Abstract
The effects of exogenous thiocyanate (SCN−) on amino acids composition, content of mineral nutrients and antioxidative systems in plants were investigated. Young rice seedlings (Oryza sativa L. cv. XZX 45) were grown in nutrient solutions amended with potassium thiocyanate (KSCN). Activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) in plant materials were analyzed in vivo. Mineral nutrients and free amino acids in rice seedlings were also measured to determine metabolic responses to SCN− exposure. A significant reduction in transpiration and relative growth was recorded with all treatments (p < 0.05), while changes of total chlorophyll content in leaves was negligible (p > 0.05). SCN-induced toxicity appeared to be more sensitive to activities of POD in shoots and APX activities in roots than the others. The content of nutrient elements in rice seedlings exposed to exogenous SCN− was variable, while the effects were more evident at the highest SCN-treatment (p < 0.05). Although the change of total free amino acids in shoots of SCN-exposed seedlings was negligible (p > 0.05), responses of different amino acids to SCN− application were quite different. Among fifteen free amino acids detected, serine (Ser), proline (Pro), and methionine (Met) increased, while asparagine (Asp) decreased with an increase of the doses of SCN− supplied. Phyto-transport of SCN− was apparent and the removal rates were positively correlated to the doses, suggesting that phyto-assimilation of SCN− is an enzymatic process through a potentially un-identified degradation pathway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiocyanate (SCN−) can be naturally produced in plant cells during detoxification of free cyanide through the sulfur transferase pathway catalyzed by the enzyme rhodanese (EC 2.8.1.1) (Miller and Conn 1980; Boening and Chew 1999; Sorokin et al. 2001). Damaged and decaying tissues of Brassica spp. is another natural source of SCN−, in which indole and p-hydroxybenzyl contained in plant tissues are hydrolyzed by thioglucoside glucohydrolyase (EC 3.2.3.1) to produce unstable isothiocyanate intermediates which spontaneously form SCN− (Brown and Morra 1996). The natural occurring SCN− is typically maintained at very low levels, which did not affect the health of environment. However, anthropogenic release of SCN− to the environment is greater in amounts and intensity than natural production. SCN− is widely used in several industries: photofinishing, herbicide and insecticide production, dyeing, acrylic fiber production, manufacturing of thiourea, metal separation and electroplating (Hung and Pavlostathis 1998; Jeong and Chung 2006). The main sources of SCN− into the environment are wastewater discharged from coal conversion process (Jeong and Chung 2006). It is observed that SCN− occurs as a primary constituent in industrial coke-oven wastewater at concentrations of 50–650 mg/L (Chakraborty and Veeramani 2006).
Although SCN− is deliberately produced as a less toxic form of cyanide, a number of deleterious effects of SCN− on various animals have been documented (Watson and Maly 1987; Speyer and Raymond 1988; Bhunia et al. 2000). It is known that its strong binding tendency to protein has been considered to be the major contributor of SCN− toxicity in living organisms (Wood et al. 1998). Inhibition of halide transport to the thyroid gland, stomach, cornea and gills of fish by exogenous SCN− was observed (Katz et al. 1982; Heming et al. 1985). It also acts as non-competitive inhibitors to block a variety of enzymatic reactions (Wood et al. 1998; Lee et al. 2008). Additionally, SCN− affects the nervous system in humans, causing irritation, nervousness, hallucinations, psychosis, mania, delirium and convulsions (Boening and Chew 1999). SCN− has specific antithyroidal properties, and the bioaccumulation of SCN− has been implicated as a possible etiologic factor in the alteration of thyroid function and the development of goiter in various mammals (Lanno and Dixon 1996).
