Abstract
To attend the increasing demand for food and energy, vast monocultures such as sugarcane, soy, and corn, often adopts routinely intensive application of pesticides and chemical fertilizers, disregarding their potential effects on non-target soil organisms, which are crucial for soil functioning. In this study, we present the assessment of toxic effects of the commercial herbicide DMA® 806 BR (active ingredient: 2,4-D) and of the insecticide Regent® 800 WG (active ingredient: fipronil) to the non-target terrestrial plant Raphanus sativus var. acanthioformis (dicotyledon) and to the collembolan species Folsomia candida, in combination with different soil moisture conditions in natural and artificial tropical soils. Plant growth and biomass were severely affected by the presence of DMA alone, with significant differences from the control treatment already detected at 0.13 mg/kg/dw. Upon low soil moisture (20%, 40%, 60% WHC), the toxicity of DMA to plants was diminished, exhibiting an antagonistic pattern of interaction between DMA and soil moisture. Soil composition had a significant influence on survival and reproduction of collembola especially at high soil moisture content. In the natural soil, 80% of the water holding capacity (WHC) induced 100% collembola mortality whereas, in the tropical artificial soil (TAS), this same moisture condition had a negligible effect on survival. Reproduction was mainly affected by fipronil under drought conditions (20% WHC) at both soil types, possibly correlated with increased concentration of fipronil in the soil pore water at such conditions. Results herein presented highlight the requisite of including abiotic fluctuations in hazard assessment of pesticides to preserve soil function provided by biota.
Highlights
-
Soil moisture contribute on determining 2,4-D toxicity to terrestrial plants.
-
Soil moisture contribute on determining fipronil toxicity to Folsomia candida.
-
High-moisture levels increased plant species sensitivity to the herbicide 2,4-D.
-
Soil properties influences collembola sensitivity to pesticide contamination.
-
Dry conditions (20% WHC) + fipronil (1/8 RD) decrease F. candida
-
reproduction in 70%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the world population rises, there is a continuous attempt to meet global demands for food and energy supply, causing the vast monocultures (such as sugarcane and soy) to apply on its management routine, pesticides and chemical fertilizers (Marcato et al. 2017; de Menezes Oliveira et al. 2018). Sugarcane crops play a fundamental role in Brazil's economy, however they also represent one of the crops in which pesticides are mostly applied. Among the various active ingredients (a.i.) used in sugarcane fields to eliminate pests and maintain productivity, the herbicide 2,4-D and the insecticide/termiticide fipronil stands as some of the top 10 most applied ingredients (Christofoletti et al. 2017; IBAMA 2017).
The active ingredient 2,4-D is an auxinic substance largely pulverized on agricultural fields to eliminate broad-leaved weed species from cultures of monocotyledonous species, such as sugarcane. However, ecotoxicological tests have described growth inhibition and genotoxic effects of this substance on non-target organisms. In the presence of 0.3 mg/L of 2,4-D, bean seedlings of Phaseolus vulgaris L (dicotyledonous) suffered a 74% inhibition in root growth (Cenkci et al. 2010). Also, DNA changes in mitotic cells of Triticum aestivum L (Monocotyledon) were reported upon exposure of these plants to 50 mg/L of 2,4-D (Kumar 2010). Moreover, severe effects of the pesticide have been observed on the germination and growth of two plant species: Raphanus sativus var. acanthioformis (dicotyledonous) and Allium cepa (Monocotyledon), at environmental concentrations (< 4.7 mg 2,4-D/kg dw soil) (Triques et al. 2021).
Regarding fipronil, although its use has been restricted in Europe since 2009 (Regulation (EC) 1107/2009) (European Commission 2009) due to insufficient scientific information about its potential harmful effects on bees, it has been widely used in Brazil. Fipronil is a phenylpyrazole insecticide mainly applied to eliminate insects and termites, which are harmful to different types of crops, and to eliminate ectoparasites in animal diseases prevention. Hence, this compound may reach the soil when directly applied to agricultural fields and/or through animal excretion (Gupta and Milatovic 2014; Zortéa et al. 2018a). Negative effects of the exposure of the in-soil organisms to fipronil have already been demonstrated. Alves et al. (2014) observed that at concentrations below the recommended dose for application in agricultural fields, the insecticide can impact the survival and reproduction of non-target collembola species Folsomia candida, which was also confirmed by Triques et al. (2021). In addition, fipronil can leach from the soil into adjacent freshwater bodies, where it can affect non-target aquatic organisms, such as cladocerans (Silva et al. 2012) midges (Pinto et al. 2021b) odonates (Pisa et al. 2014) and amphibians (Boscolo et al. 2017), among other aquatic organisms (Pinto et al. 2021a).
In addition to being exposed to a cocktail of anthropogenic pollutants, including pesticides, soil organisms often face changes in abiotic factors such as temperature, UV radiation intensity, soil moisture and pH, among others. Acting as natural stressors, abiotic conditions may influence organism’s sensitivity to contaminants, especially in extreme climatic events such as drought and floods (Jänsch et al. 2005; Holmstrup et al. 2007; Ferreira et al. 2010; Krab et al. 2013). Increasing in frequency and duration of abnormal droughts, floods and higher temperatures have the potential to impact the ecological structure and functioning of terrestrial ecosystems, interfering in biotic and abiotic aspects, such as organism’s density, species composition, spatial distribution, degradation of organic matter, water content and soil available oxygen (Krab et al. 2013).
Regarding chemical sensitivity, terrestrial organisms show a broad range of responses when subjected to concomitant exposure to chemicals and varying abnormal conditions. For instance, the effects of the pesticide carbaryl on F. candida reproduction was decreased under high temperatures, whereas the terrestrial plant Brassica rapa and the terrestrial oligochaete Eisenia andrei displayed higher sensitivity to carbaryl under high temperatures and decreased sensitivity under low temperatures (Lima et al. 2011). Soil pH can also alter the dissolution rate of metal nanoparticles in the soil pore water, turning it more available for uptake by organisms (Waalewijn-Kool et al. 2014).
At the broader organizational level such as invertebrate community, Menezes-Oliveira et al. (2014) investigated the combined effects of extreme temperature conditions and copper contaminated soil on terrestrial invertebrates. The authors observed synergistic effects on some species and showed that extrapolating effects to soil organisms from non-polluted to polluted areas turn out not possible when the stress factors are combined. Considering this evidence, and the current changes in climatic conditions, it is crucial to understand the interaction between natural and chemical factors on soil organisms to better predict the environmental risks that this combination may pose to terrestrial ecosystems, especially in tropical conditions, for which information is scarce. Therefore, the present study aimed to analyze the combined effects of different soil moisture conditions and two pesticides intensively applied in sugarcane crops, 2,4-D and fipronil, on two soil species, representing different taxa and dissimilar ecosystem functions. To assess the effects of varying moisture conditions and herbicide combinations, the non-target plant species Raphanus sativus var. acanthioformis was chosen as a model organism. To investigate the possible adverse effects of different soil moisture conditions and insecticides, the chosen model species was the collembola Folsomia candida. In both cases, the assays were performed in natural and artificial tropical soil.
