Abstract
The daubed shanny Leptoclinus maculatus is a relatively common fish species in Svalbard waters, but little is known about its reproduction. In total, 207 specimens (76 females, 131 males) were collected and examined. Gonadosomatic (GSI) and hepatosomatic indices were significantly higher in females (mean 2.8 and 2.7%, respectively) than males (mean 0.3 and 1.8%) in all seasons. The GSI of females was highest during autumn and winter (18.5%) while the GSI of males was highest in winter (1.7%). Females become sexually mature at 125 mm standard length (SL) and 7 years of age while males become sexually mature at similar length and 6 years of age. The number of oocytes in gonads increased with SL and age and comprised two groups: (1) 115–124 mm SL, 5–7 g gutted weight (GW) and 5–6 years of age with potential fecundity of 950–1850 oocytes, (2) 125–134 mm SL, 7–9 g GW and 7–12 years of age with 1250–2800 oocytes. The daubed shanny seems to spawn all its ripe oocytes during one spawning event each year. Fish become sexually dimorphic after maturation, and differences in sexual morphology are likely linked to reproduction, with female parental care and territorial defence for males. The reproductive strategy of daubed shanny is characterized by late maturation, relatively low fecundity and large size of offspring, i.e. large investment in relatively few offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The daubed shanny Leptoclinus maculatus (Fries, 1838) (suborder Zoarcoidei: family Stichaeidae) is a common species of the epibenthic ichthyofauna in the Arctic waters of Norway. In the Barents Sea, this species is widely distributed on mud and pebbly bottoms at 2–475 m depth south to the Norwegian coast (Byrkjedal and Høynes 2007; Balanov et al. 2011; Wienerroither et al. 2011, 2013). The daubed shanny is commonly caught in bottom trawl surveys and it is an important prey for pelagic (as larvae) and benthic (as adults) predators. Because it is generally associated with low temperatures (around 0 °C), it also occurs north of the polar front and in the Svalbard area. From a zoogeographic point of view, it is considered an arctic-boreal species (Mecklenburg et al. 2018) and may therefore be negatively affected by ocean warming with associated borealization of fish communities in the Arctic (Fossheim et al. 2015). Thus, its populations may be subjected to increased predation by expanding distribution of Atlantic cod Gadus morhua as well as bycatch in trawling for commercial benthic species (Christiansen 2017; Haug et al. 2017).
The family Stichaeidae contains six subfamilies with 37 genera and 76 valid species (Mecklenburg and Sheiko 2004). Little is known about the reproductive behaviour of Stichaeidae (Coleman 1992). Blumer (1982) classifies Stichaeidae as having female-only or male-only parental care in which the guarding parent may be coiled around the eggs, fanning the eggs and moving the eggs if necessary. The few existing studies show that some stichaeid species exhibit male parental care (e.g. Marliave and DeMartini 1977; Green et al. 1987), which is the most common form of parental care in bony fishes (Blumer 1982), whereas others exhibit female parental care (e.g. Peppar 1965; Shiogaki 1983).
The life history of daubed shanny comprises a pelagic phase of early postlarvae and a benthic phase of late postlarvae, juveniles and adults (Meyer Ottesen et al. 2011). The transition between pelagic and benthic life mode happens when the postlarvae reach approximately 80 mm in length at 3 years of age (Meyer Ottesen et al. 2011). At that time, their lipid sac (Falk-Petersen et al. 1986) is absorbed and its body becomes densely pigmented and changes into a deeper more powerful body with larger jaws and eyes. Such changes in morphology presumably increase their survival in the benthic habitat (Meyer Ottesen et al. 2011). After settling, the daubed shanny may spend up to 2 years before the transformation from late postlarvae into juvenile is complete (Meyer Ottesen et al. 2011), and a further 1–2 years as juveniles before reaching sexual maturation. Females seem to mature when they reach 7 years of age and 125–130 mm in length (Murzina et al. 2012). The daubed shanny displays sexual dimorphism and males grow larger and have longer life span than females (Meyer Ottesen et al. 2014). Furthermore, males are larger than females of the same age and have higher growth performance than females, related to differential investment of energy into reproduction (Meyer Ottesen et al. 2014). Spawning has been indicated to occur during winter in the North Atlantic (Pethon 2005), although it may occur earlier, during the autumn, in the southern Barents Sea, as shown by capture of females with large eggs (1.5 mm Ø) in October on the Murman Shoal (Dolgov 1994). Larvae have been recorded in the south-eastern Barents Sea in April–May (1970–1972) and June–July (1968 and 1971) based on data from Russian ichthyoplankton surveys 1959–1993 (Mukhina 2005). Daubed shanny has relatively low fecundity (≤ 1000 eggs; Andriyashev 1954; Dolgov 1994; Pethon 2005) and probably exhibits parental care, as has been reported for several species within the Stichaeidae family (Baylis 1981; Gross and Shine 1981; Blumer 1982).