Recently, our preliminary study showed that over exposure of an exogenous SCN− resulted in reduced growth and transpiration rate of rice seedlings (Yu et al. 2012b). However, nothing is known yet about antioxidative responses of plants to SCN−. Reactive oxygen species (ROS) in plants are generated as intermediates of a number of metabolic reactions in cellular organelles (Dixit et al. 2002). It is evident that numerous biotic and abiotic stresses have been found to be able to stimulate the formation and accumulation of ROS. High ROS levels in plant cells can result in the damage of DNA, proteins, and pigments as well as can initiate lipid peroxidation (Panda and Khan 2003). Adequate defense against oxygen toxicity requires efficient scavenging of ROS e.g., superoxide radicals, hydrogen peroxide and hydroxyl radical (Tsang et al. 1991). Toxicity of superoxide radicals has been attributed to its interaction with hydrogen peroxide to form highly reactive hydroxyl radicals, which are thought to be largely responsible for mediating oxygen toxicity in vivo (Fridovich 1978). The enzyme antioxidant components e.g., superoxide dismutase (SOD, EC 1.1.5.1.1), peroxidase (POD, EC 1.11.1.7), catalase (CAT, EC 1.11.1.6), and ascorbate peroxidase (APX, EC 1.11.1.11) can neutralize free radicals and may reduce or even help in prevention of potential damage (Apel and Hirt 2004). Amino acids also play an important role in plant stress tolerance via regulating intracellular pH and ion transport, modulating stomatal conductance, and detoxifying ROS (Rai 2002). In this work, effects of exogenous SCN− on amino acids, mineral nutrients, and antioxidative systems in rice seedlings were evaluated to compare the toxic effects.
Materials and methods
Plant materials and experimental design
Plant materials and the exposure regime were identical to our previous work (Yu et al. 2012a, b). Fifteen-day old rice seedlings (Oryza sativa L. cv. XZX 45) with similar height and weight was transplanted to a pre-treatment solution containing 1 mM CaCl2 + 2 mM MES-Tris buffer (pH 6.0) for 4 h to clear the cell wall space of ions (Ebbs et al. 2008), after that ten rice seedlings were transferred into a 50 mL Erlenmeyer flask filled with 50 mL modified ISO 8692 nutrient solution (Yu et al. 2012a, b) with addition of 10 μM Fe-EDTA. The plants were first conditioned for 24 h for acclimatization in new environmental condition. The flasks were all wrapped with aluminum foil up to the flask mouth to prevent evaporation, and to inhibit potential growth of algae inside. All flasks were kept in a plant growth chamber with constant temperature of 25 ± 0.5 °C and a relative humidity of 60 ± 2 % under continuous artificial light. Nutrient solution in each flask was replaced by spiked solution, except for the controls. Potassium thiocyanate (KSCN) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, PR China), analytical grade with ≥98.5 % purity were used for the treatments. Six different concentrations were used as treatment groups. Twelve replicates were prepared for each treatment concentration, and all measured parameters were conducted in three replicates with exposure duration of 120 h.
The phytotoxicity determination of SCN− was quantified by measuring transpiration and biomass growth of rice seedlings. The weigh loss of the plant-flask system was expressed as the transpiration (g/d). Relative growth (%) was calculated from the weight change of individual rice seedlings with respect to the initial weight. Total chlorophyll content in shoots of rice seedlings were also measured (Yu et al. 2007).
Determination of nutrient elements
The plant materials from the treated and non-treated rice seedlings were collected at the termination of experiments and rinsed with deionized water. Plant materials were dried at 90 °C for 48 h and mixed with 8 mL of 1:1 HNO3–HClO4 solution for overnight. The samples were then place in a digestion block and heated for 2 h at 200 °C until the digested liquid was clear. The cooled residue was dissolved in 5 mL of 5 % HNO3 and deionized water was added up to 25 mL of total volume (Wang et al. 2009). The content of K, Na, Cu, Zn, Fe, Mn and Mg in plant tissues was analyzed by inductively-coupled plasma atomic emission spectrometry (ICP-AES).
Measurement of enzyme activities
In vivo enzyme measurement in different plant materials was carried out after exposures according to the methods described by Li (2006). Plant tissues (0.2 g, fresh weight) was precisely weighted and homogenized in a triturator with 1.8 mL prechilled extraction medium (pH 7.8, containing NaH2PO4, Na2HPO4, PVPP, EDTA and mercapto-ethanol). Trituration was ground in liquid N2, and centrifuged at 15,000×g for 15 min at 4 °C. The supernatant was collected and analyzed. Each enzyme assay was analyzed in three separate tubes. Assays of SOD, POD and CAT and APX activities were performed as described previously (Yu et al. 2007; Boominathan and Doran 2002).