Material and Methods
Test Organisms
The chosen test organisms were the terrestrial plant species Raphanus sativus var. acanthioformis (dicotyledon) and the collembola species Folsomia candida. For plant bioassays, organic seeds (i.e., with no pesticide coating) were obtained from a local agro centre, presenting satisfactory purity and germination potential (> 99% and 88%, respectively). As for soil invertebrate tests, organisms were obtained from stabilized laboratory cultures, which have been maintained for several generations under controlled conditions of temperature (20 ± 2ºC) and photoperiod (16:8 h light: dark). Folsomia candida organisms were maintained in a Plaster of Paris substrate and fed on Saccharomices cerevisae twice a week.
Test Chemicals
Commercial formulations of the herbicide DMA® 806 BR, hereafter referred to as DMA, and the insecticide Regent®, hereafter Regent, were obtained from a local commerce supplier. Formulations were applied in this study to reproduce a more realistic condition of exposure of both pesticides in the field. DMA is an auxinic herbicide from the chemical group of Aryloxyalkanoic acid. Marketed by Dow AgroSciences, this soluble concentrate has as active ingredient 2,4-D dimethylamine salt (80.6% m/v) with 67.0% (m/v) equivalent to the 2,4-D acid and 41.9% (m/v) corresponding to inert ingredients. Classified by the Brazilian environmental agency as extremely toxic to human health and dangerous to the environment, DMA application in sugarcane monocultures is recommended to be done through pulverization over the crops or in the moist soil in the pre and/or post-emergence seasons of the target plants, depending on the weed species. The herbicide mode of action consists in modifying the cell wall plasticity, protein synthesis and ethylene production of dicotyledonous (broad-leaved) plant species meristematic cells, causing abnormal growth, senescence, and death of the plant (Song 2014; Foloni 2016).
Regent is a phenylpyrazole insecticide/termiticide commercialized by the BASF company. The dispersible granulate formulation has 80% fipronil as an active ingredient (g/kg) with the other 20% composed of inert ingredients (Aubin and Elbeuf 2018). Highly toxic to human health and very dangerous to the environment, according to the manual of instructions of the product, this commercial formulation is applied in sugarcane planting furrow aiming to eliminate insects such as Diatraea saccharalis (Lepidoptera) and Migdolus fryanus (Coleoptera), among other arthropods considered as pests in agricultural systems (Boscolo et al. 2017; Christofoletti et al. 2017). The toxic action of fipronil is activated via ingestion, interfering in the central nervous system of arthropods, such as bees, collembola, and beetles. It prevents neurotransmitter γ-aminobutyric acid (GABA) from acting on chloride channels of neural cells, which causes neuronal hyperactivity, overexcitation, severe paralysis and death of the organism (Gunasekara et al. 2007; Nogueira Cardoso and Lopes Alves 2012; Gupta and Milatovic 2014; Pisa et al. 2014).
Test Soils
Soil types used in all assays were tropical artificial soil (TAS) and natural soil (NS). TAS was prepared in the laboratory following the adaptation from Römbke and García (2000) and consisted of a mixture of 75% fine sand, 20% kaolinitic clay and 5% coconut fibre powder. The natural soil was obtained from an experimental field at the São Paulo Agribusiness Technology Agency (APTA) located in Brotas municipality, the eastern countryside of the state of São Paulo – Brazil. The natural soil characteristics were: 89.8% sand; 8.2% clay and 2.0% silt, with 2.5% organic matter content, average pH = 4.3, cation exchange capacity (CEC) = 37.8 mmolc/dm3 and 27% of maximum water holding capacity (WHC). After collection, the natural soil was sieved to 2 mm and dried at 60 °C overnight.
Soil Spiking
The soil spiking procedure followed the method described in our previous work (Triques et al. 2021). Stock solutions of the pesticide were mixed into premoistened soil. The total amount of soil needed for all replicates of a given concentration was spiked at the same time. After homogenization, subsamples were transferred to the corresponding test vessel. The amount of soil used in each vessel varied according to the test organism. The concentration of the pesticides used in each experiment was chosen based on both commercial products effects observed in previous single tests with both test species, on the same soil types (Triques et al. 2021). For the previous experiments, concentrations were derived from the recommended dose (RD) for application in sugarcane fields, as established by each commercial product’s (c.p) manufacturer. For the herbicide DMA, the recommended dose is 7 µL of c.p/kg of dw soil, which corresponds to 4.7 mg of 2,4-D/kg dw soil (active ingredient), used to control broad-leaved weeds during their pre-emergence period. Regarding the insecticide Regent, the recommended dose is 1.3 mg of c.p./kg of dry weight (dw) soil (1.04 mg of fipronil/kg of dw soil), indicated to control the beetle Migdolus fryamus (Aubin and Elbeuf 2018). From those assays, effect concentrations (EC) [EC10, EC20 e EC50] were determined, which originated the concentration ranges of the present work. Details of each experiment are presented in the following sections. Although commercial formulations were applied in this study, all nominal concentrations applied in the ecotoxicological assessment of the herbicide DMA and the insecticide fipronil are presented as active ingredient.
Plant Growth Tests
Following the procedures of the guideline ISO 11269–2 (ABNT 2012) seeds of Raphanus sativus var. acanthioformis were exposed to the herbicide DMA. The concentrations of DMA applied were different for each type of soil, as we previously observed that soil composition influences the sensitivity of R. sativus to the herbicide DMA (Triques et al., 2021). For NS, concentrations of DMA were: 0; 0.125; 0.25; 0.5; 1.0 and 2.0 mg of 2,4-D/kg dw soil. For the TAS, where plants displayed higher sensitivity to DMA, concentrations applied were: 0; 0.001; 0.005; 0.01; 0.1 and 1.0 mg of 2,4-D/kg dw soil. For both types of soils, five different soil moisture conditions were combined with each herbicide concentration, being them 20%; 40%; 60%; 80% and 100% of each soil water holding capacity (WHC). In a full factorial design, for each moisture condition, three replicates for the control group (no pesticide) and two replicates for the different herbicide concentrations were used. By decreasing the number of replicates, it is possible to use more treatments (combinations of stressors) in each experiment, to acquire a full analysis of the response surface. This has been advocated as a way to increase both reliability and power of the analysis (Jonker et al. 2005) as the response surface analysis is based on a regression model. Conical polystyrene test vessels (8.9 cm height; 6.7 cm bottom diameter; 10.0 cm top diameter) containing 250 g of spiked soil for each replicate were used, where ten seeds of the test species were planted. Soil moisture was maintained through daily replenishment of water, based on test vessels weight loss of all treatments, except for the 80% WHC, for which a self-watering system was used. This system consisted of a fiberglass wick (5–10 mm Ø) located at the bottom of the test vessels and connected to independent conical polystyrene containers (5.5 cm height; 5.0 cm bottom diameter; 7.0 cm top diameter), filled with distilled water. The 80% WHC was considered as the control condition for water content. The test started when 70% of the seeds have emerged in the control group and lasted for 14 days, under controlled conditions. After that period, different endpoints were measured in all experimental treatments: individual shoot length (SL), fresh weight (FW) biomass (B) and hydric content (HC).