Even though the daubed shanny is common in Svalbard waters and the Barents Sea, little data have been obtained on the life-history aspects of this species. The study by Murzina et al. (2012) determined oogenesis and lipids in gonads and livers of females from Svalbard, and focussed on histological changes during oocyte development. Lipid classes and fatty acids in liver, muscle and gonads of females were further assessed in Murzina et al. (2013). In this paper, we examine sex distribution and determine gonadosomatic (GSI) and hepatosomatic (HSI) indices with length and age of females and males over seasons and length groups (mature vs. immature specimens) in Svalbard waters. While some aspects of GSI and HSI were treated in Murzina et al. (2012), our study compared indices for males and females based on seasonal samples from several locations in Svalbard and further described reproductive aspects and sexual dimorphism. Our aim is to determine how gonadal development and sexual dimorphism change prior to spawning and thereby infer spawning time for this species as an integral part of its life history with regard to reproduction. We further describe potential fecundity (eggs in ovary) and ripe oocytes (% of total in ovary) with size and age and with GSI and HSI, and discuss how maturation influences morphometric characters.
Materials and methods
Sampling
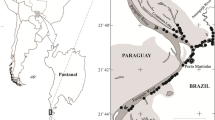
Daubed shannies were sampled during fish surveys in Svalbard fjord and shelf areas (Fig. 1) during several cruises in 2006–2008 (April–May–July, August, September and October) with R/V Helmer Hanssen, UiT The Arctic University of Norway. Juvenile and adult specimens were sampled on soft bottoms (mud, clay or silt) by a Campelen 1800 shrimp trawl (mesh size ~ 10 mm) with towing speed of 3 knots for ~ 20 min at depths 150–400 m. The specimens were immediately frozen (− 40 °C) in labelled plastic zip-lock bags for subsequent laboratory analyses at UiT and the Norwegian Polar Institute.
Svalbard with sampling stations (black stars) on the coast and inside Isfjorden and Kongsfjorden, for collections in Table 1. Depth contours are 200 m
Processing of material
The six sampling months were joined into four seasons: winter (April), spring (May), summer (July and August) and autumn (September and October). A total of 207 specimens were analysed: 76 females and 131 males (Table 1). Intact fish were weighed (i.e. total body weight, 0.1 g) and the standard length (SL, mm) was measured with a ruler (from tip of the snout to end of the caudal peduncle; tail fin was not included because it was often eroded or damaged during trawling). Morphometric measurements were done on 152 specimens (51 females and 101 males) as described in Meyer Ottesen et al. (2011), with the added measurement of pectoral fin length. The abdominal cavity was opened with scissors and cut from the gill cavity to approximately 5 mm behind the anus. Sex was determined from macroscopic analysis of the gonads. The gonads and liver were dissected and weighed (0.01 g) separately. Maturation stages (5 for females, 3 for males) were assigned to gonads based on their appearance: general size of ovary or testis, colour of ovary or testis, oocyte size, colour of oocyte, presence of lipid droplets in oocyte and consistency of testis (Table 2). After removal of the remaining internal organs in the abdominal cavity, gutted weight (GW) was determined (0.1 g), and gonadosomatic index (GSI) and hepatosomatic index (HSI) were calculated as percent of GW. The HSI was included because energy (as lipids) stored in liver may be mobilized during development of gonads. Gonad length (mm) was measured: ovary length and width for females and testis length for males. Gonad length index (GLI) was determined as gonadal length in percentage of SL. Oocyte size was measured in samples from different parts of the ovary in order to get their approximate size-range for different gonad stages (Table 2). Finally, the otoliths were removed, cut in half, mounted on black clay and cleared in glycerol for age readings using a stereo microscope (2–4 × magnification). The interpretation of the annuli followed the “Manual on generalized age determination procedures for groundfish” (CARE 2000).
Fecundity for 14 females (size-range 103–134 mm) from Isfjorden, sampled in October 2007, was estimated using the gravimetric method in which potential fecundity is determined as the product of gonad weight and oocyte density (Murua et al. 2003). Oocyte density was determined by counting number of oocytes in a weighed sub-sample (Murua et al. 2003) from the middle of the ovary lobe. Oocytes were divided into ripe (large, round oocytes with lipid droplets in the centre) and unripe (small, uneven oocytes without visible lipid droplets). The number of ripe oocytes in sub-sample from middle of the ovary was expressed as percent of total number of oocytes in that sub-sample.
Data treatment
Statistical treatments and graphical presentation of the data were done in SYSTAT12 (2007 version), with specimens from all sampling areas pooled for analyses. Based on the result of GSI, as well as the conclusions of Meyer Ottesen et al. (2014), two maturation groups were identified: immature specimens (85–124 mm) and mature specimens (125–155 mm). Two-sample t test was used to test for significant differences in GSI and HSI between sex and stage/length group within each season. ANOVA was used to test for significant differences in GSI and HSI among seasons for each sex and stage (length group). A two-sample t test was used to test for differences in potential fecundity and percent ripe oocytes (of total) between length groups. A linear regression was used to test GSI–HSI relationships of adult females and males. Morphological differences between sexes were tested with discriminant analysis, using a complete model with all variables entered simultaneously into the model (Online Resource 1). The length groups were tested by two-sample t test to determine differences between sexes with regard to morphometric measurements before and after maturation.
