Abstract
The acute toxicity of potassium thiocyanate (KSCN) and ammonium thiocyanate (NH4SCN) to rice seedlings was tested. Hydroponically-grown plants showed different responses to the two species of thiocyanate. NH4SCN caused more severe stress to rice seedlings than KSCN. A significant reduction in transpiration and relative growth was observed with all NH4SCN treatments (p < 0.01), while the effect of KSCN on rice seedlings was more evident at greater than 100 mg SCN/L (p < 0.01). Both chemicals had a negligible effect on total chlorophyll content in shoots of rice seedlings (p > 0.05). Although phyto-transport of thiocyanate was apparent, rice seedlings showed significantly higher removal potential for NH4SCN than KSCN.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
A natural source of thiocyanate (SCN−) has been observed in plants during the in vivo biological detoxification of cyanide (Boening and Chew 1999; Sorokin et al. 2001). However, the majority is derived from photofinishing, herbicide and insecticide production, dyeing, acrylic fiber production, manufacturing of thiourea, metal separation and electroplating (Hung and Pavlostathis 1998). The extensive output from industrial activities has led to its widespread occurrence in wastewater, which may result in serious environmental problems (Lee et al. 2008). It is evident that thiocyanate occurs as a primary constituent in industrial coke-oven wastewater at concentrations of 50–650 mg/L (Chakraborty and Veeramani 2006). The toxicity of thiocyanate to various animal species is well documented (Watson and Maly 1987; Speyer and Raymond 1988; Bhunia et al. 2000). Because of its strong binding tendency to protein, thiocyanate causes inhibition of halide transport to the thyroid gland, stomach, cornea and gills of fish (Katz et al. 1982; Heming et al. 1985). It also acts as non-competitive inhibitors to block a variety of enzymatic reactions (Wood et al. 1998; Lee et al. 2008). Additionally, thiocyanate affects the nervous system in humans, causing irritability, nervousness, hallucinations, psychosis, mania, delirium and convulsions (Boening and Chew 1999). However, nothing is known yet about the phytotoxicity of thiocyanate. The objectives of this study were to investigate responses of plants to thiocyanate using rice seedling grown hydroponically, and to estimate phyto-removal potential for thiocyanate.
Materials and Methods
Seeds of rice (Oryza sativa L. cv. XZX 45) from the Hunan Academy of Agricultural Sciences, PR China after cleaning were planted in sandy soils at 25°C until shoots appeared. After another 15 days of growth, young rice seedlings with similar height and weight were transferred to a pre-treatment solution containing 1 mM CaCl2 + 2 mM MES-TRIS buffer (pH 6.0) for 4 h to clear the cell wall space of ions (Ebbs et al. 2008), and then ten rice seedlings were transferred into a 50 mL Erlenmeyer flask filled with 50 mL modified ISO 8692 nutrient solution (Yu and Gu 2007). The plants were first conditioned for 24 h to allow adaptation to the new environmental conditions. The flasks were all wrapped with aluminum foil at the flask mouth to prevent escape of water, and the flask wall to inhibit potential growth of algae inside. All flasks were housed in a plant growth chamber with constant temperatures of 25 ± 0.5°C and a relative humidity of 60% ± 2% under continuous artificial light. Then, the nutrient solution in each flask was replaced by spiked solution, except for the controls. Potassium thiocyanate (KSCN) and ammonium thiocyanate (NH4SCN) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, PR China) of analytical grade with ≥98.5% purity were used. For each testing chemical, six difference concentrations were applied. For each treatment concentration, four replicates were prepared. Exposure periods were 48 h.
The concentrations of thiocyanate in solutions were determined spectrophotometrically by a standard method (State Environmental Protection Administration of China, 1989, method number GB 7487-87).
Mean measured initial concentrations of SCN− in treatments spiked with KSCN: 0, 25.3(SD: 0.68), 50.3(SD: 0.79), 100(SD: 8.47), 150(SD: 13.0), and 200(SD: 11.5) mg SCN/L. Mean measured initial concentrations of SCN− in treatments spiked with NH4SCN: 0, 25.2(SD: 0.71), 50.1(SD: 2.76), 100(SD: 7.39), 150(SD: 4.34), and 200 (SD: 8.05).