Collembola Survival and Reproduction Tests
Tests followed the procedures of the ISO 11267:2011 guideline (ABNT 2018), using both natural (NS) and artificial (TAS) soils. Folsomia candida was exposed to the insecticide/termiticide Regent at concentrations of 0; 0.06; 0.13; 0.26 mg of fipronil/kg dw soil. Following the same experimental design of the plant assays, five different soil moisture contents were combined with each fipronil concentration (20%; 35%; 50%; 65% and 80% WHC), with the 50% WHC soil moisture considered as the control condition for soil moisture. For each moisture condition, there were five replicates for the control group and four replicates for all other treatments, each containing 50 g of the spiked soils. At the starting of the experiment, each test vessel received ten organisms of Folsomia candida, within the same age interval (10–12 days). The reproduction test lasted for 28 days, food and water replenishment were weekly performed. The experiment was conducted at 20 ± 2 ºC and a photoperiod of 16:8 h (light: dark). After 28 days of exposure, the test vessels were fulfilled with tap water and the content of each vessel was transferred to a larger (10 cm Ø) recipient. Water-soluble stamp ink was added to each recipient and carefully stirred with a spatula, allowing animals to float. Finally, digital pictures were taken, and the total number of organisms in each test vessel was calculated using the software ImageJ (Schneider et al. 2012).
Soil Chemical Analysis
Soil samples preparation for analytical measurements of DMA and fipronil were conducted according to Triques et al. (2021). Soil samples (~ 50 g) spiked with the different concentrations tested, of both pesticides in the different soils (NS and SAT), were processed by the Environmental Chemistry Laboratory of the State University of Campinas (UNICAMP—São Paulo, Brazil). Fipronil and 2,4-D were quantified by the 6410B Triple Quadrupole Liquid Chromatograph/Mass Spectrometer. Procedures for the extraction of the analytes were adapted from De Amarante et al. (2003). The solid–liquid extraction was performed through three cycles (10 min each) with the addition of 20 ml of dichloromethane (degree HPLC > 99.8%) each. Recovery rates in NS and SAT, respectively, were 50 and 33% for fipronil and 34 and 72% for 2,4-D. The limits of detection were 0.00001 mg/kg (dw soil) for fipronil and 0.0007 mg/kg (dw soil) for 2,4-D.
Statistical Analysis
A one-way analysis of variance (ANOVA) was used to detect differences between treatments against the control group on isolated exposures. A two-way analysis of variance was used to assess interactions between both soil moisture content and pesticide concentration factors (α = 0.05). Kruskal–Wallis ANOVA on Ranks was performed whenever data were not normally distributed, and data transformation did not correct for normality (SPSS, 1997). A non-linear regression model was used to determine the EC50 values of the pesticides in different soil moisture conditions (ymax = (1 + Conc/EC50)b, where: ymax is the maximum response (number of juveniles produced or survival rate at control conditions); Conc is the concentration of the pesticide; EC50 is the effect-concentration for 50% of exposed organisms and b is the slope of the curve. A generalized likelihood test was used to compare predicted EC50 values at different exposure conditions, on SPSS v. 27 (IBM Corp 2020).
Results and Discussion
Soil Chemical Analysis
Recovery rates of fipronil were 50% in NS and 33% in TAS whereas the recovery rates for DMA were 34% in NS and 72% in TAS. Similarly to our previous work (Triques et al. 2021), the low recovery rates of both pesticides are believed to be linked to extraction procedures during the analytical procedure. To simplify comparisons and data report, results herein are presented and discussed based on nominal concentrations.
For both plants and collembola, all tests fulfilled the legitimacy criteria stated in each guideline adopted.
Plant Growth Tests
Isolated Exposures
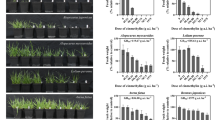
Under flood conditions, represented by 100% soil WHC, no plant emergence was observed at both soil types. In the natural soil, the presence of DMA alone (i.e., under control moisture condition of 80% WHC) caused a significant decrease in plant performance, measured by the parameters shoot length, fresh weight, dry weight, and hydric content, as shown on Fig. 1 (values of ANOVA testing are represented in the Figure). Plant growth and biomass gain were highly affected by the presence of DMA alone, with significant differences from the control treatment already detected in the first tested concentration (0.13 mg/kg/dw). The hydric content, however, was only affected at the DMA concentration of 0.5 mg/kg/dw. These results agree with our previous study where single effects of DMA on R. sativus were evaluated and which revealed the herbicide impairment on plant parameters such as fresh weight, dry weight, and hydric content at environmentally relevant concentrations of DMA (Triques et al. 2021).
Isolated effects of DMA.® 806 BR in Natural Soil (NS) on plant growth (A), biomass (B), fresh weight (C) and hydric content (D). Dark blue bars represent average measurements of the replicates, and the error bars represent the standard error of the average measurements. Asterisks indicate statistical differences (α = 0.05) against the control condition (0 mg/kg DMA BR and 80% moisture)
Considering different moisture conditions in the natural soil (Fig. 2), plant growth (2A) and fresh weight (2C) were only significantly affected at the extremely dry condition, represented by the 20% WHC. However, the biomass gain (2B) was significantly lower when compared to the control treatment (80%) at water percentages of 60, 40 and 20. In the tropical artificial soil, DMA impaired plant growth at all concentrations tested (Fig. 3) while plant fresh weight (3A) was only reduced at 1 mg DMA /kg dw soil. Moreover, the dry weight (3B) of plants exposed to DMA alone in tropical artificial soil was not affected and the hydric content (3C) of plants was significantly different from the control at DMA concentrations of 0.001 and 1 mg/kg dw soil. Considering the effects of different soil moisture conditions on plants, in the tropical artificial soil (Fig. 4), growth was reduced at the 20% treatment, while varying water content in the soil exerts no effect on plant fresh weight. On the other hand, the biomass was significantly lower at the 60%, 40% and 20% conditions while the hydric content of plants was higher when compared to the control at those same conditions. This suggests that under water limiting conditions, R. sativus, like other plant species, tend to accumulate solutes in the root, thus attracting water to it (Liu and Stützel 2004) or decreasing stomatal respiration.