Results
Sex distribution with season
In general, our samples were dominated by males (~ 63%; Table 1). Males dominated with 57–80% for most of the year except for the spring (36%) and summer (35%) samples from Sørkapp and Kongsfjorden, respectively (Table 1).
Gonad morphology and classification
The gonads were paired, elongated and oriented longitudinally within the abdominal cavity (Fig. 2). The ovary consisted of two lobes that were tightly joined together so that they appeared to be one structure. The ovary contained oocytes of different sizes and stages (Table 2). Numerous small (≤ 0.3 mm Ø), immature oocytes were always present. They did not contain lipid droplets, but rather were filled with a clear liquid. During ovary development, the ovaries changed from white to yellow (Stages 1–2) and subsequently to darker orange and red (Stages 4–5), while the oocyte sizes increased from 0.3 mm to a maximum of 1.7 mm (Table 2). The cortical alveoli formation happened during the secondary growth stage (Stage 3, Table 2), when lipid droplets started to appear in oocytes (Murzina et al. 2012). In autumn, the ovary of large females (SL ≥ 125 mm) occupied approximately 70–80% of the abdominal cavity (Fig. 2a). The ovary of the largest females was filled with dark orange oocytes of different sizes in a sticky mass. Large oocytes (≈ 1.5–1.7 mm Ø) appeared more often in ovaries of large females, although the eggs were not fully hydrated and running. The oocytes contained either several small lipid droplets or one large lipid droplet in the centre.
The male gonads were small and classified based on colour and appearance. The testes occupied approximately 5–10% of the abdominal cavity. The testes were often small and thin and consisted of two string-like lobes, most often with one of the lobes longer than the other (Fig. 2b). They changed from yellow and thin (Stage 1) to orange and swollen (Stage 2) during the early part of maturation (Table 2). In autumn, the testes of large males (SL ≥ 125 mm) were broader, plumper and light pink or milky white with a yoghurt-like consistency (Stage 3, Table 2).
ANOVA tests showed that the gonad stages used for classification of female gonads (Table 2) differed significantly in GSI (p = 0.001) and GLI (p < 0.001), with summer and autumn females having higher GSI and GLI than those from spring and winter. Kruskal–Wallis one-way analysis showed that the male gonad stages did not differ in GSI (p = 0.269), but the GLI was significantly higher (p = 0.008) in summer and autumn than in spring.
Gonad stage varied over the season with highest frequency of ripe, dark orange to red oocytes during summer and autumn and small light to dark yellow oocytes in spring and winter (Fig. 3a). We also observed two spent females (117 and 124 mm SL, Stage 6) with small, white gonads in April. Males had large, light orange (or light pink) to milky-white testes in summer and autumn while in winter and spring the testes were thin and translucent, with yellow to orange colour (Fig. 3b).
Seasonal GSI and HSI with SL and age
Gonadosomatic index (GSI) and hepatosomatic index (HSI) were significantly higher in females (mean 2.8 and 2.7%, respectively) than males (mean 0.3 and 1.8%). The female GSI ranged from 0.5 to 18.5% of GW (Fig. 4a, b) while male GSI ranged from < 0.1 to 1.7% of GW (Fig. 4c, d). The summer and particularly the autumn material showed large variability in GSI in females of the same size. Female GSI was low in all seasons for specimens < 125 mm (Fig. 4a) ≤ 7 years of age (Fig. 4b), at which point GSI increased sharply during summer and autumn. The GSI of females varied significantly over the seasons (p = 0.035), with mean GSI being highest (18.5% of GW) in autumn (October). Males showed similar patterns of GSI with SL and age. Male GSI was low in all seasons for specimens < 125 mm (Fig. 4c) and ≤ 6 years of age (Fig. 4d), at which point GSI increased sharply in the summer, autumn and winter seasons. The GSI of males also differed between seasons (p < 0.001), with the highest GSI (1.7% of GW) during late winter (April). Thus, our results indicated that females become sexually mature at 125 mm and 7 years of age while males become sexually mature at similar length and 6 years of age; the material was therefore divided into immature (length group 86–124 mm) and mature specimens (length group 125–155 mm).
The female HSI ranged from 0.5 to 8.3% of GW (Fig. 5a, b) while male HSI ranged from 0.6 to 3.6% of GW (Fig. 5c, d). Female HSI slowly increased until specimens reached approximately 125–130 mm (Fig. 5a) and 7–8 years of age (Fig. 5b), at which point HSI surged during the autumn. Male HSI also increased during the autumn, when specimens reached approximately 120 mm (Fig. 5c) and 5–6 years of age (Fig. 5d).
The GSI and HSI values varied seasonally for the immature (SL < 125 mm) and mature (SL ≥125 mm) length groups. Females showed significant differences in mean GSI between length groups both in spring (p = 0.021) and autumn (p = 0.004) (Fig. 6a). Males similarly showed significant differences in mean GSI between length groups both in spring (p < 0.001) and autumn (p = 0.002) (Fig. 6c). Females had significantly higher GSI than males in all seasons in both length groups. With regard to HSI, female length groups differed in autumn (p = 0.012) (Fig. 6b) while length groups of males had similar HSI values (Fig. 6d). Mean HSI differed between mature females and males (length group ≥125 mm) during spring (p = 0.012), summer (p = 0.006) and autumn (p = 0.005), while the winter season had insufficient data for testing. The GSI increased with increasing HSI in females (r2 = 0.764, p < 0.001; Fig. 7a), but not in males (r2 = 0.017, p = 0.207; Fig. 7b).
