Abstract
Migration and reproduction of the Caspian Lamprey, Caspiomyzon wagneri, in the Shirud River were investigated during late-March to early-May at water temperatures ranging from 11 to 21.25°C. We examined the effect of water temperature on timing of spawning migrations. There was a significant negative relationship between temperature and intensive migration of Caspian Lamprey (p < 0.05). The most intensive migration of lampreys was at night (21:00–3:00 h) and when the water temperatures averaged 16°C (34.43%). The overall sex ratio (male to female) was 1.07 to 1. The individual absolute fecundity was 31 ‘758–51’ 198 eggs (mean±SD—41,924 ± 5,382). The egg diameter was 0.780–1.151 (0.92 ± 0.081) mm. The individual relative fecundity varies from 80.3 to 148.1 (107.2 ± 15.1) eggs per 1 mm of length and from 260.8 to 677.4 (397.6 ± 93) eggs per 1 g of weight. The gonadosomatic index (GSI) of females was 5.83–31.44 (11.22 ± 4.30).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout their distribution, lampreys (Petromyzontiformes) are of significant ecological, cultural and economic importance (Hardisty 1986; Renaud 1997; Kelly and King 2001; Lucas and Baras 2001; Jang and Lucas 2005). Only the parasitic species migrate upstream from lakes or the sea where they spend the feeding stage. At the time when migration starts, lampreys stop growing and begin to mature sexually (Larsen 1980). Spawning areas are normally stony or gravelly stretches in running water. They spawn in pairs or groups, laying eggs in shallow depressions created by lifting away stones with their suckers (Maitland et al. 1994; Salewski 2003).
The Caspian Lamprey is a Eurasian species. It occurs in the Ponto-Caspian province (North Ponto-Caspian and South Ponto-Districts) of the European-Mediterranean Subregion within the Holarctic Region. Caspiomyzon wagneri is endemic to the Caspian Sea and rivers in its northern, western, and southern watershed (Holčík 1986), and migrates to the Volga, Ural, Terek, and Kura rivers (Holčík and Oláh 1992; Coad 2008). The Caspian Lamprey in the southern Caspian basin (Iran) migrates to such rivers as Shirud, Talar, Babolrud, Gorganrud, Tajan, Haraz, Sardabrud, Aras, Tonekabon, Polrud, Sefidrud and Anzali Lagoon (Kiabi et al. 1999; Nazari 2007). The habitat of this species in the southern Caspian Sea proper is unknown although some specimens have been caught in the Caspian Sea at 600–700 m (Jolodar and Abdoli 2004; Coad 2008).
Data and analysis of Pravdin (1913b) by Holčík (1986) clearly indicates that in the autumn and winter of 1911, at least two schools of Caspian Lamprey entered the Volga Estuary. The first entered on September 28, and the second, on October 10. Movement upriver only occurs at night, near the surface when dark and on the bottom when the moon is out. During the day, the lampreys hide among stones (Holčík 1986; Coad 2008). Ginzburg (1969, 1970) examined the reproduction of this species below the Volgograd Dam on the Volga River and similar conditions may occur in Iran (Coad 2008). Caspiomyzon wagneri spawns from the end of March to the beginning of July (Pravdin 1913a, b; Abdurakhmanov 1962; Ginzburg 1969, 1970; Agamaliev 1970, 1971; Koblitskaya 1981) according to geographical location of the river and the distance of the spawning sites from the estuary (Holčík 1986). Females release all their eggs but males may spawn again with other females, ammocoetes hatch after 8–10 days at 17–23°C and metamorphosis of ammocoetes occurs at 8.0–11.0 cm in October in Iran (Coad 2008). The duration of the Caspian Lamprey larval stage is estimated to be 3 years in the Volga River (Dyuzhikov 1956; Ginzburg 1970) and 2 to 4 years in the Kura River System (Holčík 1986). The adult life of this species is at least 1 year and 5 months.
Over half of all lamprey species are considered to be endangered, vulnerable, or extinct in at least a portion of their range (Renaud 1997). The Caspian Lamprey is vulnerable in Europe generally (Lelek 1987; Maitland 1991; Renaud 1997); sharply declining numbers in Russia (Pavlov et al 1985; Renaud 1997); extirpated from the Sefid River and rare in Anzali Lagoon and tributaries, Iran (Renaud 1997). It is vulnerable because it migrates into rivers which are polluted and dammed and because of its restricted and declining distribution (Coad 2008). These conditions apply particularly in Iran, although there is some evidence for spawning based on captures in the 1990s (Holčík and Oláh 1992). The loss of spawning grounds is the major cause of decline in the population size of Caspian Lamprey in Iranian water bodies (Kiabi et al. 1999), and this species is in the “near threatened” category (IUCN 1996; Kiabi et al. 1999). These problems have led to the need for special protection of the species in many localities.
