Abstract
A study was conducted to investigate the contribution of β-cyanoalanine synthase (CAS) to the botanical metabolism of free cyanide and iron cyanides. Seedlings of rice (Oryza sativa L. cv. XZX 45) were grown hydroponically and then amended with free cyanide (KCN) or ferri-cyanide [K3Fe(CN)6] into the growth media. Total cyanide, free cyanide, and Fe3+/Fe2+ in aqueous solution were analyzed to identify the speciation of K3Fe(CN)6. Activity of CAS in different parts of the rice seedlings was also assayed in vivo and results indicated that dissociation of K3Fe(CN)6 to free cyanide in solution was negligible. Almost all of the applied KCN was removed by rice seedlings and the metabolic rates were concentration dependent. Phyto-transport of K3Fe(CN)6 was apparent, but appreciable amounts of cyanide were recovered in plant tissues. The metabolic rates of K3Fe(CN)6 were also positively correlated to the concentrations supplied. Rice seedlings exposed to KCN showed a considerable increase in the CAS activity and roots had higher CAS activity than shoots, indicating that CAS plays an important role in the botanical assimilation of KCN. However, no measurable change of CAS activity in different parts of rice seedlings exposed to K3Fe(CN)6 was detected, suggesting that K3Fe(CN)6 is likely metabolized by rice directly through an unknown pathway rather than the β-cyanoalanine pathway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elevated input of cyanide derived from anthropogenic sources to the environment imposes significantly higher ecological risk of ecosystems. Not surprisingly, problems of discharge and catastrophic accidents occur repeatedly worldwide (Korte et al. 2000; Mudder and Botz 2001). It is evident that several cyanide-containing compounds have been frequently detected in the environment, namely free cyanide, weak-acid dissociable cyanide, iron cyanides, and thiocyanate (Ebbs et al. 2008). Chemical speciation of cyanide in the environment is variable, highly depending on solution/soil pH, redox potential, the intensity and spectrum of solar radiation, biological activity, and community, and the presence of organic matter etc. (Meeussen et al. 1995; Rennert and Mansfeldt 2002; Ghosh et al. 2004). However, iron cyanides, e.g., ferro-cyanide [FeII(CN)6]−4 and ferri-cyanide [FeIII(CN)6]−3, are the most commonly found chemical forms in soils and groundwater contaminated with cyanide because of reactivity and stability (Mansfeldt et al. 2004).
An endogenous source of cyanide has been widely observed in higher plants during the ethylene biosynthesis from 1-aminocyclopropane-1-carboxylic acid (Peiser et al. 1984). However, cyanide produced does not accumulate in plant tissues (Goudey et al. 1989; Maruyama et al. 2001), since cyanide degradation capacity in plant materials is several orders of magnitude higher than that cyanide of production (Manning 1988). The evidence of cyanide metabolism by β-cyanoalanine synthase (CAS) is clear (Miller and Conn 1980; Goudey et al. 1989; Maruyama et al. 2001), in which CAS catalyzes the conversion of cyanide and cysteine to β-cyanoalanine and sulfide (Maruyama et al. 2001), and then β-cyanoalanine is converted into asparagine in the presence of the enzyme β-cyanoalanine hydrolyase (Castric et al. 1972). Because of its exclusive localization in mitochondria, the main physiological role of CAS has been considered to be the removal of toxic cyanide produced in plant tissues to avoid toxicity (Hendrickson and Conn 1969; Maruyama et al. 2001).
Unlike botanical uptake of free cyanide, ferri-cyanide is unable to move through biological membranes by simple diffusion mechanism due to its physical–chemical properties (Federico and Giartosio 1983; Dzombak et al. 2005; Ebbs et al. 2010). The evidences available suggest plants may be able to transport and assimilate this iron complex to less extent (Ebbs et al. 2003; Samiotakis and Ebbs 2004; Larsen and Trapp 2006; Ebbs et al. 2008; Yu and Gu 2009). However, a complete picture on the degradation pathway of ferri-cyanide in plants is still unknown.