Gonadosomatic index (GSI, as % of GW) compared with hepatosomatic index (HSI, as % of GW) of L. maculatus a females and b males. The dotted lines represent linear relationships between GSI and HSI for females (regression values: r2 = 0.764, p = 0.001) and males (regression values: r2 = 0.017, p = 0.207)
Fecundity
Potential fecundity increased with increasing size and age, and was here split into two length groups. The first group included 115–124 mm, 5–7 g GW and 5–6 years of age with 950–1850 (mean 1530) oocytes in the ovaries (Table 3). The second group was 125–134 mm SL, 7–9 g GW and 7–9 years of age and had 1250–2800 (mean 2260) oocytes in the ovaries. Small, young females had 26–73% ripe oocytes (mean 52%), whereas larger and older females had 24–95% ripe oocytes (mean 59%; Table 3). Because of the large range in % ripe oocytes, the means were not significantly different. The largest fish (134 mm SL, 9 years) had low number of oocytes (1400) but the highest percentage of ripe ones (95%). Fecundity increased with increasing GSI and HSI (Fig. 8a, b).
Sex-specific size and age
Females and males were notably different in morphometric characters and the differences became more prominent after maturation (Online Resource 1). The number of significant morphometric differences between the sexes increased from four to 10 with sexual maturation. The daubed shanny was sexually dimorphic in pectoral fin length, head length, pre-dorsal length and head depth for both immature (85–124 mm SL) and mature specimens (125–155 mm SL). The classification matrix from the discriminant analysis showed that specimens were better assigned to correct sex based on morphometry after maturation (Online Resource 1).
Sketch drawings based on the mean value of each morphometric measurement illustrate differences between females and males (Fig. 9). Females had a sleeker body with relatively larger eyes and longer pectoral and caudal fins than males, which in turn had a larger head and a deeper, more powerful body. Body depth, pre-dorsal length and caudal fin length were larger in mature males. Moreover, adult males had large canines in the upper jaw (Fig. 10a, b); these are much smaller in females.
Discussion
The sex ratio in a population is important with regard to life-history strategy and reproduction. Most of our samples were dominated by males. The percentage of males varied between 35 and 80% (mean 63%) with no apparent relationship to season or location. The large bottom trawl used to obtain samples in this study is not selective for similar-sized fish. Thus, our samples reflect the sex ratio for the species at the trawling sites. Distribution patterns and behaviour of fishes are often gender-specific, and variations in sex ratio may occur spatially as well as seasonally (Trippel 2003). Thus, differences in behaviour and bathymetrical distribution between the sexes may result in different sex ratios in samples than for the population at large. More extensive sampling can partly resolve this problem, although trawling is typically limited to deep soft-bottom habitats. In contrast to our results, a domination of females has been reported for several Stichaeidae species, e.g. Stichaeus grigorjewi Herzenstein, 1890 (Kolpakov and Klimkin 2004; Kalchugin et al. 2006), S. nozawae Jordan and Snyder, 1902 (Kolpakov and Klimkin 2004) and longsnout prickleback Lumpinella longirostris (Evermann and Goldsborough, 1907; Antonenko et al. 2004).
The daubed shanny is sexually dimorphic in length, weight and age distribution with males outgrowing and having longer life span than females, with maximum of 12 years for males and 10 years for females (Meyer Ottesen et al. 2014). Analyses have been extended in the present study with morphometric measurements of sexes to classify males and females of immature as well as mature fish (Online Resource 1). These traits seem common among Stichaeidae and has been reported for several species, such as S. grigorjewi (Kolpakov and Klimkin 2004; Kalchugin et al. 2006), S. nozawae (Kolpakov and Klimkin 2004), radiated shanny Ulvaria subbifurcata Storer, 1839 (LeDrew and Green 1975) and pighead prickleback Acantholumpenus mackayi Gilbert, 1896 (COSEWIC 2003). The faster growth, larger body size and longer life span of males are likely related to reproduction because they defend territories during spawning and, thus, allocate more energy towards muscle growth (Meyer Ottesen et al. 2014).
The presence of oocytes of different sizes and stages within one and the same ovary indicates that daubed shanny has a group-synchronous gonad organization, where both larger oocytes of similar size and a heterogeneous combination of smaller and larger oocytes are recognized (Murua and Saborido-Rey 2003). The large oocytes will be spawned during the current breeding season while the smaller oocytes may be retained for future spawning (Murua and Saborido-Rey 2003) or regressed. Many demersal species inhabiting cold marine waters tend to have this type of gonad organization (Murua and Saborido-Rey 2003). However, because our latest samples were taken some time before the spawning time, the smaller oocytes could still develop until Stage 5 (Table 2; Fig. 2a), and ripe eggs would be spawned in a single event. An alternative explanation is that daubed shanny is either a bi-annual spawner or skip spawning some years, given that two size-groups of yolky oocytes were present in the ovary, low GSI for part of the mature-sized females in October, and that the percentage of ripe oocytes was rather similar (50–60%) in both size-groups of fish. Skipped spawning is relatively widespread among fishes (Rideout and Tomkiewicz 2011). Although it has not been demonstrated for Arctic marine fishes, bi-annual maturation of gonads has been observed among Antarctic notothenioids, and e.g. mackerel icefish Champsocephalus gunnari (Lönneberg, 1905) may skip spawning in some years (Kock and Kellermann 1991).