Measurement of free amino acids
Free amino acids in leaves were quantified by the method described previously (Di Martino et al. 2003; Wang et al. 2007). Plant tissues (0.2 g, fresh weight) from the treated and non-treated rice seedlings were ground in liquid N2, homogenized with 1.8 mL cooled ethanol (80 %, v/v), and left for 10 min. The homogenate was collected and then centrifuged at 12,000×g for 15 min at 4 °C. The supernatants were pooled and used for analyses.
Chemical analysis
The concentrations of SCN− in solutions were determined spectrophotometrically by a standard method (State Environmental Protection Administration of China, 1989, method number GB 7487-87). One to five milliliters of aliquot solution samples were pipetted into a 25-mL colorimetric cylinder (depending on the concentrations of SCN− in solution), and 0.2 mL HNO3 solution (1:1) was added. After that, 0.5 mL of 0.21 M Fe(NO3)3 color reagent solution (Dissolve 41.725 g Fe(NO3)3·9H20 in about 250 mL deionized water, and add 12.5 mL concentrated HNO3, finally dilute with deionized water to 500 mL) was introduced. The content was diluted with deionized water to 25 mL and mixed thoroughly. It should be noted that the pH of solution was adjusted to 1.0–2.0 with HNO3 solution (1:1). The absorption of light at 460 nm was quickly measured in a cuvette with an optical path of 10 mm against deionized water as a reference.
Mean measured initial concentrations of SCN− in treatments spiked with KSCN: 0, 20.15(SD: 0.90), 40.30 (SD: 2.05), 80.38 (SD: 5.97), 140.04 (SD: 4.78), 180.34 (SD: 7.39).
Calculation of the botanical removal rate of thiocyanate by rice seedlings was identical to our previous work (Yu et al. 2012b).
Statistical methods
Analysis of variance (ANOVA) and Tukey’s multiple range tests was carried out to determine the statistical significance at 0.01 or 0.05 between the treatments (Sachs 1992). The partial correlation was used to determine whether a relationship between two variables was due to a common correlation to a third variable, with the equation
where r xy.z is the partial correlation coefficient between variables x and y under the assumption of a constant variable z, and rxy is the bivariate Pearson correlation coefficient between variables x and y etc.
Results
Responses of rice seedlings to exogenous SCN−
The changes of selected parameters of rice seedlings exposed to SCN− are shown (Table 1). All rice seedlings showed positive growth, however a remarkable decline trend in relative growth and transpiration rate of rice seedlings was observed in treatments with increased SCN− concentrations (p < 0.05). Visible toxic symptoms of chlorosis were not found in any of the treatments at the termination of 120 h exposure. The change in total chlorophyll content was negligible in shoots of SCN-exposed rice seedlings at lower than 180.34 mg SCN/L (p > 0.05), compared with seedlings in the absence of SCN−.
Content of nutrient elements in plant materials related to SCN−
The content of nutrient elements in rice seedlings exposed to exogenous SCN− was variable within treatments significantly (Table 2). The content of K in both roots and shoots of treated rice seedlings was significantly higher than non-treated plants (p < 0.01). It is also obvious that, in both control and treated rice seedlings, roots accumulated more K than shoots (p < 0.05). Na content in roots gradually decreased with an increase of SCN−, while a significant reduction was only observed with rice seedlings exposed to SCN− at higher than or equal to 180.34 mg SCN/L. No detectable effect was observed on the content of Na in leaves. When exposed to the highest treatment of SCN−, Mg content decreased significantly in both roots and shoots (p < 0.05), compared with control. Treated rice seedlings accumulated more Fe and Mn in plant materials than non-treated plants. More Fe was detected in roots of SCN-exposed rice seedlings than control, while the application of SCN− showed a negligible effect on Fe accumulation in shoots. Compared with non-treated plants, SCN-exposed rice seedlings were able to accumulate more Mn in shoots rather than roots. Accumulation of Cu and Zn was not significantly affected by SCN−.