Isolated effects of different soil moisture conditions, on Natural Soil (NS), on plant growth (A), biomass (B); fresh weight (C) and hydric content (D). Dark blue bars represent the average measurements of the replicates, and the error bars represent the standard error of the average measurements. Asterisks indicate statistical differences against the control condition (80% moisture)
Isolated effects of DMA® 806 BR in Tropical Artificial Soil (TAS) on plant growth (A), biomass (B), fresh weight (C) and hydric content (D). Dark blue bars represent average measurements of the replicates, and the error bars represent the standard error of the average measurements. Asterisks indicate statistical differences (α = 0.05) against the control condition (0 mg/kg DMA BR and 80% moisture)
Isolated effects of different soil moisture conditions, on Tropical Artificial Soil (TAS), on plant growth (A), biomass (B); fresh weight (C) and hydric content (D). Dark blue bars represent the average measurements of the replicates, and the error bars represent the standard error of the average measurements. Asterisks indicate statistical differences against the control condition (80% moisture)
The overall pronounced plant growth increase according to soil water content conditions is related to the importance of water as a limiting factor in terrestrial plants development. Subject to constant water loss through evapotranspiration, plants in soils with adequate water supply present higher photosynthetic rates, with greater protein synthesis and cell expansion, more stomatal conduction, and less solute and abscisic acid accumulation, which greatly influence their growth (Lazar 2003). On the other hand, the excessive water content may harm plant emergence and growth due to seed rot by soil saturation and consequent anaerobiosis in the substrate (Lima et al. 2011). The present results alert for the damaging effects of droughts and floods on the development of terrestrial plants. As reported by Trenberth (2011), alterations in rainfall regimes as well-hydrological cycle, are considered as some of the consequences of climate change on Earth’s water distribution and are considered, whenever at greater frequency and magnitude, as extreme events. The increase in temperature generates higher rates of evaporation and, thus, dryness of surfaces. In this way, drought events can become longer and intense, potentially leading to wildfires and heatwaves (Williams et al. 2019). In addition, increasing atmosphere moisture causes more frequent and intense precipitation events (e.g., tropical storms and cyclones, thunderstorms, orographic rainfall), increasing the risk of flooding (Trenberth 2011).
Furthermore, we could detect that the influence of soil moisture on plant growth was soil dependent. Differences in organic matter source and texture of both soils may have contributed to the magnitude of effects observed concerning the varied soil moisture conditions. At the artificial soil, powder of coconut fibre (source of organic content) and the lack of components of natural soils, such as silt and clay, may have contributed to decreased water absorption by soil particles and consequently increasing water availability to plants, which occurred even at driest condition on the artificial soil (Lazar 2003).
Plant Test – Combined Exposure
To identify the influence of soil moisture on DMA toxicity to plants, an EC50 of DMA was estimated (non-linear regression model, 3-parameter equation, see statistical analysis section) for each plant development parameter (SL, FW, B and HC), at each soil moisture condition (Table 1). On the natural soil, an extremely low EC50 value (3 × 10–11 mg/kg a.i) of DMA was estimated for plant fresh weight and biomass at control moisture condition (80% WHC), which, when compared to the EC50 value for those same parameters at lower soil water percentages (20%, 40% and 60%), indicates an antagonistic pattern of combination between DMA and water availability. This means that under lower water availability, DMA effects on plant fresh weight and biomass (i.e., fresh and dry weight) is soothed, as suggested by higher EC50 values of DMA at such conditions (Fig. 5). This tendency was confirmed by the two-way ANOVA (supplementary material- Fig. S2) which revealed that the effects of DMA on all plant parameters are dependent on the moisture of the soil which plants were exposed to (p-value < 0.001).
Growth parameters of the terrestrial plant R. sativus var. acanthioformis exposed to the herbicide DMA® 806 BR (active ingredient 2,4-D), at different concentrations (x axis), under varying soil moisture conditions on natural soil, for 14 days. Error bars correspond to standard errors of the replicate measurements [nº replicates = 3 (control); nº replicates = 2 (treatments)]
The antagonistic pattern was also observed at the tropical artificial soil (TAS) when considering plant shoot length. Fresh weight and biomass were not differently affected by DMA under variable water content in soil, which is demonstrated by the interpolation of the 95% confidence intervals of the EC50 value for DMA under control condition (80% WHC) and the lower moisture conditions (20%, 40% and 60%—Table 1, Fig. 5). Considering that contaminant exposure to plants is made through the soil pore water, these outcomes were possibly due to 2,4-D high water solubility and, consequently, higher bioavailability to plant uptake as soil water content increases. This possibly explains the absence or less pronounced effects of 2,4-D under lower soil moisture conditions (20% WHC), in which soil sorption of the compound is likely greater, thereby decreasing bioavailability for uptake by plants (Luiz Lonardoni Foloni 2016) Fig. 6.
Growth parameters of the terrestrial plant R. sativus var. acanthioformis exposed to the herbicide DMA® 806 BR (active ingredient 2,4-D), at different concentrations (x axis), under varying soil moisture conditions on Tropical Artificial Soil, for 14 days. Error bars correspond to standard errors of the replicate measurements [nº replicates = 3 (control); nº replicates = 2 (treatments)]
Combined effects of natural and chemical stress factors such as soil moisture and pesticides have been investigated by other authors. For instance, the toxic effect of the insecticide carbaryl to Triticum aestivum and Brassica rapa shoot length was increased at simulated flood conditions, indicating a synergistic interaction between the insecticide and soil water content (Lima et al. 2011). As for fresh weight and biomass, the authors suggested an antagonistic interaction between the two stress factors when carbaryl was present in the soil with low water content, thus corroborating the findings of the present study. Furthermore, antagonistic interaction, in this case, can be explained by the fact that under contaminated soil and low water availability, soluble xenobiotics such as 2,4-D become less bioavailable to the plants (Bonmatin et al. 2015). Our results show inhibitory effects of DMA® 806 BR (2,4-D) on Raphanus sativus var. acanthioformis even at concentrations five times (for TAS) and 40 times (for NS) lower than its recommended dose of application (4.7 mg 2,4-D / kg dw soil). This may represent an added environmental risk, considering that drifts from spray applications of DMA can reach adjacent sensitive crops, such as tobacco, cotton, tomato, and grape crops (Foloni 2016), potentially leading to negative effects on such species. Considering the high solubility of DMA (900 mg/L) and its easy transportation from application site to adjacent water bodies through leaching (Islam et al. 2018), one should consider the different factors influencing pesticides toxicity on soil organisms to better predict their effects on the environment and be able to propose different methods and or conditions for the application of the pesticides without harming the terrestrial biota.