The stability of ferri-cyanide in plant growth media has been considered to be the most important factor affecting phyto-removal to take place (Yu and Gu 2010). [FeIII(CN)6]−3, HCN and CN− may occur naturally together in solution in variable proportions, particularly under an acidic condition (Meeussen et al. 1992). Free cyanide is readily released during photo-induced decomposition of ferri-cyanide (Yu et al. 2011). Additionally, phytosiderophores excreted by the roots of graminaceous plants may elicit the release of free cyanide from ferri-cyanide, due to the formation of Fe3+-phytosiderophore complex under Fe-deficiency (Römheld and Marschner 1986). In this study, hydroponically-grown rice seedlings were kept in flasks with a stable solution pH that preserves cyanide speciation. Activities of CAS in different parts of the rice seedlings exposed to either KCN or ferri-cyanide were assayed to elucidate the contribution of the β-cyanoalanine pathway to the metabolism of both cyanide species in plants.
Materials and methods
Rice and exposure regimes
Seeds of rice (Oryza sativa L. cv. XZX 45) from the Hunan Academy of Agricultural Sciences, P.R. China after cleaning were planted in sandy soils under laboratory condition at 25°C until shoots appeared. After another 15 days of growth, young rice seedlings with similar height and weight were transferred to a pre-treatment solution containing 1 mM CaCl2 + 2 mM MES-TRIS buffer (pH 6.0) for 4 h to clear the cell wall space of ions (Ebbs et al. 2008), and then used in the subsequent experiments. Ten rice seedlings were transferred into a 50 ml Erlenmeyer flask filled with 50 ml of aerated hydroponic solution spiked with cyanide. The flasks were all wrapped with aluminum foil at the flask mouth to prevent escape of water, and the flask wall to inhibit potential growth of algae inside. All flasks were housed in a plant growth chamber with constant temperatures of 25 ± 0.5°C and a relative humidity of 60 ± 2% under continuous artificial light (illumination intensity: 20,000 lux). A modified ISO 8692 nutrient solution (Yu and Gu 2009) was used to support seedling growth.
Five different concentrations of KCN (0, 0.45, 0.97, 1.53, 2.04 mg CN/l) were prepared by adding the required aliquots of 1.0 g CN/l stock solution of KCN to the modified ISO 8692 nutrient solution. For the treatments amended with ferri-cyanide, five different concentrations (0, 2.70, 5.33, 7.95, 10.65 mg CN/l) were applied. For each treatment concentration, six replicates were prepared. One control in three replicates (the flasks were also wrapped with aluminum foils) contained testing chemicals without rice seedlings to quantify the loss and speciation during handling, hydrolysis and/or degradation by microorganisms. Exposure periods of all treatments were 48 h.
Potassium ferri-cyanide [K3Fe(CN)6] or potassium cyanide KCN (Sinopharm Chemical Reagent Co., Ltd., Shanghai, PR China) of analytical grade with ≥95% purity were used. It should be noted that 1 mg K3Fe(CN)6 and KCN equals 0.474 and 0.400 mg CN, respectively.
Chemical analysis
Analysis of free cyanide and total cyanide
The presence of total cyanide and free cyanide in solution was analyzed just prior to application and at the termination of exposure. Total cyanide in roots and shoots of rice seedlings was also analyzed after the exposure period.
The concentrations of free cyanide and total cyanide in the aqueous solution were determined spectrophotometrically by a standard method (State Environmental Protection Administration of China, 1989, method number GB 7487-87) as described previously (Yu and Gu 2010).
The analysis of total cyanide in plant tissues was also analyzed by a standard distillation method (State Environmental Protection Administration of China, 1989, method number GB 7486-87). After the end of exposure, plant materials were taken out and rinsed with deionized water. The remaining procedures were identical to those described previously (Yu and Gu 2010).
Analysis of Fe3+/Fe2+ in solution
Soluble Fe2+ concentration was determined by the ferrozine method as described previously (Gibbs 1979). The total soluble Fe including soluble Fe2+ and Fe3+ was measured by reducing total soluble Fe into Fe2+, and then Fe2+ was determined by the ferrozine method without addition of sodium fluoride (NaF) solution (Greenberg et al. 1992). The content of soluble Fe3+ in solution was obtained by taking the difference between total soluble Fe and soluble Fe2+.