Oxidative status of rice seedlings exposed to exogenous SCN−
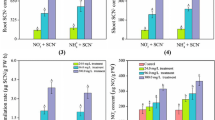
In both control and treated rice seedlings, significantly higher SOD activities were observed in shoots than roots (Fig. 1a). Although an increasing trend in SOD activities was recorded in plant materials with an increase of SCN− application, a significant increase was only observed with rice seedlings exposed to SCN− at 180.34 mg SCN/L (p < 0.05), compared with non-treated rice seedlings. It is interesting to note that exogenous SCN− had slightly higher positive effect on SOD activity in shoots than roots, but the difference was not significant (p > 0.05). Activities of CAT (Fig. 1b), POD (Fig. 1c) and APX (Fig. 1d) in both shoots and roots of seedlings were elevated with an increase of exogenous SCN−, however remarkable increase were only observed in 180.34 mg SCN/L treatment (p < 0.05).
a Measured SOD activity in shoots (upper part) and roots (lower part) of rice seedlings exposed to SCN−. The exposure period was 120 h. The values are mean of three individual replicates. Vertical lines represent standard deviation. Asterisk symbol refers to the significance difference between KSCN treatments and control (p < 0.05). b Measured CAT activity in shoots (upper part) and roots (lower part) of rice seedlings exposed to SCN−. The exposure period was 120 h. The values are mean of three individual replicates. Vertical lines represent standard deviation. Asterisk symbol refers to the significance difference between KSCN treatments and control (p < 0.05). c Measured POD activity in shoots (upper part) and roots (lower part) of rice seedlings exposed to SCN−. The exposure period was 120 h. The values are mean of three individual replicates. Vertical lines represent standard deviation. Asterisk symbol refers to the significance difference between KSCN treatments and control (p < 0.05). d Measured APX activity in shoots (upper part) and roots (lower part) of rice seedlings exposed to SCN−. The exposure period was 120 h. The values are mean of three individual replicates. Vertical lines represent standard deviation. Asterisk symbol refers to the significance difference between KSCN treatments and control (p < 0.05)
Accumulation of amino acids in shoots of rice seedlings exposed to exogenous SCN−
Fifteen free amino acids were detected in shoots of rice seedlings exposed to exogenous SCN− (Table 3). The most abundant free amino acids in shoots of non-treated rice seedlings were asparagine (Asp), threonine (Thr), serine (Ser), glutamine (Glu), alanine (Ala), which accounted for more than 60 % of the total free amino acids detected. The pools of other amino acids (proline-Pro, glycine-Gly, valine-Val, isoleucine-Ile, leucine-Leu, Tyrosine-Tyr, phenylalanine-Phe, lysine-Lys and arginine-Arg) were approximate 34 % of overall. Only traces of methionine (Met) were found. Content of Thr, Glu, Ala, Val, Ile, Leu, Tyr, Phe, Lys, Arg and total amino acids in shoots of rice seedlings was not significantly affected by SCN− exposure (p > 0.05), compared with non-treated plants. Ser, Pro, and Met increased, while Asp decreased in the SCN-treatments. A remarkable difference in Asp content was observed with rice seedlings exposed to SCN− at 140.04 mg SCN/L or higher (p < 0.05). Measurable accumulation of Gly was detected in shoots of SCN-exposed rice seedlings, however no clear relationship between SCN doses applied and Gly content was observed.
Botanical removal of SCN− from hydroponic solution by rice seedlings
The removal rates of SCN− from the rice seedling growth media are presented in Fig. 2. Amount of applied SCN− in hydroponic solution was removed by the presence of rice seedlings. More than 50 % of SCN− added in the plant growth media was removed by plants over a period of 120 h exposure. SCN− uptake rate by plants was highly correlated to SCN− concentrations (the linear trend line and the R-square value are given), which agreed with our previous work (Yu et al. 2012b).
Discussion
Phytotoxicity reduced plant growth which has been related to physiological changes due to over accumulation of ROS in cellular organelles (Scandalios 1993). ROS scavenging can be achieved by antioxidative enzyme systems in plants. In our observation, activities of all selected enzymes were elevated with an increase of exogenous SCN−, however remarkable increase were only observed in the treatment of 180.34 mg SCN/L (p < 0.05), compared with control, indicating non-deleterious effects on rice seedlings due to SCN− exposure. Results of all enzymatic assays measured were plotted and analyzed (data not shown). All linear trends were significant, judged by the critical r for a given n (α = 0.05) (Sachs, 1992). Activities of POD in shoots (r 2 = 0.985) and APX activities in roots (r 2 = 0.984) were more sensitive to the changes in SCN− than the others, respectively.