There are surprisingly few papers and information about the migration and reproductive ecology of Caspian Lamprey in the southern Caspian Sea basin rivers, and few studies have been conducted (Noori 1990; Ghasempouri 1993; Kiabi et al. 1999; Shirazinejad and Saremi 2000; Jolodar and Abdoli 2004).
The purpose of this paper is an investigation of reproductive biology and migration of the Caspian Lamprey in the Shirud River during the spring migration in 2006 for conservation purposes. The main objectives of this study were: (1) investigation of correlation between water temperature and spawning migration; (2) determination of sex ratio, gonadosomatic index (GSI), egg size and fecundity; and (3) investigation of total length, total weight, length–weight relationship and size distribution.
Materials and methods
Study area
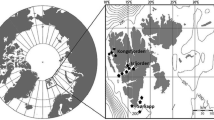
The study was carried out in the Shirud River (34° 44 ′–36° 51′ N, 50° 48′–50° 49′ E; Fig. 1). The Length of this river is about 36 km, width in the estuary 50–80 m, and depth 1.5–2.5 m. The upper substrate is composed of pebbles mixed with gravel and sand and below them it is mostly sand and mud. The River has a high water flow and high water clarity.
The study site was located at Shirud Bridge (∼200 m upstream from the river mouth). Caspian Lamprey alternate between swimming activity and resting, fixed by means of the oral sucker to the concrete bottom under Shirud Bridge or other convenient support structures. Among the rivers along the southern Caspian Sea basin (Iran) that are still colonized by the Caspian Lamprey, the Shirud River has the largest population of this species (pers. obs.). This river supports the largest fisheries of Caspian Lamprey and southern Caspian Kutum (Rutilus frisii kutum) in Iran (Nazari 2007, pers. obs).
Lamprey count, collection and investigation of reproduction
Caspian Lamprey spawners initiate their migration in the Shirud River in mid-March depending on the meteorological conditions. We observed a migratory group of Caspian Lampreys for the first time in the Shirud River from September 21 to late-October 2006. Their migration period is shorter than the spring group migration period (Nazari 2007; H. Nazari and A. Abdoli, unpublished data). This observation was strengthened by information gathered from interviews with local fishermen and poachers used to gain a better understanding of the species status.
Adult lampreys moved upstream exclusively at night. Only night migrating lampreys were caught and counted. Although we monitored lampreys for their upstream migration from 12 March–10 May in 2006, specimens were only collected from 26 March–2 May for other reproductive characteristics of this species. Visual counts of adult lampreys under the Shirud Bridge were made daily (from sunset to sunrise). A total 575 of lampreys were counted during the study period. Of these, 364 were returned to the river some 300 m above the study site. Of the 575 lampreys counted, ∼15 (2.6%) were collected by hand net (cast net, mesh size 8 mm). For further study, 211 were preserved in 10% formalin to facilitate sex determination and measurements.
The sex was determined by external sex characters (male with well-developed urogenital papillae and sperm (number: 11), female with obvious eggs in the abdominal cavity and/or swollen base of second dorsal fin and a crescent-shaped extra ‘anal fin’ (number: 15), Holčík 1986; Cochran et al. 1993; Yamazaki et al 1998; Gibson 1953; Kelly and King 2001) or by dissection in the case of weakly expressed sexual dimorphism (number: 185). Secondary sex characters first appeared at the end of March. The cloaca1 swelling among females was observed first on 29 March before spawning. A urogenital papilla was observed first among some males on 7 April.
We determined total length (TL ± 0.01 mm), total weight (W t ± 0.01 g), and female gonad weight (±0.01 g). The length–weight relationships were determined according to the allometric equation (Biswas 1993):
where W is the total body weight (g), L the total length (mm), and a and b are constants.
The individual absolute fecundity (IAF) was determined by the counting–weighing method, the number of eggs in 1 g being counted (Kucheryavyi et al. 2007). The fecundity was determined according to the following formula:
where N and n are the total and a partial number of eggs, respectively, and W and w the total and a partial weight of eggs, respectively (Yamazaki et al. 2001).
Total length is used in this study as the measure of size to relate to fecundity (Docker and Beamish 1991). The individual relative fecundity (IRF) was calculated in relation to the body weight and total length (Kucheryavyi et al. 2007). Gonads of female lampreys were dissected out and weighed for calculation of gonadosomatic index (GSI = gonad weight/body weight × 100) (Fukayama and Takahashi 1985). The gonadosomatic index (GSI) was used to determine the spawning time of the species. The GSI value shows that spawning occurs when the GSI reaches its highest level (Biswas 1993; Abdoli et al. 2002). It was used in 59 female lampreys for determination of fecundity and egg diameter. The egg diameter was determined using a binocular Nikon microscope at magnification 4X. Measurements of 40 eggs from each ovary were obtained.