The size, colour and consistency of gonads changed with season and body size; female ovaries changed from white in late winter and early spring to dark orange in late autumn (October) while, during the same time period, male testes changed from small, thin and translucent yellow to relatively large, broader and milky white or light pink with a yoghurt-like consistency. Gonads of both females and males seem nearly ripe by October based on their large size and colour. The orange colouration of eggs is typical for benthic fishes, whereas eggs of pelagic fishes tend to be transparent (e.g. Graham and Hop 1995). Immature eggs are generally whitish, as seen in ovaries containing more than one cohort of eggs (Marshall 1953). The female ovary and its seasonal morphology have been determined based on histological analyses by Murzina et al. (2012) and their results support our findings.
Body shapes of stichaeids are generally elongated or eel-like (e.g. snakeblenny Lumpenus lumpretaeformis Walbaum, 1792), and all are small (generally < 40 cm) benthic species. Thus, most species in this family probably show similarities rather than differences in reproduction and spawning behaviour. The ovary of daubed shanny seems similar to that described in Pseudalectrias tarasovi (Popov, 1933); a two-lobe-shaped ovary that is fused at the base (Miki et al. 1987). The eggs of P. tarasovi are demersal, spherical and adhesive, and other stichaeids, such as Dictyosoma burgeri van der Hoeven, 1855, and black prickleback Xiphister atropurpureus (Kittlitz 1858), have similar types of eggs (Miki et al. 1987). The black prickleback has been observed to shape the roe into a spherical mass, performed in cooperation between female and male (Wourms and Evans 1974). As egg envelopes hardened, they adhered to one another to form a coherent mass. Thus, the eggs and spawning behaviour of the daubed shanny may be similar to some of the other stichaeids, such as the black prickleback.
The oocyte diameter in autumn ranged between 0.7 and 1.7 mm, which encloses previously published data with egg size of about 1.5 mm in diameter recorded for the daubed shanny (122 mm total length; Christiansen et al. 1998). Similar sizes of eggs have been found in other Stichaeidae species (Farwell et al. 1976; Miki et al. 1987; Kyushin 1990). However, spawned eggs may be somewhat larger than the sizes of oocytes because of water uptake and hardening (Kjesbu et al. 1992), as described for P. tarasovi (Miki et al. 1987).
The relationship of GSI with SL and age indicated that females become sexually mature at 125 mm and 7 years of age, which concurs with Murzina et al. (2012). However, the percentage of ripe oocytes in our samples was similar for the two length groups, which may indicate that some of the young females classified as immatures (63% correct classification based on morphology) may also be able to spawn. This implies that part of the population may become sexually mature at < 125 cm in length. Males become sexually mature at similar length as females, but at 6 years of age. The difference in age at maturity is likely related to faster growth rates in males (Meyer Ottesen et al. 2014). The size and age of maturity in daubed shanny seem high for a fish that to our knowledge reaches a maximum length of 160 mm (Andriyashev 1954) and 10–12 years of age (Meyer Ottesen et al. 2014). Thus, females likely die within 2–3 years after first spawning.
According to Pethon (2005), the daubed shanny spawns in shallow waters during early winter, although some surveys have indicated earlier spawning during autumn (Dolgov 1994). The increase in GSI and the change in the maturity of the gonads of females and males from May to October indicate that spawning takes place during late autumn or winter, most likely from November to February or early March. The high percentage of ripe oocytes in the largest female in our study could indicate that this fish was close to spawning, although the low number of oocytes could also result from egg loss during capture. In April (late winter), one specimen had a GSI similar to that seen in large specimens during autumn, possibly a late spawner. However, spent females caught in April suggested that the spawning season had ended in March. The spawning season likely extends over some time (weeks or months), as has been found for other Stichaeidae species (Shiogaki 1981; COSEWIC 2003; Kolpakov and Klimkin 2004; Rose 2005). In species with seasonal reproduction, a major adaptation concerns spawning time, which is adjusted in such a way that the specific food requirements of larvae coincide with the seasonal availability of suitable prey. Thus, time of spawning of daubed shanny is most likely decided by matching the arrival of pelagic larvae to the secondary production in the pelagic zone (Jalabert 2005; Søreide et al. 2010).
The HSI of daubed shanny increased in the autumn for larger specimens (≥125 mm) of both sexes, which may be related to energy storage during sexual maturation. Even though the livers are much smaller than those of gadoid fishes, they contain high amounts of lipids (35% of dry weight for females), particularly triacylglycerols (65%), during autumn (Murzina et al. 2012, 2013). Females likely mobilize energy in the form of lipids from livers for oocyte development, whereas males may utilize stored energy for sperm production and defence of territories. Murzina et al. (2012) found that neutral lipids were transferred to developing oocytes during summer and autumn. The summer and particularly the autumn material showed a large variability in GSI and HSI in females of the same size, but with the highest values for large specimens during autumn. Thus, females need larger energy reserves than males, especially for the later stages when gonads and oocytes rapidly expand (Stages 4–5, Table 2).