Relative growth rate
The weight change of seedlings was the toxicity criteria. Rice seedlings were weighed prior to application and then at the termination of exposure. The relative growth rate (RGR, %) was calculated from
where M (I) is the initial weight of seedlings (g) and M (F) is the final weight of seedlings (g).
Transpiration rate
Inhibition of transpiration is a rapid measure for the toxic effect of a chemical to trees (Trapp et al. 2000). The weight loss of the plant-flask system was expressed as the absolute transpiration rate (g/d).
Determination of the metabolism rate
The metabolism rate v (μg CN/g FW h) was calculated from
where m (I) is the cyanide (μg) in hydroponic solution initially, m (F) is the final cyanide (μg) in hydroponic solution, and m (R) and m (S) are the total cyanide (μg) in roots and shoots at the termination of exposure. M is the biomass of seedlings (g), and ∆t is the time period (h).
Assay of β-cyanoalanine synthase activity
The activity of CAS was measured in fresh tissues of roots or shoots of rice seedlings at the termination of exposure. Fresh plant materials were taken from non-treated and treated seedlings. 0.2 g of tissues materials (fresh weight) was precisely weighted and placed in a triturator. 5.0 ml of extraction buffer (pH 9.5, containing 2 mM EDTA-Na2, 10 mM cysteine, 0.1 M Tris–HCl) was added before trituration. Trituration was performed in an ice-bath and then centrifuged at 15000×g for 10 min, the supernatant was collected for the enzyme assay. The measurement of CAS activity in plant tissues was determined in crude extracts using the N, N-dimethyl-p-phenylenediamine (DMPDA) method (Liang 2003). The measurement of protein contents in plant materials was similar to the previous study (Yu and Gu 2009), with Albumin Bovine V (Sigma-Aldrich Inc., St. Louis, Missouri) as a standard. CAS activity is expressed in nmol H2S/h mg Pr, which refers to 1 nmol H2S produced in 1 h by CAS sample containing 1 mg protein (Liang 2003).
Statistical methods
Analysis of variance (ANOVA) and Tukey’s multiple range test was used to determine the statistical significance at 0.01 or 0.05 between the treatments (Sachs 1992).
Results
Responses of rice seedlings to cyanide
Responses of rice seedlings to free cyanide
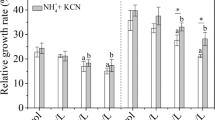
Figure 1 shows the changes of relative growth rate and transpiration rate of rice seedlings exposed to free cyanide (KCN). Compared with the non-treated seedlings, a negligible reduction in transpiration was observed (P > 0.05), with elevation of KCN. Although all plants showed positive growth, a declining trend in relative growth of rice seedlings was observed with an increase of KCN concentrations (P < 0.05), compared with the non-treated rice seedlings. Visible toxic symptom of chlorosis was not observed in all treatments during the exposure period.
Responses of rice seedlings to ferri-cyanide
Transpiration of rice seedlings varied with the doses of ferri-cyanide (Fig. 2). A slightly higher transpiration was observed from the rice seedlings exposed to ferri-cyanide (≤7.95 mg CN/l) than that from the non-treated plants (P > 0.05), while rice seedlings exposed to ferri-cyanide of 10.65 mg CN/l showed a negligible reduction in transpiration (P > 0.05). Both non-treated and treated rice seedlings showed positive growth. However, a decline trend in relative growth of rice seedlings was found with an increase of ferri-cyanide doses. It was also observed that 10.65 mg CN/l of ferri-cyanide caused a significant decrease in relative growth compared with the control plants (P < 0.05).
Removal of cyanide by rice seedlings
Phyto-removal of free cyanide
No obvious change of the cyanide concentration in aqueous solution in the control with KCN without rice seedlings was observed over a 48-h period of exposure (data not shown), indicating that the disappearance of KCN from aqueous solution in testing groups can be accounted for by the uptake by plants. Results showed that large fraction of the applied KCN transported into plant materials in all treatment groups, and trace amounts remained in hydroponic solution after 48-h of exposure. Indeed, more than 97% (mean 97.5%, S.D. 0.99, n = 4) of the applied KCN were taken up from the solution by rice seedlings (Fig. 3).