Nutrient elements have different roles in plant growth, development and yield. For example, Mg is a key component of chlorophyll, and Cu, Fe and Mn play a vital role in synthesis or stability of chlorophyll (Wang et al. 2009). Zn is the only metal represented in several electron transport enzymes (Broadley et al. 2007). K is an important enzyme activator involved in synthesis of protein and sugar, and also functions in osmotic modulation in plants (Rai 2002). It is evident that excess of heavy metals usually affects mineral nutrient homeostasis, which results from the effects on availability, absorption and transport of nutrients within plants (Ali et al. 2002). Slight change in the content of Cu and Zn in shoots of rice seedlings exposed to SCN− was observed, while significant changes in the content of other nutrient elements were detected in higher doses of SCN− supplied. The content of assimilation of pigments is a principal parameter responsible for photosynthesis and directly reflects physiological status. However, significant influence on the chlorophyll content was not observed in any of the treatments, indicating that exogenous SCN− did not cause severe effects on rice seedlings.
It has been found that the synthesis and accumulation of low molecular weight metabolites, such as free amino acids, is an ubiquitous mechanism for reducing various biotic and abiotic stresses in plants (Di Martino et al. 2003; Cuin and Shabala 2007). Although a slight change of total amino acids in shoots of rice seedlings was observed, individual amino acids showed different responses to exogenous SCN− in our observation. Among the amino acids observed, majority showed negligible changes to the application of SCN−, more than likely due to its less adverse effect. In order to find out the most sensitive species of amino acids involved in phytotoxicity of SCN− to rice seedlings, it is interesting to make a comparison between sensitive species (Asp, Ser, Pro and Met). All linear trends were significant (data not shown), judged by the critical r for given n (α = 0.05). The best correlation was obtained for Met content in shoots (r 2 = 0.978). The susceptibility of these free amino acids to change of SCN-exposure was in the order: Met > Ser > Pro > Asp.
Phytohormones ethylene can be naturally produced by all plants from Met and in trace amounts elicits many physiological responses (Yang and Hoffman 1984). Met is an S-containing amino acid. A positive relationship between Met content in shoots and exogenous SCN− supplied was found in this study. Therefore, we have a good reason to propose that 1) Met may be a possible intermediate or final product of botanical assimilation of SCN−; 2) botanical assimilation of SCN− may stimulate the production and accumulation of Met in shoots of rice seedlings. Ser has been considered as precursors of nucleic acids (Rai 2002). Accumulation of Ser in plants has been reported under osmotic stress in maize cultivars (Handa et al. 1983). Pro, one of the most common N-containing compatible solutes in plants is responsible for stabilizing both the quaternary structure of proteins and membranes against the adverse effects (Sakamoto and Murata 2000). Plants engineered to synthesis Pro also showed an increase protection from photoinhibition or from ROS (Hasegawa et al. 2000). Abundant literature shows that heavy metals considerably stimulated increase in Pro, which is largely related to an increase of nitric oxide (NO) content in plant (Zhang et al. 2008). It was not surprising to note that an obvious increase trend in Pro content in shoots of rice seedlings exposed to SCN− was observed (y = 0.016x +12.1, r 2 = 0.944, significant at α = 0.05). It is known that Asp plays central role in N storage and transport in plants (Lea et al. 2007). Accumulation in plant materials under water stress has been reported (Costa and Morel 1994). A measureable decrease in Asp content in SCN-treated rice seedlings was observed, suggesting that the exogenous SCN− may disturb N metabolism and transport in rice seedlings. Indeed, a remarkable decreasing trend in relative growth of plants was also noted (p < 0.05). In the family of free amino acids, Gly and Glu are fundamental metabolites involved in the process of chlorophyll synthesis (Zhang et al. 2012). In this current work, more or less changes in the content of both amino acids were observed with all treatments, suggesting that exogenous SCN− did not cause deleterious effects on rice seedlings. Data from total chlorophyll content in shoots also provide additional evidence to support this conclusion, where no significant decrease occurred in total chlorophyll in shoots due to the application of SCN− (p > 0.05).