Fecundity in fishes is often proportional to size or age as well as condition (Murua et al. 2003). This correlation was also apparent for the daubed shanny in that the larger, mature females had significantly higher fecundity. Larger fishes produce more eggs, and females in better condition exhibit the higher fecundity (Murua et al. 2003). Based on our results, the potential mean fecundities of daubed shanny were 1530 eggs for small females (< 125 cm) and 2260 eggs for large females (≥ 125 cm). However, the actual fecundity may be lower if some eggs are retained and regressed in ovaries. According to Murua et al. (2003), sampling of fish for maturity and fecundity estimates should include a wide range in body length, the total distribution area and account for variations in timing of maturation and spawning of different populations. Thus, our limited data should only be used as indication of fecundity of daubed shanny in Svalbard waters.
Indicators of nutritional status, such as condition and HSI, may be predictive of potential fecundity (Kamler 2005). If the energy used for gonad growth in females is mostly obtained from lipid reserves in the liver (Brooks et al. 1997), then the size of the gonads should be dependent on, and thus be correlated with, size of the liver. We found a positive correlation between GSI and HSI only in females, presumably because males invest little energy into testes growth and may rather spend the energy on territorial defence. Fecundity was also correlated with GSI as well as HSI, which further substantiates the importance of liver as energy source for reproduction in females.
The reproductive strategy of Stichaeidae is categorized as being oviparous with nest spawning. The daubed shanny likely exhibits female parental care and male territorial defence based on (1) the higher HSI of mature females, which gives them an energy reserve to help them survive the starvation that often is associated with the reduced feeding opportunity during guarding of eggs, and (2) differences in morphometry between females and males. Sexual dimorphism in morphology likely reflects different roles of females and males during the breeding season. Moreover, adult males had prominent canines in the upper jaw. Although sharp teeth also were observed in the upper jaw of females, they were less protruding than those of adult males. Males may be territorial and fight for and defend spawning sites and females. Thus, daubed shanny may have female mate choice that is based on male morphology (stout, powerful body) and quality of occupied spawning site. Our morphometric measurements showed that mature females have longer fins, larger eyes and a thinner body than males. The long fins are better suited for fanning/aeration of eggs and the slimmer body of females may be better suited for coiling around the egg mass.
Territorial behaviour was not part of our study, but has been observed in males for other Stichaeidae species. The Arctic shanny Stichaeus punctatus (Fabricius, 1780) displays a threat action by erecting its dorsal fin, arching its body and turning the head upwards (Coad et al. 1995). It further displays shaking of the body, gaping and slow wagging of the caudal fin, and this is followed by nipping and chasing if these threats are unsuccessful. The high cockscomb Anoplarchus purpurescens Gill, 1861 males also defend their territories during spawning season and they do not move more than 15 m from the spawning site (Coad et al. 1995).
Winemiller (1992) suggested three possible life-history strategies based on the interplay between maternal age at maturity, fecundity and egg size (and thus also survivorship of offspring): (1) “equilibrium” strategy with late maturity, low fecundity and large size of offspring, thus a large investment in relatively few offspring, (2) “opportunistic” strategy with early maturity, low fecundity, small size of offspring and rapid maturation, and (3) “periodic” strategy with late maturity, high fecundity and a pulsed production of large numbers of small offspring. The strength of sexual selection is influenced by which life-history strategy each fish species possesses (Winemiller 1992). Conspicuous males that are larger than the females, like the daubed shanny in this study, are common among equilibrium and opportunistic strategists, but are rare among species with periodic reproductive strategy and high fecundity. This, in addition to late maturity (6–7 years) and relatively low potential fecundity (< 3000 oocytes), would put daubed shanny in the “equilibrium” life-history category.