Collembola Reproduction Tests
Isolated Exposures – Fipronil
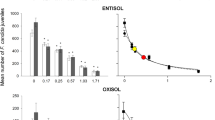
The impact of fipronil and moisture conditions, applied as single stressors, on the survival and reproduction of springtails, are summarized in Table S1. The exposure to fipronil alone, at the control soil moisture condition (50% WHC) induced F. candida adult mortality in a concentration-depended manner, with 38% mortality observed at the highest concentration of fipronil (0.26 mg/kg dw) in both types of soil. In the natural soil, reproduction was not affected by any of the concentrations of fipronil, when compared to the control treatment (0 mg/kg dw), whereas in the artificial tropical soil, reproduction of F. candida was significantly reduced at 0.26 mg fipronil/kg dw (Dunnet-t test, p = 0.008) (Fig. 7). These results are in accordance with our previous study where a LC50 value for fipronil was obtained as 0.21 mg/kg/dw soil. Furthermore, reproduction was equally affected at both soil types [EC50 (NS) = 0.27 (0.13–0.4); EC50 (TAS) = 0.26 (0.19–0.34)]. Zortéa et al. (2018b) also observed a significant decrease in offspring production by F. candida at concentrations of 0.3-mg fipronil/kg/dw in tropical artificial soil, corroborating the results of the present study.
Single effects of soil moisture (A & B) and Fipronil concentrations (C & D) on survival and reproduction of the collembola Folsomia candida exposed to the two types of soil (NS Natural Soil, TAS Tropical Artificial Soil). Asterisk on figure D indicate significant difference in the number of juveniles, compared to control treatment (0 mg/kg/dw). Vertical bars represent average values of the percentage of surviving animals (A and B) and average number of juveniles produced at each treatment. Error bars represent standard error of the averages. [Number of replicates (control) = 5; (treatments) = 4]
Isolated Exposures – Soil Moisture
The treatment with 20% water content in the soil induced 12% of adult mortality in the natural soil and 4% on the tropical artificial soil, whereas the other extreme condition (80% water content) induced 100% mortality of organisms in the natural soil and 12% in the artificial soil (Fig. 7). Different soil moisture conditions in the artificial tropical soil induced no effect on collembola mortality or reproduction. However, on the natural soil, the 80% WHC moisture condition produced 100% mortality of adult organisms. Therefore, no reproduction output is available for the 80% WHC condition in the natural soil. A strong decrease in survival and reproduction of the collembola species exposed to 75% WHC of an artificial soil (10% organic matter, 20% kaolin clay and 70% quartz sand) was observed by Van Gestel and Van Diepen (1997).
The accentuated differences in collembola sensitivity to soil moisture between the two types of soil studied here are related to soil characteristics promoting distinctive reactions at the organism level. Domene et al. (2011) compared life-traits of F. candida in different soil compositions and reported a significantly lower reproduction of organisms in the more fine-textured soils (with higher silt and fine sand content, like the natural soil used in this study). In an avoidance study, where different textured soils were examined in combination with varying levels of organic matter (2%, 5% and 10%), Natal-Da-Luz et al. (2008) demonstrated that springtails never avoided the coarser-textured soil with 10% organic matter, which suggests that organisms have somehow a preference for those type of soil. Accordingly, Van Gestel and Mol (2003) described higher mortality rates of F. candida exposed to cadmium in two natural soils (with 2.5% and 1.4% clay content) when compared to OECD and Lufa 2.2, which are more likely sandy soils. Considering that the natural soil used in the present study is rather loamy and that collembola has a permeable integument (Højer et al. 2001; Skovlund et al. 2006), the higher sensitivity to greater water content in the natural soil may be a function of increased fipronil toxicity, which becomes more bioavailable for organisms as water percentage increases, as uptake can occurs via soil pore water through collembola integument. In addition, an osmoregulatory mechanism has been described for those animals, a process in which cell membrane plays a vital role (Skovlund et al. 2006). In an event of environmental hydric stress, the osmo-regulation may be hampered thus leading to organisms reduced tolerance.
Combined Exposures—Survival
Upon combined exposures to fipronil and different soil moisture conditions, adult survival rates were lower in the natural soil, when compared to the artificial soil, with 100% mortality observed for the 80% water content in the natural soil (Fig. 8A).
Percentage Adult survival (A) and juvenile production (B) of Folsomia candida exposed to the insecticide Regent® 800 WG (a.i. fipronil) and different moisture contents on natural soil) for 28 days. Error bars represent standard error of the average. [Number of replicates (control) = 5; (treatments) = 4]
In the tropical artificial soil, 80% moisture condition did not influence fipronil toxicity, as observed by the similar survival rates at all concentrations tested (Fig. 9A). At the 20% water content, however, springtail mortality seemed to be determined by fipronil concentration in the soil, with a major difference in mortality rates between natural and artificial soil observed at the concentration of 0.06 mg/kg/dw soil of fipronil (NS – 38%; TAS – 24%). However, at this same fipronil concentration and upon “control” soil moisture (50%) the situation is reversed, and a higher mortality rate is observed in the artificial soil in comparison with the natural soil (TAS – 32%; NS – 24%). This corroborates the premise that soil composition plays a major role in governing pesticide mobility, sorption and thereby, bioavailability to organisms (Spark and Swift 2002). Overall, F. candida perform better and display less sensitivity to xenobiotics in coarsely sand soils with low organic matter content (Domene et al. 2011; de Menezes Oliveira et al. 2018; Natal-Da-Luz et al. 2008; Triques et al. 2021) likely because those soil characteristics are not suitable for pesticide sorption (Rodríguez-Cruz et al. 2006). As argued by de Menezes Oliveira et al. (2018), the feeding habits of edaphic species such as F. candida can contribute to higher exposure to contaminants in natural soils. By feeding on soil microorganisms and dead organic material (which are present in a higher percentage in natural soils), organisms are prone to ingest pesticides that are adsorbed onto the soil organic material. In the case of fipronil, the sorption coefficients (kd) for soils with different compositions were reported to be closely related with the soil organic carbon content rather than to clay content [kf = 1.94 (soil composition of 5% organic matter, 10% clay, 4% silt, 84% sand); kf = 4.89 (soil composition of 1.67% organic matter, 33% clay, 27% silt, 40% sand)]. Moreover, fipronil sorption on soil particles tends to increase with increasing clay content and organic matter in the soil (Ying and Kookana 2001) whereas desorption seems to be inversely correlated with soil organic carbon (Singh et al. 2016). Cogitated together, data regarding fipronil behavior in soils point out to a higher adsorption and retention behavior on soils with relatively high organic carbon and less clay, such as the natural soil-applied in the present study. Thus, we speculate that the higher toxicity of fipronil observed in the natural soil is attributed to the ingestion of organic carbon particles with adsorbed fipronil, by springtails.