Water and air temperatures were recorded by a hand-held thermometer at intervals of six hours. The relationship between the frequency of migration and water temperature was determined daily. River temperatures are variable during the migratory period, with an average river temperature of 16.31°C from beginning to end. Furthermore, diurnal fluctuations of the temperature were observed (see Fig. 2). To estimate potential migration timing thresholds, we assessed cumulative lamprey passage distributions in relation to water temperature using 1°C increments (Keefer et al. 2009).
Data analysis
SYSTAT version 9.0 and Excel 6.0 were used to carry out the statistical analyses. Significance differences in the sex ratio were estimated with the Chi-Square test. Significance differences in mean length and weight in both of sexes were estimated with the Student’s t-test and Covariance, respectively. Mean dates were compared using ANOVA test. All analyses were performed with a significance level of P = 0.05.
Results
Spawning migration
During the spring spawning migration periods (16 March–2 May) in 2006, we observed adult Caspian Lamprey migration and swimming behaviors. Numbers of lampreys observed per night ranged from 1 to 60, averaging 17 individuals during the sampling period. The peak migration was between 26 March–10 April and 15 April–25 April (Fig. 3).
The patterns of lamprey migration are shown in Fig. 3. During the sampling period, 56% of lampreys migrated between 26 March and 7 April before ending movement on 2 May. Lampreys are apparently absent here during the summer months but start to appear on 21 September. They occur sporadically during the 16 March until 26 March, but they were not caught; after 26 March, the numbers rise again to 2 May and then none are seen until the following autumn.
Cumulative lamprey passage distributions at Shirud Bridge showed that 37% lampreys passed before water temperatures reached 13°C (Fig. 4). On average, about half the run had passed before ∼14°C. About 75% of the run had passed by the time water temperatures reached 16–17°C. A migration pause occurred when Shirud River water temperatures exceeded 21°C, usually on ∼2 May.
During the study periods, the water and air temperatures were 11–21.25°C (mean±SD—16.31 ± 2.63) and 10–19.25°C (14.40 ± 2.29), respectively. The most intensive migration lamprey was in the water temperature 16°C (34.43%) (Fig. 5). A linear relationship between the frequency of migratory lampreys and water temperature was observed (p < 0.05). The linear regression revealed that mass migration negatively correlated to the increasing water temperature during the sampling period.
Our specimens showed strong daily patterns of movement during the spawning migration, moving mainly at night. Peak migratory activity was reached about 2 hours after sunset (∼21:00) and generally declined until about 03:00.
Total length, total weight and length–weight relationship
The body length of males of this species varied within 271–451 (mean±SD—383.4 ± 30.6) mm, of females within 310–485 (386.5 ± 44.95) mm. The body weights of males and females were 34.5–145.15 (103.6 ± 19.9) g and 59.36–164.1 (106.7 ± 18.3) g, respectively. The largest specimen was observed on 18 April. The length–weight relationships were separately evaluated for females and males. The exponent b demonstrated allometric growth. Comparing the length–weight relationships of the sexes using covariance analysis, no significant difference was found (p > 0.05); but positive correlation was observed between total length and weight in both sexes. The equation for the relationship was W = 0.0005 × L2.044 (R2 = 0.74) for females, and W = 0.0008 × L2.367 (R2 = 0.80) for males. The size distributions of the migrating lampreys are shown in Fig. 6.
Gonadosomatic index, fecundity, egg size and sex ratio
Mean gonad weight of females varies within 6.15–19.67 g (mean±SD—11.72 ± 2.85). The gonad weight suddenly increases in mid-April. The gonadosomatic index (GSI) of females was 5.83–31.44 (11.22 ± 4.30). Mean GSI values in the female lampreys rose from a mean of 8.85 ± 1.13 in late March rose from to a peak (20.49 ± 6.82) in mid-April (significantly different, p < 0.05; Fig. 7). Lower GSI values after mid-April were related to slower maturing of some unspent individuals. The increase of GSI values was well in accord with an increase in the absolute weight of ovaries which averaged 13.85 ± 3.36 g in mid-April. The GSI results revealed that spawning occurred after mid-April, when the GSI reached its highest level. However, the presence of mature individuals in late April showed that reproduction may continue at a reduced rate during May. The appearance of female post-anal fins (or cloaca1 swelling) and a change in the form of dorsal fins occur in females on 7 April. Also, two fully spent female lampreys were collected on 21 April (18.5°C) near the study site (∼50 m downstream), still alive, the maximum temperature reached by the river before then being 21°C on 14 April. No dead spent lampreys were observed.