References
Andriyashev AP (1954) Fishes of the northern seas of the U.S.S.R. Keys to the fauna of the U.S.S.R. Opredeliteli po Faune SSSR, Zoologicheskiĭ institut Akademii nauk SSSR 53 (in Russian). (Translated from Russian by the Israel Program for Scientific Translations). Jerusalem 1964, pp 266–269
Antonenko DV, Pushchina OI, Kalchugin PV (2004) Distribution and biological features of the Long-snouted blenny Lumpenella longirostris (Stichaeidae) in waters of Primorye (the Sea of Japan). J Ichthyol 44:747–751
Balanov AA, Zhemukhov VV, Antonenko DV (2011) Leptoclinus maculatus diaphanocarus (Schmidt, 1904) (Zoarcoidei: Stichaeidae) in the northern Japan Sea. J Ichthyol 51:188–194 (in Russian)
Baylis JR (1981) The evolution of parental care in fishes, with reference to Darwin’s rule of male sexual selection. Environ Biol Fish 6:223–251
Blumer LS (1982) A bibliography and categorization of bony fishes exhibiting parental care. Zool J Linn Soc 76:1–22
Brooks S, Tyler CR, Sumpter JP (1997) Egg quality in fish: what makes a good egg? Rev Fish Biol Fish 7:387–416
Byrkjedal I, Høynes Å (2007) Distribution of demersal fish in the south-western Barents Sea. Pol Res 26:135–151
CARE (Committee of Age Reading Experts) (2000) Manual on generalised age determination procedures for groundfish. http://care.psmfc.org/docs/CareManual2006.pdf
Christiansen JS (2017) No future for Euro-Arctic ocean fishes? Mar Ecol Prog Ser 575:217–227
Christiansen JS, Fevolden SE, Karamushko OV, Karamushko LI (1998) Maternal output in polar fish reproduction. In: di Prisco G, Posano E, Clark A (eds) Fishes of antarctica. A biological overview. Springer, Milan, pp 41–52
Coad BW, Waszczuk HI, Labignansd I (1995) Encyclopaedia of Canadian fishes. Canadian Museum of Nature and Canadian Sportfishing Productions Inc., Singapore
Coleman RM (1992) Reproductive biology and female parental care in the cockscomb prickleback, Anoplarchus purpurescens. Environ Biol Fish 35:177–186
COSEWIC (Committee on the Status of Endangered Wildlife in Canada) (2003) COSEWIC Assessment and update status report on the pighead prickleback (Acantholumpenus mackayi) in Canada. http://dsp-psd.pwgsc.gc.ca/Collection/CW69-14-356-2004E.pdf
Dolgov AV (1994) Some aspects of biology of non-target fish species in the Barents Sea. ICES C.M. 12:23
Falk-Petersen S, Falk-Petersen I-B, Sargent JR (1986) Structure and function of an unusual lipid storage organ in the Arctic fish Lumpenus maculatus (Fries). Sarsia 71:1–6
Farwell MK, Green JM, Pepper VA (1976) Distribution and known life history of Stichaeus punctatus in the Northwest Atlantic. Copeia 3:598–602
Fishbase: www.fishbase.org
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat Clim Change 5:673–678
Graham M, Hop H (1995) Aspects of reproduction and larval biology of Arctic cod (Boreogadus saida). Arctic 48:130–135
Green JM, Mathisen A-L, Brown JA (1987) Laboratory observations on the reproductive and agonistic behaviour of Ulvaria subbifurcala (Pisces: Stichaeidae). Nat Can 114:195–202
Gross MR, Shine R (1981) Parental care and mode of fertilization in ectothermic vertebrates. Evolution 35:775–793
Haug T, Bogstad B, Chierici M, Gjøsæter H, Hallfredsson EH, Høines ÅS, Hoel AH, Ingvaldsen RB, Jørgensen LL, Knutsen T, Loeng H, Naustvoll L-J, Røttingen I, Sunnanå K (2017) Future harvest of living resources in the Arctic Ocean north of the Nordic and Barents Seas: a review of possibilities and constraints. Fish Res 188:38–57
Jalabert B (2005) Particularities of reproduction and oogenesis in teleost fish compared to mammals. Reprod Nutr Dev 45:261–279
Kalchugin PV, Pushchina OI, Panchenko VV, Solomatov SF (2006) The distribution and some biological features of Stichaeus grigorjewi (Stichaeidae) in the waters of Northern Primorye (the Sea of Japan). J Ichthyol 46:447–453
Kamler E (2005) Parent–egg–progeny relationships in teleost fishes: an energetics perspective. Rev Fish Biol Fish 15:399–421
Kjesbu OS, Kryvi H, Sundby S, Solemdal P (1992) Buoyancy variations in eggs of Atlantic cod (Gadus morhua L.) in relation to chorion thickness and egg size: theory and observations. J Fish Biol 41:581–599
Kock K-H, Kellermann A (1991) Reproduction in Antarctic notothenioid fishes. Antarct Sci 3:125–150
Kolpakov NV, Klimkin AF (2004) Specific features of biology of shannies Stichaeus grigorjewi and S. nozawae (Stichaeidae) in waters of Northern Primorye. J Ichthyol 44:592–599
Kyushin K (1990) Embryonic development and larvae of long shanny, Stichaeus grigorjewi Herzenstein. Bull Fish Sci Hokkaido Univ 41:13–17
LeDrew BR, Green JM (1975) Biology of the radiated shanny Ulvaria subbifurcata Storer in Newfoundland (Pisces: Stichaeidae). J Ichthyol 7:485–495
Marliave JB, DeMartini EE (1977) Parental behavior of intertidal fishes of the stichaeid genus Xiphister. Can J Zool 55:60–63
Marshall NB (1953) Egg size in Arctic, Antarctic and deep-sea fishes. Evolution 7:328–341