Concentrations of total cyanide in plant tissues were also determined after the incubation period (Fig. 4). The background of total cyanide in non-treated plant materials was all below the detection limit. Cyanide concentrations in plant materials were observed to increase for the treated plants with an increase of the applied KCN, but the difference between the treatments was not significant (P > 0.05). It is noted that a slight difference existed in the distribution of total cyanide in plant materials, in which more cyanide was recovered in roots than shoots. However, the difference was also not significant enough (P > 0.05).
No cyanide transpired by plants was reported previously (Ebbs et al. 2008), therefore, the mass balance for KCN was calculated using the tissue total cyanide and the solution cyanide. At 0.45 mg CN/l, the total cyanide recovered from plant biomass accounted for 5.68% (S.D. 0.47, n = 3) of the cyanide loss from the plant growth media, whereas 1.60% (S.D. 0.04, n = 3) was detected in tissues of plants exposed to 2.04 mg CN/l. The calculated metabolic rates of KCN are shown in Fig. 5. The rates increased significantly with the applied KCN doses (the linear trend is significant at α = 0.01, R 2 = 0.999). The highest metabolic rate was 1.11 μg CN/g FW h at 2.04 mg CN/l, which is more than five fold higher than that at 0.45 mg CN/l.
Phyto-removal of ferri-cyanide
After analysis of free cyanide and soluble Fe in the control solution spiked with ferri-cyanide (without rice seedlings), trace amounts of free cyanide and soluble Fe3+ was detected in the solution after 48-h of exposure in the absence of light (data now shown), likely due to handling. This indicates that dissociation of ferri-cyanide into free cyanide in the hydroponic solution is a negligible process and the majority of the solution cyanide remained as ferri-cyanide, which is consistent with previous studies (Samiotakis and Ebbs 2004; Larsen and Trapp 2006; Ebbs et al. 2008; Yu and Gu 2010). Thus, the disappearance of the applied ferri-cyanide in hydroponic solution could be attributed to the uptake by rice seedlings. Significant amounts of ferri-cyanide were removed in the presence of rice seedlings (Fig. 6), in which between 34.7 and 50.7% of the applied mass was taken up from the hydroponic solution by rice seedlings over the 48-h period of exposure.
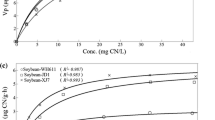
Figure 7 shows the concentrations of total cyanide in plant materials at different treatments after the 48-h period of exposure. Concentrations of total cyanide in different plant materials were strongly correlated to the applied ferri-cyanide concentrations (Figures not shown), judged by the critical r for a given n. The trends were all significant at α = 0.05 (the regression for roots y = 7.0759x − 10.793, R 2 = 0.9441; the regression for shoots y = 0.1959x + 0.0985, R 2 = 0.9749). Substantial differences in the distribution of total cyanide in plant materials were observed (P < 0.01), in which roots were the major sink for cyanide accumulation rather than shoots.
A mass balance of the total cyanide in plant tissues and the solution was made. Between 21.7 and 43.4% of the ferri-cyanide lost from the hydroponic solution was detected in the plant materials at the termination of 48-h exposure. Loss from controls without plants was negligible; as a result, majority of the ferri-cyanide was likely metabolized by rice seedlings. The calculated metabolic rates of ferri-cyanide are shown in Fig. 8. The rate increased linearly with the ferri-cyanide application (R 2 = 0.8766, significant at α = 0.05). The metabolic rate at 10.65 mg CN/l (1.02 μg CN/g FW h) was 2 times higher than that at 2.70 mg CN/l (0.47 μg CN/g FW h).
Activities of β-cyanoalanine synthase in plant materials of rice seedlings
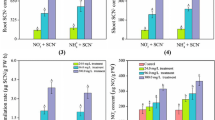
Activities of CAS in plant materials of rice seedlings exposed to KCN were measured at the termination of exposure (Fig. 9). CAS activity in roots from non-treated rice seedlings was 20.48 nmol H2S/h mg Pr. A remarkable increase in CAS activity occurred in the rice roots with an increase of the applied KCN (P < 0.05). A similar pattern of CAS activity was also observed in shoots at the termination of 48-h exposure. It was of interest to note, in both non-treated and treated rice seedlings, that roots always showed significantly higher CAS activity than shoots (P < 0.05).