The individual absolute fecundity was 31 758–51 198 eggs (mean±SD—41 924 ± 5,382). The egg diameter was 0.780–1.15 (0.92 ± 0.081) mm. The individual relative fecundity varied from 80.3 to 148.1 (107.2 ± 15.1) eggs per 1 mm of length and from 260.8 to 677.4 (397.6 ± 93) eggs per 1 g of weight. There was no significant relationship between absolute fecundity and total length. Relative fecundity decreased with total length and differed significantly (Fig. 8, p < 0.05).
The overall sex ratio of male to female lampreys (1.07:1) was not significantly different (p > 0.05). The male to female ratios for each week are presented in Table 1. The proportion of females was lower at the beginning and end of the spawning migration, but there is no significant difference in sex ratio each week (p > 0.05).
Discussion
Caspian Lamprey spawners initiate their migration in the Shirud River on the 16 March, with the migration peak between 26 March–10 April and 15 April–25 April. In the Kura River of Azerbaijan, the peak of this run is in December and January. The spawning migration in the Volga takes place from the middle of September to the end of December (Holčík 1986; Coad 2008). Ginzburg (1969) demonstrated the maximum migration at Volgograd in February. Holčík (1986) reported that construction of river barriers in the Volga and Kura Rivers negatively influenced the upstream migration of Caspian Lamprey and evidently caused a change in its season.
In this study we observed most lamprey migration during the night (21:00–3:00 h) and lampreys often alternate between swimming activity and resting, fixed by means of the oral sucker to the concrete bottom under Shirud Bridge. Similar observations have been shown for some other lampreys species (e.g., Malmqvist 1980; Kelly and King 2001; Almeida et al. 2002; Quintella et al. 2004; Almeida et al. 2007; Andrade et al. 2007; Binder and McDonald 2008; Quintella et al. 2009). A similar pattern of migration has been described for the landlocked Sea Lamprey, whose peak of migratory activity was reached about 2 hours after nightfall and generally declined until about 02:00 hours (Hardisty and Potter 1971; Almeida et al. 2002). Robinson and Bayer (2005) reported that swimming activity of Pacific Lamprey (Lampetra tridentata) occurred only during the 8 to 12 hours of darkness each day. The light–dark cycle plays a crucial role in shaping the activity of Sea Lamprey (Binder and McDonald 2007).
In this study we found a significant relationship between mass migration of Caspian Lamprey and temperature, as increasing water temperature was negatively correlated to the mass migration. Similar results have been reported by Keefer et al. (2009) for the adult Pacific Lamprey (Lampetra tridentata) in the Columbia River. In the Volga and Kura Rivers, migration is stimulated by decreasing temperature and increasing water level (Holčík 1986). In the northern Caspian Sea, the most intensive migration was observed at a water temperature of 6 to 11°C (Pravdin 1913a, b; Abdurakhmanov 1962; Holčík 1986), but in this study the most intensive migration was in the water temperature 16°C (34.43%) and about 75% of the run had passed by the time water temperatures reached 16–17°C. Binder and McDonald (2007) reported that temperature plays a role in controlling daily activity in upstream migrant Sea Lamprey. Applegate (1950), who studied the spawning migration of the landlocked Sea Lamprey, Petromyzon marinus, noted a strong correlation between temperature and migration. Tesch (1967) did not find any correlation between numbers of migrants and hydrographical factors, but suggested lunar influence initiated the migration.
Holčík (1986) and Coad (2008) reported that spawning begins of Caspian Lamprey at 15–16°C, usually in early June but sometimes at the end of March through to the beginning of July, and temperatures during spawning are usually 15–23°C. Full sexual maturity occurs at 13 to 18°C in the Kura basin (Abdurakhmanov 1962). Many lampreys are known to spawn in waters 10–12°C (e.g., Hardisty and Potter 1971; Kelly and King 2001; Takayama 2002), 10–15°C (Close et al. 2002; Almeida et al. 2002; Robinson and Bayer 2005), 15–24°C (Cochran and Gripentrog 1992) and rarely in waters at 22–24°C (Hardisty and Potter 1971). The water temperature during this study for Caspian Lamprey was 11–21°C.
Holčík (1986) reported that female Caspian Lamprey are slightly larger than males, but in this study no significant difference was observed between total length and weight in both sexes. Similar result has been reported for the Eastern brook lamprey (Lethenteron reissneri) by Takayama (2002). The mean total length of males and females from the Volga River is 360 and 369 mm, respectively (Dyuzhikov 1956). In the Kura River, total length of male varies from 426 to 432 mm, and females from 436 to 440 mm (Smirnov 1952), but in this study total length of males and females of this species varied within 271–451 and 310–485 mm, respectively. Caspian Lamprey from the Kura River are larger, as 81% of the whole catch is composed of specimens 410 to 460 with mean 432 mm (Abdurakhmanov 1962) (Table 2).