Detoxification of SCN− has been identified in various microorganisms (Hung and Pavlostathis 1998; Sorokin et al. 2001; Kwon et al. 2002; Lee et al. 2008). Carbonyl sulfide (COS)-mediated conversion of SCN− by soil microorganisms has been proposed (Bremner and Steele 1978), where thiocyanate hydrolase (SCNase) catalyzes hydrolysis of the nitrile bond of SCN−, with the formation COS and ammonia (Bezsudnova et al. 2007; Arakawa et al. 2007). A part of sulfur moiety of SCN− degraded has been found as COS during the degradation of SCN− by T. thioparus TH115, and the amounts of COS produced vary considerably, depending on the culture (Katayama et al. 1993; Kim and Katayama 2000). Stratford et al. (1994) also described another possible pathway for the degradation of SCN−, in which cyanate and sulfide are produced by a heterotrophic bacterium strain 26B, when SCN− serves as the sole of either nitrogen and/or sulfur. In the second enzymatic step, the cyanate undergoes further hydrolysis to form ammonia and carbon dioxide in the presence of the enzyme cyanase (EC 4.3.99.1) (Kelly and Baker 1990). It is evident that SCN− occurs ubiquitously in plants (Katayama et al. 2006). Two different sources of endogenous SCN− have been observed in different species of plants (Miller and Conn 1980; Brown and Morra 1996; Boening and Chew 1999; Sorokin et al. 2001). The organisms carrying thiocyanate-degrading ability are considered to be distributed widely (Katayama et al. 2006). In this study, phyto-transport of SCN− was apparent and removal rates were positively correlated to the doses supplied. This raises the hypothesis that plants would be able to metabolize SCN− in vivo. Known that SCN− is a toxic anion. If botanical assimilation of SCN− does not occur, plants should accumulate more SCN− within plant materials. Indeed, visible toxic symptoms were not observed in any of the treatments, suggesting that accumulation of SCN− is not a major sink involved in phyto-assimilation of SCN− and phyto-degradation of SCN− is suggestive. Further in-depth investigations are needed to elucidate the detailed specific mechanism and the degradation pathway of SCN− involved in plants.
The variation in SCN− application affects both kinetics of phyto-removal and transpiration rate. Subsequently, the observed change of SCN− in hydroponic solution could either be due to an increase in uptake of compound into root cells together with the transpiration of water, or by diffusion, or due to an increase of metabolism. Both correlations between the phyto-removal rate and the initial SCN− concentrations r 2 = 0.982, significant at α = 0.01), and between the initial SCN− concentrations and the transpiration rate were significant (r 2 = 0.810, significant at α = 0.05). Additionally, the correlation between the phyto-removal rate and transpiration rate was remarkable (r 2 = 0.761, significant at α = 0.05). However a partial correlation between SCN− supply, removal rate and transpiration rate unveiled that the correlation between initial SCN− concentrations and the removal rate, assuming transpiration a constant, would still be 0.934 (significant at α = 0.01). On the other hand, the partial correlation between the removal rate and transpiration rate (assuming SCN− supply a constant) is weaker (r 2 = 0.114) and insignificant (significant at α = 0.05). It can be concluded that the uptake of SCN− from the solution is highly dependent on SCN− application rather than transpiration rate, suggesting that phyto-removal of SCN− is an enzymatic process rather than a passive process.
Conclusions
Although visible toxic symptoms of chlorosis were not observed in any of the treatments, hydroponically-grown rice seedlings showed remarkable decline in relative growth and transpiration with increased SCN− concentrations. All measured antioxidative enzyme activities in plant materials were positively correlated to the doses of SCN− supplied, however the effect was more evident at the highest SCN-treatment. The content of nutrient elements in SCN-exposed plants was variable. Among free amino acids observed, Met in shoots was the most sensitive indicator for the plants exposed to SCN− application. Phyto-uptake of SCN− is a relevant sink process involved in the botanical assimilation of SCN−.