The measured activities of CAS in plant tissues of rice seedlings exposed to ferri-cyanide are shown in Fig. 10. Results indicated that CAS activities in both roots and shoots showed negligible change with elevation of ferri-cyanide in plant growth media compared with the non-treated plant materials (P > 0.05). Results from this test indicated that metabolism of KCN in rice seedlings is largely through the β-cyanoalanine pathway, while ferri-cyanide is assimilated through an unreported degradation pathway.
Discussions
Although rice seedlings showed different responses to both KCN and ferri-cyanide, deleterious effects on plant physiological functions due to addition of both cyanide species were not observed within 48 h of exposure. The results of measured toxic effects were plotted and analyzed. The linear trends from the treatments with KCN were significant (the regression between KCN and relative growth rate y = −4.5722x + 21.725, R 2 = 0.8649; the regression between KCN and transpiration y = −0.5866x + 5.7594, R 2 = 0.9603), judged by the critical r for a given n (α = 0.05) (Sachs 1992). For the treatment with ferri-cyanide, the correlation between the ferri-cyanide concentrations and the relative growth was also significant (R 2 = 0.849, significant at α = 0.05), while the linear trend between ferri-cyanide and transpiration was not significant (R 2 = 0.041, significant at α = 0.05). Data presented here suggest that KCN caused more severe stress to rice seedlings than ferri-cyanide due to the readily bioavailability and transport of the former.
Ferri-cyanide has been identified to be quite stable chemically against decomposition in the absence of light under a neutral pH condition (Ghosh et al. 1999). Indeed, the half-life of iron cyanides in soils was estimated to be approximately 100 years at pH 6.5 (Meeussen et al. 1992). In this study, the solution pH was quite stable during the 48-h of exposure, within a range of 7.2–7.6, and trace amounts of free cyanide and soluble Fe3+ was detected in the solution by the termination of incubation, probably due to handling, suggesting that dissociation of ferri-cyanide into free cyanide in the solution is negligible. Another possibility is plants and their associated microorganisms may facilitate dissociation of ferri-cyanide into free cyanide due to the presence of phytosiderophores and siderophores secreted under Fe-deficiency conditions (Römheld and Marschner 1986). It is known that the equilibrium constant (logK) of ferri-cyanide is 52.63 (Meeussen et al. 1992), while the logK value of Fe-phytosiderophores is only 18.1 (Mino et al. 1983). These give rise to the conclusion that cyanide in the solution remained principally in the form of ferri-cyanide rather than free cyanide (CN− or HCN) before uptake by rice seedlings.
In our observation, rice seedlings were able to efficiently take up both cyanide species. More than 97% of the applied KCN was eliminated from the hydroponic solution by rice seedlings after 48 h of exposure, whereas between 34.7 and 50.7% of ferri-cyanide was removed by rice seedlings. The difference is most likely attributed to the fact that rice seedlings take up the two cyanides by dissimilar mechanisms. Indeed, KCN has been found to be able to move through biological membranes mainly achieved by simple diffusion (Dzombak et al. 2005; Ebbs et al. 2010). Although iron cyanide has long been considered membrane-impermeable (Federico and Giartosio 1983), our study presented here and also others’ (Samiotakis and Ebbs 2004; Larsen and Trapp 2006; Ebbs et al. 2008; Yu and Gu 2010) collectively suggested phyto-transport of ferri-cyanide is possible.
After entering the plant materials, the biological fate and transport of both cyanides are also quite different. Indeed, a very small fraction of KCN loss from the hydroponic solution was recovered in plant tissues, judged by the total cyanide detected, and the distribution in different parts of plant materials was slightly different, indicating rice seedlings are able to metabolize KCN readily during transport within plant tissues. Slight higher cyanide concentrations was detected in roots than that in shoots in all treatments with an increase of the applied KCN, indicating that roots are the major site for KCN metabolism because of the transport route. Since liberation of ferri-cyanide into free cyanide was negligible, rice seedlings were able to take Fe3+ and CN− as a complex. Between 21.7% and 43.4% of the ferri-cyanide removed from the plant growth media was detected in plant materials after 48 h of exposure. The ferri-cyanide (%) recovered in rice biomass was significantly related to the concentrations of ferri-cyanide applied (the linear regression between the initial concentrations of ferri-cyanide and the ferri-cyanide (%) recovered in biomass: y = 2.6388x + 13.72, R 2 = 0.8177, significant at α = 0.05). However, the quantity of ferri-cyanide accumulated varied significantly in different parts of plant tissues (P < 0.01), in which the cyanide accumulated in roots accounted for 96.3% (S.D. 0.86, n = 4) of the total cyanide detected in biomass, while only 3.70% (S.D. 0.86, n = 4) was recovered in shoots.