Percentage adult survival (A) and juvenile production (B) of Folsomia candida exposed to the insecticide Regent.® 800 WG (a.i. fipronil) and different moisture contents on Tropical Artificial Soil (TAS) for 28 days. Error bars represent standard error of the average. [Number of replicates (control) = 5; (treatments) = 4]
Combined Exposures—Reproduction
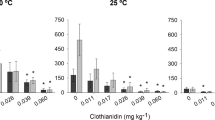
The influence of soil moisture conditions on the toxicity of fipronil on collembola reproduction is summarized in Figs. 8B and 9B for the natural soil and artificial tropical soil, respectively, where the average number of juveniles produced at each pesticide concentration and soil moisture content is displayed. At the control condition (0 mg/kg/dw fipronil and 50% WHC), there was no difference in the number of juveniles produced between the two soil types (t-test; p = 0.8). Also, a concentration of fipronil causing a 50% effect on juvenile production (EC50) was estimated for each soil moisture (Table 2). For the natural soil, at the 65% WHC, the EC50 value of fipronil was extrapolated by the non-linear regression model, due to the lack of dose–response relationship between fipronil concentrations and juveniles produced by the springtails in that condition.
For the remaining soil moisture conditions, the lack of the 95% confidence intervals for the EC50 estimates does not allow inferences to be made concerning an increase or decrease in toxicity of fipronil as soil moisture changes. Therefore, we compared the EC50 of fipronil between control moisture (50%) and drought condition (20%) in the natural soil, using a generalized likelihood ratio (GLR). The X2 value of 1.6 (< 3.84) indicates no difference between the EC50 values in both conditions which suggest that the toxicity of fipronil, in the natural soil, is not altered under drought conditions. However, it is worth considering the accentuated decrease in reproduction observed at the 20% WHC condition in the natural soil, at a fipronil concentration of 0.06 (1/8 of recommended dose) (Fig. 8). Based on the similar EC50 values and the interpolation of the 95% confidence intervals, the toxicity of fipronil to collembola reproduction was not modified in the tropical artificial soil at varying soil moisture conditions (Fig. 9).
In Fig. 10, the reproduction output of collembola is represented at extreme conditions of drought (20% WHC) and high moisture (80% WHC) on both types of soils, comparatively to the control soil moisture (50% WHC) for each fipronil concentration. Under drought conditions, F. candida seems to be more sensitive in the natural soil, compared with the TAS as indicated by the 74% decrease in the number of juveniles (comparatively to the control moisture of 50% WHC) observed at 0.06 mg fipronil/kg dw soil, while this same percentage decrease in the number of juveniles was observed in TAS at 0.26 mg fipronil/kg dw soil (Fig. 10).
Reproductive output of Folsomia candida exposed to fipronil in simulated extreme conditions of drought (A & B) and high moisture (C) at two soil types: natural soil and tropical artificial soil. White vertical bars represent control condition for moisture content (i.e., 50% WHC of soil) at each fipronil concentration values in red represent the percentage decrease in nº of juveniles produced at each fipronil concentration, in relation to control values
Thus, our data suggest that under dry soil conditions, the effect of fipronil on F. candida reproduction might be intensified at low doses of contamination and could be soothed as concentration increases. Other authors have investigated how soil moisture influences pesticides or metal toxicity to collembola. Bandow et al. (2014) found that the effects of the fungicide pyrimethanil on the reproductive output of F. candida were intensified in low soil moisture conditions in artificial OECD soil (5% peat, 20% kaolin, 74–74.9% quartz sand), which was associated with a greater concentration of the fungicide in the soil pore water. Furthermore, the effect of imidacloprid on collembola reproduction was assessed under different moisture conditions and soil types (loamy and sandy) in the study of Braúlio Hennig et al. (2020). In this case, it was observed that the toxicity of imidacloprid was accentuated in the drier condition for the loamy soil, whereas in the sandy soil, no correlation was observed between soil moisture and imidacloprid toxicity. Those observations somehow corroborate our findings, given that collembola displayed greater sensitivity to fipronil in drought conditions in the natural soil (loamy) when compared to the TAS (sandy soil).
There is a direct relationship between climate change and precipitation regimes. In summary, rise in temperature increases water evaporation thus leading to drought conditions (Trenberth 2011). The present data (in alignment with previously mentioned studies) reveals that F. candida could have difficulties producing enough offspring under extreme conditions such as drought. In the other extreme condition where a high-water percentage in the soil might be present, organisms may not survive at all, depending on soil characteristics, as we observed 100% mortality of collembola on natural soil at 80% of WHC. This highlights the urgent need to consider environmental variables arising from climate change (such as the fluctuations in soil moisture) on the management and pesticide application on crops, to preserve soil function.
Conclusions
In this study, we performed combined exposures of two broadly used sugar cane pesticides in Brazil [the herbicide DMA® 806 BR (a.i. 2,4-D) and the insecticide Regent® 800 WG (a.i fipronil)] with varying soil moistures in two soil types (natural and tropical artificial soil). We aimed to unravel the effects of such pesticides in non-target organisms (the terrestrial plant Raphanus sativus var. acanthioformis and the soil invertebrate Folsomia candida) when combined with varying soil moisture conditions. Our results demonstrated that soil moisture content plays a role in determining the toxicity of both studied pesticides to plant and collembola. Our model plant species showed increased sensitivity to DMA under high-moisture levels, likely because of the facilitated herbicide solubility in such conditions and consequently, increased bioavailability for uptake by plants.