References
Ali NA, Bemal MP, Ater M (2002) Tolerance and bioaccumulation of copper in Phragmites asutralis and Zea mays. Plant Soil 239:103–111
Apel K, Hirt H (2004) Reactive oxygen species: metabolism oxidative stress, and signaling transduction. Annu Rev Plant Biol 55:373–399
Arakawa T, Kawano Y, Kataoka S, Katayama Y, Kamiya N, Yohda M, Odaka M (2007) Structure of thiocyanate hydrolase: a new nitrile hydratase family protein with a novel five-coordinate cobalt(III) center. J Mol Biol 366:1497–1509
Bezsudnova EY, Sorokin DY, Tikhonova TV, Popov VO (2007) Thiocyanate hydrolase, the primary enzyme initiating thiocyanate degradation in the novel obligately chemolithoautotrophic halophilic sulfur-oxidizing bacterium Thiohalophilus thiocyanoxidans. Biochem Biophys Acta 23:1563–1570
Bhunia F, Saha NC, Kaviraj A (2000) Toxicity of thiocyanate to fish, plankton, worm and aquatic system. Bull Environ Contam Toxicol 64:197–204
Boening DW, Chew CM (1999) A critical review: general toxicity and environmental fate of three aqueous cyanide ions and associated ligands. Water Air Soil Pollut 109:67–79
Boominathan R, Doran M (2002) Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol 156:205–215
Bremner JM, Steele CG (1978) Role of microorganisms in the atmospheric sulfur cyanide. Adv Microb Ecol 2:155–201
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Brown PD, Morra MJ (1996) Hydrolysis products of glucosinolates in Brassica napus Tissues as inhibitors of seed germination. Plant Soil 181:307–316
Chakraborty S, Veeramani H (2006) Effect of HRT and recycle ratio on removal of cyanide, phenol, thiocyanate and ammonia in an anaerobic-anoxic-aerobic continuous system. Process Biochem 41:96–105
Costa G, Morel JL (1994) Water relations, gas exchange and amino acid content in Cd-treated lettuce. Plant Physiol Biochem 32:561–570
Cuin TA, Shabala S (2007) Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 225:753–761
Di Martino C, Delfine S, Pizzuto R, Loreto F, Fuggi A (2003) Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol 158:455–463
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactive electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L. cv. Azad) root mitochondria. Plant Cell Environ 25:687–693
Ebbs SD, Piccinin RC, Goodger JQD, Kolev SD, Woodrow IE, Baker AJM (2008) Transport of ferrocyanide by two eucalypt species and sorghum. Int J Phytorem 10:343–357
Fridovich I (1978) The biology of oxygen radical. Science 39:522–526
Handa S, Bressan RA, Handa AK, Caprita NC, Hasegawa PM (1983) Solutes contributing to osmotic adjustments in cultured cells adapted to water stress. Plant Physiol 73:834–843
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 55:373–399
Heming TA, Thurston RV, Meyn EL, Zajdel RK (1985) Acute toxicity of thiocyanate on trout. Trans Am Fish Soc 114:895–905
Hung CH, Pavlostathis SG (1998) Fate and transformation of thiocyanate and cyanate under methanogenic conditions. Appl Microbiol Biotechnol 49:112–116
Jeong YS, Chung JS (2006) Biodegradation of thiocyanate in biofilm reactor using fluidized-carriers. Process Chem 41:701–707
Katayama Y, Kanagawa T, Kuraishi H (1993) Emission of carbonyl sulfuide by by Thiobacilius Thioparus grown with thiocyanate in pure and mixed cultures. FEMS Micorbiol Let 114:223–228
Katayama Y, Hashimoto K, Nakayama H, Mino H, Nojiri M, Ono TA, Nyunoya H, Yohda M, Takio K, Odaka M (2006) Thiocyanate hydrolyase is a cobalt-containing metalloenzyme with a cysteine-sulfinic acid ligand. J Am Chem Soc 128:728–729
Katz U, Lau KR, Ramos MMP, Elloy JC (1982) Thiocyanate transport across fish intestine (Pleuronectes platessa). J Membrane Biol 66:9–14
Kelly DP, Baker SC (1990) The organosulfur cycle: aerobic and anaerobic process leading to the turnover of C1-sulfur compounds. FEMS Microbiol Rev 87:223–228
Kim SJ, Katayama Y (2000) Effect of growth conditions on thiocyanate degradation and emission of carbonyl sulfide by Thiobacilius thioparus TH115. Water Res 34:2887–2894