The gonadosomatic index (GSI) of females reported by various authors (Pravdin 1913a, b; Smirnov 1953; Holčík 1986; Noori 1990; Shirazinejad and Saremi 2000) varies for pre-spawning from 2.67 to 11.7 and for spawning from 12.12 to 33.55. These are similar to our observations (Table 2). Ginzburg (1969) found that the gonadosomatic index (GSI) in both sexes is rather stable during the winter, but it strongly increases in the spring months, correlated with increasing water temperature. The gonadosomic index of females showed a steady rise from a mean of 8.85 in late March to 20.49 in middle April. Holčík (1986) showed in the fully ripened females in the Kura River, GSI value rose to between 28 and 29. Fukayama and Takahashi (1985) observed for the Japanese River Lamprey (Lampetra japonica) during the period of upstream migration, mean GSI value in female lampreys increasing gradually as vitellogenesis progressed, reaching 14.03 ± 1.49 in April when ovarian oocytes were near to the end of exogenous vitellogenesis.
Caspian Lamprey have been reported to die after spawning (Holčík 1986). Death after spawning is probably closely correlated with exhaustion of body reserves (Larsen 1980). Although, Michaels (1980; 1982) reported that some lampreys survived to spawn a second time. The spent Caspian Lamprey captured in a downstream migration of Shirud River were still alive at the time of capture. During this study no dead spent lampreys were observed. Chase (2001) reported that it is not known if any were able to successfully readapt to a saltwater existence and survive to spawn a second time.
The sex ratio (male: female) of Caspian Lamprey reported in the Volga River range from 1.13:1 to 1.94:1 (Pravdin1913a, b; Dyuzhikov 1956; Ginzburg 1969; Holčík 1986). Noori (1990), Ghasempouri (1993) and Shirazinejad and Saremi (2000) reported that the sex ratio of Caspian Lamprey in the Babolrud, Talar and Shirud Rivers is 2.33:1, 2.97:1 and 2:3, respectively. In this study, a sex ratio of around 1:1 and increase in the number of females compared to males in second and third weeks were found. Smirnov (1953) reported that males predominate at the beginning of the migration. This was similar to our results. A similar sex ratio (∼1:1) have been reported for some other lampreys species (e.g., Mundahl and Sagan 2005; Jang and Lucas 2005; Binder and McDonald 2008; Beaulaton et al. 2008).
The fecundity of Caspian Lamprey reported by various authors (Pravdin 1913a, b; Berg 1948; Smirnov 1953; Ginzburg 1969; Holčík 1986; Noori 1990; Shirazinejad and Saremi 2000) varies from 14,000 to 60,000. Our study result agrees with these findings. Fecundity for Pacific Lamprey (Lampetra tridentata) ranged from 98.000 to 238.400 eggs per female (Close et al. 2002) and for the River Lamprey (Lampetra planeri) ranged from 7,500 to 40,000 per female (Kelly and King 2001). In present study, the individual relative fecundity varies from 260.8 to 677.4 (397.6 ± 93) eggs per gram body weight. Relative fecundity for Pacific Lamprey (Lampetra tridentata) ranged from 417.94 to 522.15 eggs per gram body weight (Close et al. 2002) and for Brook Lamprey (Icchthyomyzon jossor) ranged from 500 to 700 eggs per gram body weight (Vladykov 1951; Beamish 1982). Absolute fecundity in many species is positively correlated with female size (Bagenal 1966, 1973). Our data did not find any correlation (R2 = 0.04) between the total length of lamprey and the absolute fecundity. Similarly, Noori (1990) and Shirazinejad and Saremi (2000) did not find any correlation either, whereas Holčík (1986) obtained significant correlation between the total length of lamprey and the absolute fecundity as well as Beamish (1982) and Docker and Beamish (1991) obtained such significant correlation in other lamprey species. In this study, relative fecundity decreased with total length. The decrease in relative fecundity with female length has been observed for the Ichthyomyzon gagei by Beamish (1982) and for the Lampetra aepyptera by Docker and Beamish (1991).
In conclusion, we observed that increasing water temperature negatively correlated to the mass migration. Further studies are required to examine the effect of other environmental factors in the migration of this species. We were unable to observe Caspian Lamprey at spawning time and perform assessments of spawning habitat and spawning behavior in this study. Future investigations should address the role of Shirud River in Caspian Lamprey spawning and larval rearing. Also, further investigations are necessary on the upstream migration distance, habitat requirement and spawning grounds and downstream migration distance of spent lampreys.