The use of two soil types in this study highlighted the importance of soil composition as a limiting factor for collembola survival and reproduction, as well as sensitivity to pesticide contamination. This was evidenced by the complete lack of survival of F. candida in the 80% WHC condition in the natural soil, whereas in the tropical artificial soil, the survival rate of organisms in the same moisture conditions was negligible. As for reproduction output, under dry conditions represented by 20% WHC, and in the presence of fipronil at 1/8 of the recommended dose of application, the reproduction of F. candida suffered a 70% decrease. This data underlines the importance of taking into account soil composition in ecotoxicological assessments, especially for broadly used pesticides in tropical regions. Considering the fast pace at which climatic related events are occurring, this work also highlights the significance of including varying abiotic conditions in the assessment of chemical contamination in soils for a consistent prevision of effects, thus improving environmental protection programs.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
ABNT AB, de NT (2012) ABNT NBR ISO 16387:2012. Qualidade do solo - Efeitos de poluentes em Enchytraeidae (Enchytraeus sp.) - Determinação de efeitos sobre reprodução e sobrevivência
ABNT AB, de NT (2018) ABNT NBR ISO 11267:2019. Qualidade do solo — Inibição da reprodução de Collembola (Folsomia candida) por poluentes do solo
Alves PRL, Cardoso EJBN, Martines AM, Sousa JP, Pasini A (2014) Seed dressing pesticides on springtails in two ecotoxicological laboratory tests. Ecotoxicol Environ Saf 105:65–71. https://doi.org/10.1016/j.ecoenv.2014.04.010
Aubin S, Elbeuf L (2018) Regent ® 800 WG 1–10
Bandow C, Karau N, Römbke J (2014) Interactive effects of pyrimethanil, soil moisture and temperature on Folsomia candida and Sinella curviseta (Collembola). Appl Soil Ecol 81:22–29. https://doi.org/10.1016/j.apsoil.2014.04.010
Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EA, Noome DA, Simon-Delso N, Tapparo A (2015) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22:35–67. https://doi.org/10.1007/s11356-014-3332-7
Boscolo CNP, Felício AA, Pereira TSB, Margarido TCS, Rossa-Feres D, Almeida EA, Freitas JS (2017) Comercial insecticide fipronil alters antioxidant enzymes response and accelerates the metamorphosis in Physalaemus nattereri (Anura: Leiuperidae) tadpoles. Eur J Zool Res. 5:1–7
Braúlio Hennig T, Ogliari Bandeira F, Dalpasquale AJ, Cardoso EJBN, Baretta D, Lopes Alves PR (2020) Toxicity of imidacloprid to collembolans in two tropical soils under different soil moisture. J Environ Qual 49:1491–1501. https://doi.org/10.1002/jeq2.20143
Cenkci S, Yildiz M, Ciĝerci IH, Bozdaĝ A, Terzi H, Terzi ESA (2010) Evaluation of 2,4-D and Dicamba genotoxicity in bean seedlings using comet and RAPD assays. Ecotoxicol Environ Saf 73:1558–1564. https://doi.org/10.1016/j.ecoenv.2010.07.033
Christofoletti CA, Pereira C, Ansoar Y (2017) O emprego de agrotóxicos na cultura de cana-de-açúcar. In: Fontanetti CS, Bueno OC (Eds.), Cana-de-Açúcar e Seus Impactos : Uma Visão Acadêmica. Canal6, Rio Claro, pp. 51–61
de Amarante OP, Brito NM, dos Santos TCR, Nunes GS, Ribeiro ML (2003) Determination of 2,4-dichlorophenoxyacetic acid and its major transformation product in soil samples by liquid chromatographic analysis. Talanta 60(1):115–121. https://doi.org/10.1016/S0039-9140(03)00113-9
de Menezes Oliveira VB, de Oliveira Bianchi M, Espíndola ELG (2018) Hazard assessment of the pesticides KRAFT 36 EC and SCORE in a tropical natural soil using an ecotoxicological test battery. Environ Toxicol Chem 37(11):2919–2924. https://doi.org/10.1002/etc.4056
Domene X, Chelinho S, Campana P, Natal-da-Luz T, Alcañiz JM, Andrés P, Römbke J, Sousa P (2011) Influence of soil properties on the performance of Folsomia candida: Implications for its use in soil ecotoxicology testing. Environ Toxicol Chem 30:1497–1505. https://doi.org/10.1002/etc.533
European Commission (2009) Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC
Ferreira ALG, Serra P, Soares AMVM, Loureiro S (2010) The influence of natural stressors on the toxicity of nickel to Daphnia magna. Environ Sci Pollut Res 17:1217–1229. https://doi.org/10.1007/s11356-010-0298-y
Gunasekara AS, Truong T, Goh KS, Spurlock F, Tjeerdema RS (2007) Environmental fate and toxicology of fipronil. J Pestic Sci. https://doi.org/10.1584/jpestics.R07-02
Gupta RC, Milatovic D (2014) Insecticides, In: Gupta R (Ed.), biomarkers in toxicology. Elsevier Inc., pp. 389–407. https://doi.org/10.1016/B978-0-12-404630-6.00023-3
Højer R, Bayley M, Damgaard CF, Holmstrup M (2001) Stress synergy between drought and a common environmental contaminant: Studies with the collembolan Folsomia candida. Glob Chang Biol 7:485–494. https://doi.org/10.1046/j.1365-2486.2001.00417.x
Holmstrup M, Maraldo K, Krogh PH (2007) Combined effect of copper and prolonged summer drought on soil Microarthropods in the field. Environ Pollut 146:525–533. https://doi.org/10.1016/j.envpol.2006.07.013
IBAMA (2017) OS 10 ingredientes ativos mais vendidos
IBM Corp (2020) IBM SPSS Statistics for Windows, Version 27.0. IBM Corp, Armonk, NY
Islam F, Wang J, Farooq MA, Khan MSS, Xu L, Zhu J, Zhao M, Muños S, Li QX, Zhou W (2018) Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ Int. https://doi.org/10.1016/j.envint.2017.10.020
Jänsch S, Amorim MJ, Römbke J (2005) Identification of the ecological requirements of important terrestrial ecotoxicological test species. Environ Rev 13:51–83. https://doi.org/10.1139/a05-007
Jonker MJ, Svendsen C, Bedaux JJ, Bongers M, Kammenga JE (2005) Significance testing of synergistic/antagonistic, dose level-dependent, or dose ratiodependent effects in mixture dose-response analysis. Environ Toxicol Chem 24(10):2701–2713. https://doi.org/10.1897/04-431r.1
Krab EJ, Van Schrojenstein Lantman IM, Cornelissen JHC, Berg MP (2013) How extreme is an extreme climatic event to a subarctic peatland springtail community? Soil Biol. Biochem 59:16–24. https://doi.org/10.1016/j.soilbio.2012.12.012
Kumar S (2010) Effect of 2,4-D and Isoproturon on chromosomal disturbances during mitotic division in root tip cells of Triticum aestivum L. Cytol Genet 44:79–87. https://doi.org/10.3103/S0095452710020027
Lazar T, Taiz L, Zeiger E (2003) Plant physiology. 3rd ed., in: Annals of Botany. Sinauer Associates, pp. 750–751. https://doi.org/10.1093/aob/mcg079
Lima MP, Soares AM, Loureiro S (2011) Combined effects of soil moisture and carbaryl to earthworms and plants: simulation of flood and drought scenarios. Environ Pollut 159(7):1844–1851. https://doi.org/10.1016/j.envpol.2011.03.029
Liu F, Stützel H (2004) Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci Hortic (amsterdam) 102:15–27. https://doi.org/10.1016/j.scienta.2003.11.014
Luiz Lonardoni Foloni (2016) O herbicida 2,4-D. Uma visão geral, 1st edn. LabcomTotal. LabcomTotal, Ribeirão Preto
Marcato AC, Souza C, Fontanetti CP (2017) Herbicide 2,4-D: A Review of Toxicity on Non-Target Organisms. Water Air Soil Pollut. https://doi.org/10.1007/s11270-017-3301-0