Mecklenburg CW, Sheiko BA (2004) Annotated checklist of fishes: family Stichaeidae Gill 1864, pricklebacks. An electronic journal published by the California Academy of Sciences ISSN 1545-150X (CD-ROM). https://www.calacademy.org/scientists/ichthyology/cmecklenburg
Mecklenburg CW, Lynghammar A, Johannesen E, Byrkjedal I, Christiansen JS, Dolgov AV, Karamushko OV, Mecklenburg TA, Møller PR, Steinke D, Wienerroither RM (2018) Marine Fishes of the Arctic Region. Conservation of Arctic Flora and Fauna, Akureyri. https://caff.is/monitoring-series/451-marine-fishes-of-the-arctic-region-vol-1. Accessed 28 Feb 2018
Meyer Ottesen CA, Hop H, Christiansen JS, Falk-Petersen S (2011) Early life history of daubed shanny (Teleostei: Leptoclinus maculatus) in Svalbard waters. Mar Biodiv 41:383–394
Meyer Ottesen CA, Hop H, Christiansen JS, Falk-Petersen S (2014) Growth of the daubed shanny (Teleostei: Leptoclinus maculatus) in Svalbard waters. Polar Biol 37:809–815
Miki T, Yoshida H, Amaoka K (1987) Rare stichaeid fish, Pseudoalectrias tarasovi (Popov), from Japan and its larvae and juveniles. Bull Fish Sci Hokkaido Univ 38:1–13
Mukhina NV (2005) Distribution of eggs and fish larvae in the Norwegian and Barents Sea. PINRO Publishing, Murmansk (in Russian)
Murua H, Saborido-Rey F (2003) Female reproductive strategies of marine fish species of the North Atlantic. J Northw Atl Fish Sci 33:23–31
Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S (2003) Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J Northw Atl Fish Sci 33:33–54
Murzina SA, Meyer Ottesen CA, Falk-Petersen S, Hop H, Nemova NN, Polyektova OG (2012) Oogenesis and lipids in gonad and liver of daubed shanny (Leptoclinus maculatus) females from Svalbard waters. Fish Physiol Biochem 38:1393–1407
Murzina SA, Nefedova ZA, Falk-Petersen S, Hop H, Ryokolainen TR, Meyer Ottesen CA, Pipatti PO, Berge J, Nemova NN (2013) Lipids in the daubed shanny (Teleostei: Leptoclinus maculatus) in Svalbard waters. Polar Biol 36:1619–1631
Peppar JL (1965) Some features of the life history of the cockscomb prickleback, Anoplarchus purpurescens. Gill. M.Sc. Thesis, University of British Columbia, Vancouver
Pethon P (2005) Aschehougs store fiskebok, 5th edn. Aschehoug, Oslo, pp 362–363
Rideout RM, Tomkiewicz J (2011) Skipped spawning in fishes: more common than you might think. Mar Coast Fish 3:176–189
Rose GA (2005) On distributional responses to North Atlantic fish to climate change. ICES J Mar Sci 62:1360–1374
Shiogaki M (1981) Notes on the life history of the stichaeid fish Opisthocentrus tenuis. Jpn J Ichthyol 28:319–328
Shiogaki M (1983) On the life history of the stichaeid fish Chirolophis japonicas. Jpn J Ichthyol 29:446–455
Søreide JE, Leu E, Berge J, Graeve M, Falk-Petersen S (2010) Timing in blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob Change Biol 16:3154–3163
Trippel EA (2003) Estimation of male reproductive success of marine fishes. J Northw Atl Fish Sci 33:81–113
Wienerroither R, Johannesen E, Dolgov A, Byrkjedal I, Bjelland O, Drevetnyak K, Eriksen KB, Høines Å, Langhelle G, Langøy H, Prokhorova T, Prozorkevich D, Wenneck T (2011) Atlas of the Barents Sea fishes. IMR/PINRO Joint Report Series 1-2011, ISSN 1502-8828
Wienerroither R, Johannesen E, Dolgov A, Byrkjedal I, Aglen A, Bjelland O, Drevetnyak K, Eriksen KB, Høines Å, Langhelle G, Langøy H, Murashko P, Prokhorova T, Prozorkevich D, Smirnov O, Wenneck T (2013) Atlas of the Barents Sea fishes based on the winter survey. IMR-PINRO Joint Report Series 2-2013. ISSN 1502-8828
Winemiller KO (1992) Life-history strategies and the effectiveness of sexual selection. Oikos 63:318–327
Wourms JP, Evans D (1974) The annual reproductive cycle of the black prickleback, Xiphister atropurpureus, a Pacific Coast blennioid fish. Can J Zool 52:795–802
Acknowledgements
This work is supported by Statoil and Arctos. We also acknowledge the TUNU-Programme (UiT- The Arctic University of Norway) for our participation and thus opportunity for sampling of material on the TUNU-III Expedition 2007. We would like to thank the crew of RV Helmer Hanssen for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The sampling was carried out at UiT or UNIS cruises. Both institutions have strict guidelines related to minimizing environmental impacts through field-related activities, and all activities are carried out according to the current HSE guidelines at UiT and UNIS (e.g. see www.unis.no/resources/hse/). These institutions obtain permits from the Norwegian Directorate of Fisheries to conduct scientific surveys (incl. bottom trawling) in Svalbard waters. No ethical issues have been identified connected to the project.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meyer Ottesen, C.A., Hop, H., Falk-Petersen, S. et al. Reproduction and sexual dimorphism of daubed shanny (Teleostei: Leptoclinus maculatus) in Svalbard waters. Polar Biol 41, 1867–1880 (2018). https://doi.org/10.1007/s00300-018-2328-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2328-z