Transpiration and biomass growth of rice seedlings were used as endpoints for phytotoxicity determination. The weight loss of the plant-flask system was expressed as the transpiration rate (g/d). Relative growth (%) was calculated from the weight change of rice seedlings with respect to the initial weight. Total chlorophyll content in shoots of rice seedlings were also measured (Yu and Gu 2007).
Results and Discussion
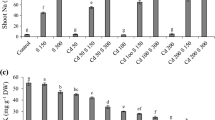
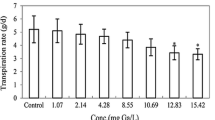
Figures 1, 2 and 3 show the changes of selected parameters of rice seedlings exposed to KSCN and NH4SCN. Compared with the non-treated seedlings, a remarkable reduction in transpiration was observed (p < 0.01) with all NH4SCN treatments (Fig. 1), while a significant reduction in transpiration was only detected with rice seedlings exposed to KSCN at greater than or equal to 100 mg SCN/L. Although all plants showed positive growth, a significant declining trend in relative growth of rice seedlings was observed in both thiocyanate treatments with increased concentrations (Fig. 2). Visible toxic symptoms of chlorosis were not observed in any of the treatments at the termination of 48-h exposure. Indeed, the changes in total chlorophyll content were negligible in shoots of rice seedlings exposed to KSCN and NH4SCN (p > 0.05), compared with non-treated seedlings (Fig. 3).
Transpiration rates of rice seedling exposed to thiocyanate for 48 h. The values are the mean of four replicates. Vertical lines represent standard deviation. “a” refers to the significance difference between KSCN treatments and the control (p < 0.01). “b” refers to the significance difference between NH4SCN treatments and the control (p < 0.01)
Relative growth of rice seedling exposed to thiocyanate for 48 h. The values are the mean of four replicates. Vertical lines represent standard deviation. “a” refers to the significance difference between KSCN treatments and the control (p < 0.01). “b” refers to the significance difference between NH4SCN treatments and the control (p < 0.01)
The removal rates of KSCN and NH4SCN from the rice seedling growth media are presented in Fig. 4. Phyto-removal rate (μg SCN/g FW. h) was positively related to doses of the applied KSCN and NH4SCN (≤150 mg SCN/L), while the highest concentrations of 200 mg SCN/L impaired the removal rate significantly in both treatments (p < 0.01).
The results presented here indicated that rice seedlings showed different responses to the two species of thiocyanate. Indeed, NH4SCN caused more severe stress to rice seedlings than KSCN, showing a significant difference in relative growth between the two thiocyanate treatments (p < 0.01).
It is known that thiocyanate can be used as a source of energy, nitrogen, and carbon by microorganisms (Hung and Pavlostathis 1998; Sorokin et al. 2001; Kwon et al. 2002; Lee et al. 2008). Biodegradation of thiocyanate is evident (Sorokin et al. 2001; Bezsudnova et al. 2007), in which thiocyanate hydrolase (SCNase) catalyzes hydrolysis of the nitrile bond of thiocyanate, with the formation carbonyl sulfide (COS) and ammonia (Bezsudnova et al. 2007; Arakawa et al. 2007). An endogenous source of thiocyanate has been observed in tissues of cyanogenic plants during cyanide detoxification (Boening and Chew 1999; Bezsudnova et al. 2007). The organisms carrying thiocyanate-degrading ability are considered to be distributed widely (Katayama et al. 2006). We therefore have good reasons to propose that plants would be able to metabolize thiocyanate in vivo. Indeed, phyto-transport of thiocyanate was apparent in our work. It is also of interest to note that rice seedlings showed significantly higher removal potential for NH4SCN than KSCN (p < 0.01). This could be an explanation why NH4SCN caused more severe effects on rice seedlings than KSCN. Results also suggest that the presence of NH +4 and K+ in nutrient solutions may play different roles on the botanical removal of thiocyanate (SCN−).