Menezes-Oliveira VB, Scott-Fordsmand JJ, Soares AMVM, Amorim MJB (2014) Development of ecosystems to climate change and the interaction with pollution—Unpredictable changes in community structures. Appl Soil Ecol 75:24–32. https://doi.org/10.1016/j.apsoil.2013.10.004
Natal-Da-Luz T, Römbke J, Sousa JP (2008) Avoidance tests in site-specific risk assessment - Influence of soil properties on the avoidance response of Collembola and earthworms. Environ Toxicol Chem 27:1112–1117. https://doi.org/10.1897/07-386.1
Nogueira Cardoso EJB, Lopes Alves PR (2012) Soil ecotoxicology, in: ecotoxicology. IntechOpen https://doi.org/10.5772/28447
Pinto TJDS, Rocha GS, Moreira RA, Silva LCMD, Yoshii MPC, Goulart BV, Montagner CC, Daam MA, Espindola ELG (2021a) Multi-generational exposure to fipronil, 2,4-D, and their mixtures in Chironomus sancticaroli: Biochemical, individual, and population endpoints. Environ Pollut 283:117384. https://doi.org/10.1016/j.envpol.2021.117384
Pinto TJDS, Moreira RA, Silva LCMD, Yoshii MPC, Goulart BV, Fraga PD, Montagner CC, Daam MA, Espindola ELG (2021b) Impact of 2,4-D and fipronil on the tropical midge Chironomus sancticaroli (Diptera: Chironomidae). Ecotoxicol Environ Saf 209:111778
Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Mcfield M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, Van Der Sluijs JP, Van Dyck H, Wiemers M (2014) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res 22:68–102. https://doi.org/10.1007/s11356-014-3471-x
Rodríguez-Cruz M, Sánchez-Martín M, Andrades M, Sánchez-Camazano M (2006) Comparison of pesticide sorption by physicochemically modified soils with natural soils as a function of soil properties and pesticide hydrophobicity. Soil Sediment Contam 15:401–415. https://doi.org/10.1080/15320380600751769
Römbke J, García M (2000) Assessment of Ecotoxicological effects of pesticides on the soil fauna and soil processes under tropical conditions. Proc Ger. 1999:543–549
Schneider C, Rasband W, Eliceiri K (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Silva LC, da Moreira RA, Rocha O (2012). A Toxicidade Aguda Do Agrotóxico Fipronil Para O Cladócero Bosmina Freyi De Melo and Hebert, 1994. Periódico Eletrônico Fórum Ambient. da Alta Paul. 8, 384–394. https://doi.org/10.17271/19800827822012267
Singh A, Srivastava A, Srivastava PC (2016) Sorption-desorption of fipronil in some soils, as influenced by ionic strength, pH and temperature. Pest Manag Sci 72:1491–1499. https://doi.org/10.1002/ps.4173
Skovlund G, Damgaard C, Bayley M, Holmstrup M (2006) Does lipophilicity of toxic compounds determine effects on drought tolerance of the soil collembolan Folsomia candida? Environ Pollut 144:808–815. https://doi.org/10.1016/j.envpol.2006.02.009
Song Y (2014) Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol 56(2):106–113. https://doi.org/10.1111/jipb.12131
Spark KM, Swift RS (2002) Effect of soil composition and dissolved organic matter on pesticide sorption. Sci Total Environ 298:147–161. https://doi.org/10.1016/S0048-9697(02)00213-9
Trenberth KE (2011) Changes in precipitation with climate change. Clim Res 47:123–138. https://doi.org/10.3354/cr00953
Triques MC, Oliveira D, Goulart BV, Montagner CC, Espíndola ELG, de Menezes-Oliveira VB (2021) Assessing single effects of sugarcane pesticides fipronil and 2,4-D on plants and soil organisms. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2020.111622
Van Gestel CAM, Mol S (2003) The influence of soil characteristics on cadmium toxicity for Folsomia candida (Collembola: Isotomidae). Pedobiologia (jena) 47:387–395. https://doi.org/10.1007/s00128-016-1792-9
Van Gestel CAM, Van Diepen AMF (1997) The influence of soil moisture content on the bioavailability and toxicity of cadmium for Folsomia candida Willem (Collembola: Isotomidae). Ecotoxicol Environ Saf 36:123–132. https://doi.org/10.1006/eesa.1996.1493
Waalewijn-Kool PL, Rupp S, Lofts S, Svendsen C, van Gestel CAM (2014) Effect of soil organic matter content and pH on the toxicity of ZnO nanoparticles to Folsomia candida. Ecotoxicol Environ Saf 108:9–15. https://doi.org/10.1016/j.ecoenv.2014.06.031
Williams AP, Abatzoglou JT, Gershunov A, Guzman-Morales J, Bishop DA, Balch JK, Lettenmaier DP (2019) Observed impacts of anthropogenic climate change on wildfire in California. Earth's Future 7:892–910. https://doi.org/10.1029/2019EF001210
Ying GG, Kookana RS (2001) Sorption of fipronil and its metabolites on soils from South Australia. J Environ Sci Heal Part B Pesdtic Food Contam Agric Wastes 36:545–558. https://doi.org/10.1081/PFC-100106184
Zortéa T, da Silva AS, dos Reis TR, Segat JC, Paulino AT, Sousa JP, Baretta D (2018a) Ecotoxicological effects of fipronil, neem cake and neem extract in edaphic organisms from tropical soil. Ecotoxicol Environ Saf 166:207–214. https://doi.org/10.1016/j.ecoenv.2018.09.061
Zortéa T, dos Reis TR, Serafini S, de Sousa JP, da Silva AS, Baretta D (2018b) Ecotoxicological effect of fipronil and its metabolites on Folsomia candida in tropical soils. Environ Toxicol Pharmacol 62:203–209. https://doi.org/10.1016/j.etap.2018.07.011
Acknowledgements
Thanks are due to Dr. Luiz Antonio Martinelli, Dr. Janaina Braga do Carmo and Dr. Leonardo Machado Pitombo for their technical contribution to the measurement of the physical-chemical parameters of the natural soil used. The authors also thank the Brazilian coordination of Superior Level Staff Improvement (CAPES) for the master’s degree scholarship conceded and the São Paulo Research Foundation (FAPESP) for the financial support conceded via the thematic project “Environmental Effects of the Pasture-Sugarcane Conversion and Pasture Intensification” (Process: 2015/187903) and the post-doctoral grant (Process PDJ: 2017/04858-0).
Funding
This work was supported by the Brazilian coordination of Superior Level Staff Improvement (CAPES) through a master degree scholarship attributed to Maria Carolina Triques and by the São Paulo Research Foundation (FAPESP) through financial support conceded via the thematic project “Environmental Effects of the Pasture-Sugarcane Conversion and Pasture Intensification” (Process: 2015/187903) and through post-doctoral grant conceded to Vanessa Bezerra de Menezes-Oliveira (Process PDJ: 2017/04858–0).
Author information
Authors and Affiliations
Contributions
MCT conceptualization, methodology, formal analysis, investigation, and writing (Original draft); FR investigation, formal analysis, writing and editing, (Original draft); DO conceptualization, methodology, formal analysis, and investigation; BVGt methodology and investigation; CCM article investigation and resources; ELGE investigation, resources, and supervision; VB de M-O article conceptualization, methodology, formal analysis, investigation, writing (Original draft and review), resources and supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Triques, M.C., Ribeiro, F., de Oliveira, D. et al. The Ecotoxicity of Sugarcane Pesticides to Non-target Soil Organisms as a Function of Soil Properties and Moisture Conditions. Int J Environ Res 16, 61 (2022). https://doi.org/10.1007/s41742-022-00433-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-022-00433-6