References
Abdoli A, Rahmani H, Rasooli P (2002) On the occurrence, diet and reproduction of Neogobius fluviatilis in Madarsoo stream, Golestan National Park (north eastern Iran). Zool Mid East 26:123–128
Abdurakhmanov YUA (1962) Rўbў presnўkh vod Azerbaidjana. Izd Akad naud Azerb SSR, Baku (in Russian)
Agamaliev AS (1970) Razmnozhenie kaspiǐskoǐ minogi v basseǐne kury v usloviyakh gidrostroiteǏstva. Trudў molodўkh uchënnўkh VNIRO 4:112–118 (in Russian)
Agamaliev AS (1971) Lichinochnyǐ period zhizni kaspiǐskoǐ minogi (Caspiomyzon wagneri Kessler). Trudў VNIRO 36:139–148 (in Russian)
Almeida PR, Quintella BR, Dias NM (2002) Movement of radio-tagged anadromous Sea Lamprey during the spawning migration in the River Mondego (Portugal). Hydrobiologia 483:1–8. doi:10.1023/A:1021383417816
Almeida PR, Póvoa I, Quintella BR (2007) Laboratory protocol to calibrate Sea Lamprey (Petromyzon marinus L.) EMG signal output with swimming effort. Hydrobiologia 582:209–220. doi:10.1007/s10750-006-0539-8
Andrade NO, Quintella BR, Ferreira J, Pinela S, Póvoa I, Pedro S, Almeida PR (2007) Sea Lamprey (Petromyzon marinus L.) spawning migration in the Vouga river basin (Portugal): poaching impact, preferential resting sites and spawning grounds. Hydrobiologia 582:121–132. doi:10.1007/s10750-006-0540-2
Applegate VC (1950) Natural history of the Sea Lamprey, Petromyzon marinus, in Michigan. US Fish Wildl Serv Spec Sci Rep Fisheries 55:1–237
Bagenal TB (1966) The ecological and geographical aspect of fecundity of plaice. J Mar Biol Assoc UK 46:161–186
Bagenal TB (1973) Fish fecundity and its relations with stock and recruitment. Rapp Pv R Cons int Explor Mer 164:186–198
Beamish FWH (1982) Biology of the southern brook lamprey, Ichthyomyzon gagei. Environ Biol Fish 7(4):305–320. doi:10.1007/BF00005566
Beaulaton L, Taverny C, Castelnaud G (2008) Fishing, abundance and life history traits of the anadromous Sea Lamprey (Petromyzon marinus) in Europe. Fish Res 92:90–101. doi:10.1016/j.fishres.2008.01.001
Berg LS (1948) Freshwater Fish of the U.S.S.R. and Adjacent Countries. Israel Program for Scientific Translation 1962–1965
Binder TR, McDonald DG (2007) Is there a role for vision in the behaviour of Sea Lampreys (Petromyzon marinus) during their upstream spawning migration? Can J Fish Aquat Sci 64:1403–1412. doi:10.1139/F07-102
Binder TR, McDonald DG (2008) The role of temperature in controlling diel activity in upstream migrant Sea Lampreys (Petromyzon marinus). Can J Fish Aquat Sci 65:1113–1121. doi:10.1139/F08-070
Biswas SP (1993) Manual of methods in fish biology. South Asian, New Delhi
Chase SD (2001) Contributions of the life history of adult Pacific Lamprey (Lampetra tridentata) in the Santa Clara River of southern California. Bull Southern California Acad Sci 100:74–85
Close DA, Fitzpatrick MS, Li HW (2002) The ecological and cultural importance of a species at risk of extinction, Pacific Lamprey. Fisheries 27:19–25. doi:10.1577/1548-8446(2002) 027<0019:TEACIO>2.0.CO;2
Coad BW (2008) Petromyzontidae. Available. http://www.briancoad.com/Species%20Accounts/Petromyzontidae.htm. Accessed 18 July 2009
Cochran PA, Gripentrog AP (1992) Aggregation and spawning by lampreys (genus Ichthyomyzon) beneath cover. Environ Biol Fish 33:381–387. doi:10.1007/BF00010950
Cochran PA, Sneen ME, Gripentrog AP (1993) Notes on the biology of the American brook lamprey (Lampetra appendix) in Wisconsin. Trans Wisconsin Acad Sci Arts Lett 81:39–46
Docker MF, Beamish FWH (1991) Growth, fecundity, and egg size of least brook lamprey, Lampetra aepyptera. Environ Biol Fish 31:219–227. doi:101007/BF00000688
Dyuzhikov AT (1956) O biologii i promўsle volzhskoǐ minogi. Uchënnўe zapiski Saratovsk. Gosuniv. Im. N. G. Chernўshevskogo, vўp Biol 51:87–101. (in Russian)
Fukayama S, Takahashi H (1985) Changes in serum levels of estradiol–17β and testosterone in the course of sexual maturation. Bull Fas Fish Hokkaido Univ 36(4):163–169
Ghasempouri SM (1993) The Caspian Lamprey. Abzeeyan 4(7):18–21 (in Farsi)