Results present here and also previous works suggest that exogenous KCN is quickly metabolized without accumulation in healthy plants when the concentration is below the inhibitory toxic level (Larsen et al. 2005). This is largely due to the fact that the rate of botanical metabolism of KCN via the β-cyanoalanine pathway is much faster than the uptake rate, resulting in no accumulation in plant materials. Indeed, activities of CAS in roots and shoots of rice seedlings were positively increased with the application of KCN in all treatments in our observation. A comparable result was found in a previous work (Liang 2003), where exogenous KCN at 10 μM was sprayed onto shoots of normally watered tobacco plants and their CAS activity in shoots increased more than onefold. However, a different conclusion was reached by Maruyama et al. (2001) and Meyer et al. (2003) in which activity of CAS was not responsive to exogenous cyanide concentrations, but positively increased with ethylene production. Based on our experimental data, the linear regression between the measured CAS activity and KCN supplied was plotted (Figures not shown). Both linear trends were significant (the regression for the roots y = 10.568x + 23.699, R 2 = 0.914, the regression for shoots y = 4.5783x + 15.497, R 2 = 0.939), judged by the critical r for a given n. It is of interest to compare the concentration sensitivity of the enzyme activity between the two parts of rice seedlings using the slope of the linear fit curve. The difference between roots and shoots of treated rice seedlings was 1.96. This indicated that (1) roots are the major site for botanical metabolism of KCN instead of shoots; (2) a less extend of KCN was translocated to shoots due to quick conversion of KCN in roots. This could be an explanation for the less change of CAS activity in shoots than that in roots, with an increase of the applied KCN from 0.45 to 2.04 mg CN/l. Our results are consistent to another study, where activity of CAS was 2- to 3-fold higher from roots of wheat than from shoots (Machingura and Ebbs 2010). However, significant amounts of cyanide analyzed as total cyanide were recovered in plant tissues of rice seedlings exposed to ferri-cyanide in this observation, implying that dissociation of ferri-cyanide into free cyanide was unlikely to occur in plant materials. Indeed, negligible changes of CAS activity were observed in both roots and shoots of rice exposed to ferri-cyanide, suggesting that in vivo phyto-dissociation of ferri-cyanide is not prerequisite for botanical metabolism. Ferri-cyanide is most likely metabolized by rice seedlings without phyto-dissociation through an undiscovered degradation pathway rather than the β-cyanoalanine pathway.
Evidence that chemical speciation of cyanide in the environment is variable (Meeussen et al. 1995). Of the cyanides detected, free cyanide and iron cyanide are frequently detected and the dynamic interconversion between the two cyanide species is most likely and especially crucial (Mansfeldt et al. 2004; Yu et al. 2011) because plants showed different responses to free cyanide and iron cyanide, depending on the concentrations and chemical forms (Larsen et al. 2005; Yu and Gu 2009). Indeed, the toxicity of cyanide to living organisms is well documented. Iron cyanides, though less toxic than free cyanide, is potentially hazardous due to toxic free cyanide liberated from them through photodecomposition (Meeussen et al. 1992; Yu et al. 2011), which led to serious concern in waste management and ecosystem restoration. It is obvious that higher concentrations of free cyanide not only cause negative ecological effects, but also significantly reduce the effectiveness of phytoremediation. The evidence offered here and also others’ (Miller and Conn 1980; Goudey et al. 1989; Maruyama et al. 2001; Larsen et al. 2005; Ebbs et al. 2010) suggests that free cyanide, within a certain concentration range, can be quickly degraded by the plant enzyme CAS. In a recent work, it has been proposed that non-toxic concentrations of exogenous free cyanide can be beneficial to plants as an alternative source of nitrogen under nitrogen-limiting conditions (Ebbs et al. 2010; Machingura and Ebbs 2010). For waste management, this has, as consequence, that botanical assimilation of free cyanide is a fast and destruction-based process and causes less adverse effects on both the environment and living organisms. Despite evidence that botanical uptake, transport and assimilation of iron cyanides is possible (Ebbs et al. 2003; Samiotakis and Ebbs 2004; Larsen and Trapp 2006; Ebbs et al. 2008; Yu and Gu 2009), research focusing on the degradation pathway of iron cyanides in plants is relatively limited. Our current work suggests that rice seedlings are likely to metabolize ferri-cyanide directly as a substrate through an undefined degradation pathway rather than the β-cyanoalanine pathway. Additionally, cyanide accumulation in plant materials (i.e., majority in roots rather than shoots) is not a major sink during phyto-removal of ferri-cyanide. Therefore, from an ecological health point of view, we have good reasons to suggest that phyto-assimilation of both free cyanide and ferri-cyanide is an environmental friendly strategy for waste management and ecosystem restoration.