Kwon HK, Woo SH, Park JM (2002) Thiocyanate degradation by Acremonium strictum and inhibition by secondary toxicants. Biotechnol Let 24:1347–1351
Lanno RP, Dixon DG (1996) The comparative chronic toxicity of thiocyanate and cyanide to rainbow trout. Aquatic Toxicol 36:177–187
Lea PJ, Sodek L, Parry MAJ, Shewry PR, Halford NG (2007) Asparagine in plants. Ann Appl Biol 150:1–26
Lee C, Kim J, Do H, Hwang S (2008) Monitoring thiocyanate-degrading microbial community in relation to changes in process performance in mixed culture systems near washout. Water Res 42:1254–1262
Li HS (2006) Principles and techniques of plant physiology and biochemistry. Higher Education, Beijing In Chinese
Miller JM, Conn EE (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65:1199–1202
Panda SK, Khan MH (2003) Antioxidant efficiency in rice (Oryza sativa L.) leaves under heavy metal toxicity. J Plant Biol 30:23–29
Rai VK (2002) Role of amino acids in plant responses to stresses. Biol Plant 45:481–487
Sachs L (1992) Angewandte Statistik. Springer, Berlin
Sakamoto A, Murata N (2000) Genetic engineering of glycinebetaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot 51:81–88
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7–12
Sorokin DY, Tourova TP, Lysenko AM, Kuenen JG (2001) Microbial thiocyanate utilization under highly alkaline conditions. Appl Environ Microbiol 67:528–538
Speyer MR, Raymond P (1988) The acute toxicity of thiocyanate and cyanate to rainbow trout as modified by water temperature and pH. Environ Toxicol Chem 7:565–571
Stratford J, Dias AEXO, Knowles CJ (1994) The utilization of thiocyanate as a nitrogen source by a heterotrophic bacterium: the degradative pathway involves formation of ammonia and tetrathionate. Microbiol 140:2657–2662
Tsang E, Bowler C, Herouart D, Villarroel R, Genetello C, Inze D (1991) Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell 3:783–792
Wang JF, Dong CX, Shen QR (2007) Effect of ammonium/nitrate ratios on the free amino acids and three kinds of enzymes of nitrogen metabolism in spinach shoot. Plant Nutr Fertilizer Sci 13:664–670 (In Chinese)
Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W, Lin ZP (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75:1469–1476
Watson SJ, Maly EJ (1987) Thiocyanate toxicity to Daphina magna: modified by pH and temperature. Aquat Toxicol 10:1–8
Wood PA, Kelly PD, McDonald RI, Jordan LS, Morgan DT, Khan S, Murrell JG, Borodina E (1998) A novel pink-pigmented facultative methylotroph, Methylobacterium thiocyanatum sp. nov., capable of growth of thiocyanate or cyanate as sole nitrogen sources. Arch Microbiol 169:148–158
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Yu XZ, Gu JD, Huang SZ (2007) Hexavalent chromium induced stress and metabolic responses in hybrid willows. Ecotoxicology 16:299–309
Yu XZ, Lu PC, Yu Z (2012a) On the role of beta-cyanoalanine synthase (CAS) in metabolism of free cyanide and ferri-cyanide by rice seedlings. Ecotoxicology 21:548–556
Yu XZ, Zhang FZ, Li F (2012b) Phytotoxicity of thiocyanate to rice seedlings. Bull Environ Contam Toxicol 88:703–706
Zhang LP, Mehta SK, Liu ZP, Yang ZM (2008) Cupper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. J Agri Food Chem 57:411–419
Zhang PP, Fu JM, Hu LX (2012) Effects of alkali stress on growth, free amino acids and carbohydrates metabolism in Kentucky bluegrass (Poa pratenis). Ecotoxicology 21:1911–1918
Acknowledgments
This study is financially supported by The Education Department of Hunan Province, People’s Republic China (Grant No: 11A047).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XZ., Zhang, FZ. Effects of exogenous thiocyanate on mineral nutrients, antioxidative responses and free amino acids in rice seedlings. Ecotoxicology 22, 752–760 (2013). https://doi.org/10.1007/s10646-013-1069-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1069-6