Gibson FA (1953) Brook lamprey, Lampetra branchialis Day, in a Westmeath stream. Ir Nat J 11:86–89
Ginzburg YI (1969) Nerestovaya populyastiya minogi (Caspiomyzon wagneri Kessler) posle zaregulirovaniyar. Volgi plotinoǐ Volgogradskoǐ GES, Vopr, Ikhtiol 9:1022–1031 (in Russian)
Ginzburg YI (1970) Razmozhenie minogi Caspiomyzon wagneri (Kessler) nizhe volgogradskoǐ. Plotinў I razvitie eë lichinok, Vopr, Ikhtiol 10:655–665 (in Russian)
Hardisty MW (1986) General introduction to lampreys. In: Holčík J (ed) The freshwater fishes of Europe. Aula-Verlag, Wiesbaden, pp 19–83
Hardisty MW, Potter IC (1971) The general biology of adult lampreys. In: Hardisty MW, Potter IC (eds) The biology of lampreys. Academic, London, pp 127–206
Holčík J (1986) Caspiomyzon wagneri (Kessler, 1870). In: Holčík J (ed) The freshwater fish of Europe. Aula-Verlag, Wiesbaden, pp 119–142
Holčík J, Oláh J (1992) Fish, fisheries and water quality in Anzali Lagoon and its watershed. Report prepared for the project—Anzali Lagoon productivity and fish stock investigations. FAO, Rome, FI:UNDP/IRA/88/001
IUCN (1996) IUCN red list of threatened animals. International Union for Conservation of Nature and Natural Resources, Gland
Jang MH, Lucas MC (2005) Reproductive ecology of the river lamprey. J Fish Biol 6:499–512. doi:10.1111/j.0022-1112.2005.00618.x
Jolodar M, Abdoli A (2004) Fish species atlas of south Caspian Sea basin (Iran waters). Iranian Fisheries Research Organization, Tehran
Keefer ML, Moser ML, Boggs CT, Daigle WR, Peery CA (2009) Variability in migration timing of adult Pacific Lamprey (Lampetra tridentata) in the Columbia River, U.S.A. Environ Biol Fish 85:253–264. doi:10.1007/s10641-009-9490-7
Kelly FL, King JJ (2001) A review of the ecology and distribution of three Lamprey species, Lampetra fluviatilis (L.), Lampetra planeri (Bloch) and Petromyzon marinus (L.): a context for conservation and biodiversity considerations in Ireland. Proc R Irish Acad 101B(3):165–185
Kiabi BH, Abdoli A, Naderi M (1999) Status of the fish fauna in the south Caspian basin of Iran. Zool Mid East 18:57–65
Koblitskaya AF (1981) Opredlitel’ molodi presnovodnўkh rўb, 2nd edn. Lëgkaya i pischevaya promyshlennost, Moskva (in Russian)
Kucheryavyi AV, Savvaitova KA, Pavlov DS, Gruzdeva MA, Kuzishchin KV, Stanford JA (2007) Variations of life history strategy of the lamprey Lethenteron camtschaticum from the Utkholok River (Western Kamchatka). J Ichthyol 47(1):37–52. doi:10.1134/s0032945207010055
Larsen LO (1980) Physiology of adult lampreys, with special regard to natural starvation, reproduction, and death after spawning. Can J Fish Aquat Sci 37:1762–1779. doi:10.1139/f80-221
Lelek A (1987) The freshwater fishes of Europe. AULA-Verlag GmbH, Wiesbaden
Lucas MC, Baras E (2001) Migration of freshwater fishes. Blackwell, Oxford
Maitland PS (1991) Conservation of threatened freshwater fish in Europe. Convention on the Conservation of European Wildlife and Natural Habitats, Council of Europe Press, Nat Environ Ser 46:6–76
Maitland PS, Morris KH, East K (1994) The ecology of lampreys (Petromyzonidae) in the Loch Lomand area. Hydrobiologia 290:105–120. doi:10.1007/BF00008958
Malmqvist B (1980) The spawning migration of the brook lamprey, Lampetra planeri Bloch, in a South Swedish stream. J Fish Biol 16:105–114. doi:10.1111/j.1095-8649.1980.tb03690.x
Michael JHJ (1980) Repeat spawning of Pacific Lamprey. Calif Fish Game 66(3):186–187
Michael JHJ (1982) Additional note on the repeat spawning by Pacific Lamprey. Calif Fish Game 66(3):186–187
Mundahl ND, Sagan RA (2005) Spawning ecology of the American brook lamprey, Lampetra appendix. Environ Biol Fish 73:283–292. doi:10/1007s10641-005-2145-4
Nazari H (2007) Evaluation of some population parameters of Caspian Lamprey (Caspiomyzon wagneri) during the migratory season in the Shirud and Talar Rivers southern Caspian Sea. Dissertation, Islamic Azad University of Tehran (in Farsi with English summery)