Conclusions
Although rice seedlings showed different responses to the application of KCN and ferri-cyanide, no deleterious effects were observed in all treatments. Rice seedlings were able to efficiently take up both cyanide species from the plant growth media, but the phyto-removal rates were different and may indicate the uptake pathways were quite dissimilar. In vivo CAS assays suggest that the β-cyanoalanine pathway is responsible for the botanical metabolism of KCN. However, rice seedlings are likely to metabolize ferri-cyanide directly as a substrate through an undefined degradation pathway rather than the β-cyanoalanine pathway.
References
Castric PA, Farnden KJF, Conn EE (1972) Cyanide metabolism in higher plants. V. The formation of asparagine from β-cyanoalanine. Arch Biochem Biophys 152:62–69
Dzombak DA, Ghosh RS, Young TC (2005) Physical–chemical properties and reactivity of cyanide in water and soil. In: Dzombak DA, Ghosh RS, Wong-Chong GW (eds) Cyanide in water and soil: chemistry, risk, and management. CRC, Boca Raton, pp 58–92
Ebbs SD, Bushey J, Poston S, Kosma D, Samiotakis M, Dzombak D (2003) Transport and metabolism of free cyanide and iron cyanide complexes by willow. Plant Cell Environ 26:1467–1478
Ebbs SD, Piccinin RC, Goodger JQD, Kolev SD, Woodrow IE, Baker AJM (2008) Transport of ferrocyanide by two eucalypt species and sorghum. Int J Phytoremediation 10:343–357
Ebbs SD, Kosma D, Nielson EH, Machingura M, Baker AJM, Woodrow IE (2010) Nitrogen supply and cyanide concentration influence the enrichment of nitrogen from cyanide in wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor L.). Plant Cell Environ 33:1152–1160
Federico R, Giartosio CE (1983) A transplasmamembrane electron transport system in maize. Plant Physiol 73:182–184
Ghosh RS, Dzombak DA, Luthy RG, Nakles DV (1999) Subsurface fate and transport of cyanide species at a manufactured-gas plant site. Water Environ Res 71:1205–1216
Ghosh RS, Nakles DV, Murarka P, Neuhauser EF (2004) Cyanide speciation in soil and groundwater at manufactured gas plant (MGP) sites. Environ Eng Sci 21:752–767
Gibbs MM (1979) A simple method for the rapid determination of iron in natural waters. Water Res 13:295–297
Goudey JS, Tittle FL, Spencer MS (1989) A role for ethylene in the metabolism of cyanide by higher plants. Plant Physiol 89:1306–1310
Greenberg AE, Clesceri LSE, Eaton AD (1992) Standard methods for the examination of water and wastewater, 18rd edn. American Water Works Association. Water Pollution Control Federation, Washington, pp 366–368
Hendrickson HR, Conn EE (1969) Cyanide metabolism in higher plants. IV. Purification and properties of the beta-cyanoalanine synthase of blue lupine. J Biol Chem 244:2632–2640
Korte F, Spiteller M, Coulston F (2000) The cyanide leaching gold recovery process is a non-sustainable technology with unacceptable impacts on ecosystems and humans: The disaster in Romania. Ecotoxicol Environ Saf 46:241–245
Larsen M, Trapp S (2006) Uptake of iron cyanide complexes into willow trees. Environ Sci Technol 40:1956–1961
Larsen M, Ucisik A, Trapp S (2005) Uptake, metabolism, accumulation and toxicity of cyanide in willow trees. Environ Sci Technol 39:2135–2142
Liang WS (2003) Drought stress increases both cyanogenesis and beta-cyanoalanine synthase activity in tobacco. Plant Sci 165:109–1115
