M, Matsuda K (2018) Neonicotinoids: molecular mechanisms of action, insights into resistance and impact on pollinators. Curr Opin Insect Sci 30:1–7
ISO (1993) International organization for standardization - 11268-1. Soil quality - effects of pollutants on earthworms (Eisenia fetida) - Part 1: Determination of acute toxicity using soil substrate. Genève, pp 26
ISO (2012) International Standardization Organization - 11268-2. Soil quality - effects of pollutants on earthworms - Part 2: Determination of effects on reproduction of Eisenia fetida/Eisenia andrei. Genève, Switzerland
ISO (2014) International Standardization Organization – 11267. Soil quality - Inhibition of reproduction of Collembola (Folsomia candida) by soil contaminants. Genève, Switzerland
Jeschke P, Nauen R, Schindler M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908
Laycock I, Lenthall KM, Barratt AT, Cresswell JE (2012) Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21:1937–1945
Mabubu JI, Nawaz M, Cai W, Zhao J, He Y, Hua H (2017) Ecotoxicity of the neonicotinoid insecticides imidacloprid and thiacloprid to the soil-dwelling arthropod Folsomia candida (Collembola). J Kansas Entomol Soc 90:323–333
Maccari AP, Baretta D, Paiano D, Leston S, Freitas A, Ramos F, Sousa JP, Klauberg-Filho O (2016) Ecotoxicological effects of pig manure on Folsomia candida in subtropical Brazilian soils. J Hazard Mater 314:113–120
Niemeyer JC, Chelinho S, Sousa JP (2017) Soil ecotoxicology in Latin America: current research and perspectives. Environ Toxicol Chem 36:1795–1810
Niva CC, Niemeyer JC, Júnior FM, Nunes ME, De Sousa DL, Aragão CW, Sautter KD, Espindola EG, Sousa JP, Römbke J (2016) Soil ecotoxicology in Brazil is taking its course. Environ Sci Pollut Res 23:11363–11378
Ogungbemi AO, van Gestel CAM (2018) Extrapolation of imidacloprid toxicity between soils by exposing Folsomia candida in soil pore water. Ecotoxicology 27:1107–1115
Peijnenburg W, Capri E, Kula C, Liess M, Luttik R, Montforts M, Nienstedt K, Römbke J, Sousa JP, Jensen J (2012) Evaluation of exposure metrics for effect assessment of soil invertebrates. Crit Rev Environ Sci Technol 42:1862–1893
Ping L, Zhang C, Zhu Y, Wu M, Dai F, Hu X, Zhao H, Li Z (2010) Imidacloprid adsorption by soils treated with humic substances under different pH and temperature conditions. J Biotechnol 9:1935–1940
Pisa LW, Amaral-Rogers LP, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiserm DP, Krupke C, Liess M, McField M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, Van der Sluijs JP, Van Dyck H, Wiemers M (2015) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res 22:68–102
Renaud M, Akeju T, Natal-da-Luz T, Leston S, Rosa J, Ramos F, Sousa JP, Azevedo-Pereira HMVS (2018) Effects of the neonicotinoids acetamiprid and thiacloprid in their commercial formulations on soil fauna. Chemosphere 194:85–93
Rutherford DW, Chiou CT, Kile DE (1992) Influence of soil organic matter composition on the partition of organic compounds. Environ Sci Technol 26:336–340
Segat JC, Alves PRL, Baretta D, Cardoso EJBN (2015) Ecotoxicological evaluation of swine manure disposal on tropical soils in Brazil. Ecotoxicol Environ Saf 122:91–97
Sharma S, Singh B (2014) Persistence behaviour of imidacloprid and its metabolites in soil under sugarcane. Environ Monit Assess 186:2281–2288
Sheng GY, Yang YN, Huang MS, Yang K (2005) Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environ Pollut 134:457–463
Sillapawattana P, Schäffer A (2017) Effects of imidacloprid on detoxifying enzyme glutathione S-transferase on Folsomia candida (Collembola). Environ Sci Pollut Res 24:11111–11119
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais, 2nd edn. Universidade Federal do Rio Grande do Sul, Porto Alegre, p 147 (Boletim Técnico, 5)
Van der Sluijs JP, Amaral-Rogers V, Belzunces LP, Bijleveld van Lexmond MFIJ, Bonmatin J, Chagnon M, Downs CA, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Simon-Delso N, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Whitehorn PR, Wiemers M (2015) Conclusions of the worldwide integrated assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environ Sci Pollut Res 22:148–154
van Gestel CAM (2012) Soil ecotoxicology: state of the art and future directions. ZooKeys 176:275–296
van Gestel CAM, de Lima ESC, Lam T, Koekkoek JC, Lamoree MH, Verweij RA (2017) Multigeneration toxicity of imidacloprid and thiacloprid to Folsomia candida. Ecotoxicology 26(3):1–9
Wang J, Wang J, Wang G, Zhu L, Wang J (2016) DNA damage and oxidative stress induced by imidacloprid exposure in the earthworm Eisenia fetida. Chemosphere 144:510–517
Wang X, Zhu X, Peng Q, Wang Y, Ge J, Yang G, Wang X, Cai L, Shen W (2019) Multi-level ecotoxicological effects of imidacloprid on earthworm (Eisenia fetida). Chemosphere 219:923–932
Wood TJ, Goulson D (2017) The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ Sci Pollut Res 24:17285–17325
Acknowledgements
The authors thank the reviewers who provided thoughtful and constructive feedback on this manuscript and also thank the National Research Coucil (CNPq) for the research grant (Project 407170/2016-2). D.B. and E.J.B.N.C thank CNPq for their research productivity grants (Processes number 307162/2015-0 and 305193/2016-3, respectively). T.B.H. thanks CNPq for a Grant of Scientific Initiation (Process number 167664/2017-4). A.S. thanks Federal University of Fronteira Sul for a Grant of Scientific Initiation (Process code PES-2018-0959). F.O.B. thanks the Coordination for the Improvement of Higher Education Personnel (CAPES) for a master grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Yanzheng Gao
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bandeira, F.O., Alves, P.R.L., Hennig, T.B. et al. Toxicity of imidacloprid to the earthworm Eisenia andrei and collembolan Folsomia candida in three contrasting tropical soils. J Soils Sediments 20, 1997–2007 (2020). https://doi.org/10.1007/s11368-019-02538-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02538-6