References
Arakawa T, Kawano Y, Kataoka S, Katayama Y, Kamiya N, Yohda M, Odaka M (2007) Structure of thiocyanate hydrolase: a new nitrile hydratase family protein with a novel five-coordinate cobalt(III) center. J Mol Biol 366:1497–1509
Bezsudnova EY, Sorokin DY, Tikhonova TV, Popov VO (2007) Thiocyanate hydrolase, the primary enzyme initiating thiocyanate degradation in the novel obligately chemolithoautotrophic halophilic sulfur-oxidizing bacterium Thiohalophilus thiocyanoxidans. Biochem Biophys Acta 23:1563–1570
Bhunia F, Saha NC, Kaviraj A (2000) Toxicity of thiocyanate to fish, plankton, worm and aquatic system. Bull Environ Contam Toxicol 64:197–204
Boening DW, Chew CM (1999) A critical review: general toxicity and environmental fate of three aqueous cyanide ions and associated ligands. Wat Air Soil Pollut 109:67–79
Chakraborty S, Veeramani H (2006) Effect of HRT and recycle ratio on removal of cyanide, phenol, thiocyanate and ammonia in an anaerobic-anoxic-aerobic continuous system. Process Biochem 41:96–105
Ebbs SD, Piccinin RC, Goodger JQD, Kolev SD, Woodrow IE, Baker AJM (2008) Transport of ferrocyanide by two eucalypt species and sorghum. Int J Phytorem 10:343–357
Heming TA, Thurston RV, Meyn EL, Zajdel RK (1985) Acute toxicity of thiocyanate on trout. Trans Am Fish Soc 114:895–905
Hung CH, Pavlostathis SG (1998) Fate and transformation of thiocyanate and cyanate under methanogenic conditions. Appl Microbiol Biotechnol 49:112–116
Katayama Y, Hashimoto K, Nakayama H, Mino H, Nojiri M, Ono TA, Nyunoya H, Yohda M, Takio K, Odaka M (2006) Thiocyanate hydrolyase is a cobalt-containing metalloenzyme with a cysteine-sulfinic acid ligand. J Am Chem Soc 128:728–729
Katz U, Lau KR, Ramos MMP, Elloy JC (1982) Thiocyanate transport across fish intestine (Pleuronectes platessa). J Membrane Biol 66:9–14
Kwon HK, Woo SH, Park JM (2002) Thiocyanate degradation by Acremonium strictum and inhibition by secondary toxicants. Biotechnol Let 24:1347–1351
Lee C, Kim J, Do H, Hwang S (2008) Monitoring thiocyanate-degrading microbial community in relation to changes in process performance in mixed culture systems near washout. Wat Res 42:1154–1162
Sorokin DY, Tourova TP, Lysenko AM, Kuenen JG (2001) Microbial thiocyanate utilization under highly alkaline conditions. Appl Environ Microbiol 67:528–538
Speyer MR, Raymond P (1988) The acute toxicity of thiocyanate and cyanate to rainbow trout as modified by water temperature and pH. Environ Toxicol Chem 7:565–571
Watson SJ, Maly EJ (1987) Thiocyanate toxicity to Daphina magna: modified by pH and temperature. Aquat Toxicol 10:1–8
Wood PA, Kelly PD, McDonald RI, Jordan LS, Morgan DT, Khan S, Murrell JG, Borodina E (1998) A novel pink-pigmented facultative methylotroph, Methylobacterium thiocyanatum sp. nov., capable of growth of thiocyanate or cyanate as sole nitrogen sources. Arch Microbiol 169:148–158
Yu XZ, Gu JD (2007) Accumulation and distribution of trivalent chromium and effects on hybrid willow (Salix matsudana Koidz × alba L.) metabolism. Arch Environ Contam Toxicol 52:503–511
Acknowledgments
This work was financially supported by The Education Department of Hunan Province, PR China (grant no: 11A047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XZ., Zhang, FZ. & Li, F. Phytotoxicity of Thiocyanate to Rice Seedlings. Bull Environ Contam Toxicol 88, 703–706 (2012). https://doi.org/10.1007/s00128-012-0545-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0545-7