Machingura M, Ebbs S (2010) Increased beta-cyanoalanine synthase and asparaginase activity in nitrogen-deprived wheat exposed to cyanide. J Plant Nutr Soil Sci 173:808–810
Manning K (1988) Detoxification of cyanide by plants and hormone action. In: Ciba Foundation (ed) Cyanide compounds in biology. John Wiley & Sons, Chichester, pp 92–110
Mansfeldt T, Leyer H, Barmettler K, Kretzschmar R (2004) Cyanide leaching from soil developed from coking plant purifier waste as influenced by citrate. Vadose Zone J 3:471–479
Maruyama A, Saito K, Ishizawam K (2001) β-cyanoalanine synthase and cysteine synthase from potato: molecular cloning, biochemical characterization, and spatial and hormonal regulation. Plant Mol Biol 46:749–760
Meeussen JCL, Keizer MG, de Haan FAM (1992) Chemical stability and decomposition rate of iron cyanide complexes in soil solutions. Environ Sci Technol 26:511–516
Meeussen JCL, van Riemsdijk WH, van der Zee SEATM (1995) Transport of complexed cyanide in soil. Geoderma 67:73–85
Meyer T, Burow M, Bauer M, Papenbrock J (2003) Arabidopsis sulfurtransferases: investigation of their function during senescence and in cyanide detoxification. Planta 217:1–10
Miller JM, Conn EE (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65:1199–1202
Mino Y, Ishida T, Ota N, Inoue M, Nomoto K, Takemoto T, Tanaka H, Sugiura Y (1983) Mugineic acid-iron (III) complex and its structurally analogous cobalt (III) complex: characterization and implication for absorption and transport of iron in gramineous plants. J Am Chem Soc 105:4611–4676
Mudder T, Botz M (2001) A guide to cyanide. Mining Environ Manag 9:8–12
Peiser GD, Wang TT, Hoffman NE, Yang SF, Walsh CT (1984) Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carbonxylic acid during its conversion to ethylene. Proc Natl Acad Sci USA 81:3059–3063
Rennert T, Mansfeldt T (2002) Sorption of iron-cyanide complexes on goethite in the presence of sulfate and desorption with phosphate and chloride. J Environ Qual 31:745–751
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophore in roots of grasses. Plant Physiol 80:175–180
Sachs L (1992) Angewandte Statistik. Springer, Berlin
Samiotakis M, Ebbs SD (2004) Possible evidence for transport of an iron cyanide complex by plants. Environ Pollut 127:169–173
Trapp S, Zambrano KC, Kusk KO, Karlson U (2000) A phytotoxicity test using transpiration of willows. Arch Environ Contam Toxicol 39:154–160
Yu XZ, Gu JD (2009) Uptake, accumulation and metabolic response of ferri-cyanide in weeping willows. J Environ Monit 11:145–152
Yu XZ, Gu JD (2010) Effect of Temperature on removal of iron cyanides from solutions by maize plants. Environ Sci Pollut Res 17:106–114
Yu XZ, Peng XY, Wang GL (2011) Photo induced dissociation of ferri and ferro cyanide in hydroponic solutions. Int J Environ Sci Technol 8:853–862
Acknowledgment
This work was financially supported by The National Science Foundation of China (NSFC: 40971256).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XZ., Lu, PC. & Yu, Z. On the role of β-cyanoalanine synthase (CAS) in metabolism of free cyanide and ferri-cyanide by rice seedlings. Ecotoxicology 21, 548–556 (2012). https://doi.org/10.1007/s10646-011-0815-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0815-x