Noori M (1990) Caspian Lamprey (Caspiomyzon wagneri). In: National Conference on Beneficiary Suitable the Caspian Sea Fisheries Resources, pp 100–115 (in Farsi)
Pavlov DS, Reshetnikov YS, Shatunovski MI, Shilin NI (1985) Rare and disappearing fishes in the USSR and the principles of there inclusion in the “Red Book”. J Ichthyol 25(1):88–89
Pravdin IF (1913a) Nablyudeniya nad kaspiǐskoǐ minogoǐ (Caspiomyzon wagneri Kessler) vesnoǐ 1912 goda. Trudў Ikhtiol Lab Uprav Kasp volzh Rўb i tyul Promўshl Astrakhań 2:1–17 (in Russian)
Pravdin IF (1913b) Osenniǐ khod minogi (Caspiomyzon wagneri Kessler) iz Kaspiǐskogo morya v reku Volga. Trudў Ikhtiol Lab Uprav Kasp volzh Rўb i tyul Promўshl Astrakhań 2:19–40 (in Russian)
Quintella BR, Andrade NO, Koed A, Almeida PR (2004) Behavioural patterns of Sea Lampreys spawning migration during difficult passage areas studied by electromyogram telemetry. J Fish Biol 65:1–12. doi:10.1111/j.1095-8649.00497.x
Quintella BR, Póvoa I, Almeida PR (2009) Swimming behaviour of upriver migrating Sea Lamprey assessed by electromyogram telemetry. J Appl Ichthyol 25:46–54. doi:10.1111/j.1439-0426.2008.01200.x
Renaud CB (1997) Conservation status of northern hemisphere lampreys. J Appl Ichthyol 13:143–148. doi:10.1111/j.1439-0426.1997.tb00114.x
Robinson TC, Bayer JM (2005) Upstream migration of Pacific Lampreys in the John Day River, Oregon: behavior, timing, and habitat use. Northwest Sci 79:106–119
Salewski S (2003) Satellite species in lamprey: a worldwide trend for ecological speciation in sympatry? J Fish Biol 63:267–279. doi:10.1046/j.1095-8649.2003.00166.x
Shirazinejad AR, Saremi A (2000) Biology of Caspian Lamprey (Caspiomyzon wagneri). Dissertation, Esfehan University of Technology (in Farsi)
Smirnov AN (1952) Vidovaya kharak–teristika kurinskoǐ minogi (Caspiomyzon wagneri Kessler). Izvestiya Akad, Naud Azerb SSR 6:51–56 (in Russian)
Smirnov AN (1953) Materialў po biologii kurinskoǐ minogi. Trudў Inst Zool Akad naud Azerb SSR 15:52–86 (in Russian)
Takayama M (2002) Spawning activities and physical characteristics of the spawning ground of Lethenteron reissneri at the headstream of the Himekawa River, central Japan. Ichthyol Res 49:165–170. doi:10.1007/s102280200022
Tesch FW (1967) Aktivitat and Verhalten wandernder Lampetra fluviatilis. Lota Iota and Anguilla anguilla im Tidegebiet der Elbe. Helgolander wiss. Meeresuntersuchungen 16:92–111 doi:10.1007/BF01620691
Vladykov VD (1951) Fecundity of Quebec lampreys. Can Fish Cult 10:1–14
Yamazaki Y, Sugiyama H, Goto A (1998) Mature dwarf males and females of the arctic lamprey, Lethenteron japonicum. J Ichthuol Rec 45(4):404–408. doi:10.1007/BF02725194
Yamazaki Y, Konno S, Goto A (2001) Interspecific differences in egg size and fecundity among Japanese lampreys. Fisheries Sci 67:375–377. doi:10.1046/j.1444-2906.2001.00257.x
Acknowledgements
We thank Gh. Vossoughi, B. Kiabi, F. Kaymaram, R. Ghorbani, A. M. Hajimoradloo and R. Patimar for their advice, M. Molaee for the use of lab equipment, local professional fishermen and personnel of the Center for Propagation and Culture of Fish Shahid Rejaee-e-Sari (particularly, Ch. Makhtumi) for their assistance in the field. We also thank W. B. Coad (Research Scientist, Canadian Museum of Nature, Ottawa, Ontario) and B. H. Kiabi (associated professor of Shahid Beheshty University-Tehran) to edit the paper. This research was made possible by Shahid Beheshti University (G, C) and Islamic Azad University, Science and Research Branch of Tehran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reproductive Caspian Lamprey in Iran
Rights and permissions
About this article
Cite this article
Nazari, H., Abdoli, A. Some reproductive characteristics of endangered Caspian Lamprey (Caspiomyzon wagneri Kessler, 1870) in the Shirud River southern Caspian Sea, Iran. Environ Biol Fish 88, 87–96 (2010). https://doi.org/10.1007/s10641-010-9620-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-010-9620-2