Abstract
Background and Aim
Aberrant DNA methylation has been shown to be associated with the growth, development, metastasis, and prognosis of tumors. Methylated DNAs may be suitable biomarkers for cancer patients. Here, we investigated whether circulating methylated MINT2 DNAs represent a potential poor prognostic factor in gastric cancer (GC).
Methods
MINT2 methylation was detected by real-time methylation-specific PCR in tumor tissues, pairing preoperative peritoneal lavage fluid (PPLF) and blood from 92 GC patients. The theory meaning and clinical practicality value of MINT2 methylation in different specimens were analyzed.
Results
The methylation status of the MINT2 gene was found to be significantly higher in tumor tissues (44.6 %, 41/92) than in adjacent normal tissues (3.3 %, 3/92). No MINT2 methylation was found in healthy controls, and partial MINT2 methylation was observed in three (6.25 %, 3/48) patients with chronic atrophic gastritis. The frequency of MINT2 methylation in pairing PPLF and blood samples from 92 GC patients was 40.2 % (37/92) and 39.1 % (36/92), respectively. Methylated MINT2 in tumor tissues, pairing PPLF, and blood samples were very approximate. Aberrant MINT2 methylation in tumor tissues and pairing PPLF or blood samples were closely related to peritoneal dissemination, tumor progression, and poor prognosis (all P < 0.0001).
Conclusions
Aberrant MINT2 methylation in PPLF/blood may predict peritoneal micrometastasis for GC patients, which is a potential poor prognostic factor in GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common causes of death worldwide [1]. Although the prognosis of patients with advanced gastric cancer has been improved by early detection, effective chemotherapy, adjuvant radiotherapy, and development in surgical techniques such as extensive lymphadenectomy, it still displays a poor prognosis with a 5-year survival rate of less than 35 % [2]. Early gastric cancers can have metastasis and poor prognosis even if they undergo curative resection, suggesting that systemic micrometastasis may have already existed at the time of surgery [3]. Peritoneal metastasis is the most frequent event in recurrent gastric cancers [4, 5]. The cytological examination of preoperative peritoneal lavage fluid (PPLF) using Papanicolaou staining is commonly performed during surgery to detect peritoneal metastasis. However, peritoneal metastasis sometimes occurs even in cases that show a negative cytological examination. Therefore, various examinations are used to detect micrometastasis to the peritoneal cavity in gastric cancer, such as immunocytology [6, 7], tumor markers [8, 9], telomerase activity [10], reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of carcinoembryonic antigen (CEA), and cytokeratin (CK) mRNA [5, 11–16]. Recently, methylation-specific PCR (MSP) analysis of bisulfite-treated DNAs has been conducted to detect peritoneal micrometastasis using PPLF samples [17].
Accumulating data strongly suggested that DNA methylation could be a useful and powerful biomarker in cancer risk evaluation [18], early diagnosis [19], prediction of patient prognosis [17, 19–22], evaluation of sensitivity to chemotherapeutic drugs [23], etc. DNA methylation could be examined in primary tumor tissues, as well as in PPLF or blood samples. Our previous study demonstrated that detecting circulating methylated DNA in PPLF/blood could be a possible marker for the detection of peritoneal micrometastasis in gastric cancer patients [17].
MINTS are multimodular adapter proteins encoded by a member of the X11 protein family in functioning membrane transport and organization. It is a neuronal adapter protein that interacts with the Alzheimer’s disease amyloid precursor protein (APP). It stabilizes APP and inhibits production of proteolytic APP fragments, including the A beta peptide that is deposited in the brains of Alzheimer’s disease patients. MINTS are believed to be involved in signal transduction processes, and are also regarded as a putative vesicular trafficking protein in the brain that can form a complex with the potential to couple synaptic vesicle exocytosis to neuronal cell adhesion [24, 25]. Multiple transcript variants encoding different isoforms have been found for this gene. Recently, the MINT2 transcription start site and the complete sequence of exon 1 were identified. The MINT2 promoter was located upstream of exon 1 and was more active in neurons. The core promoter contains several CpG dinucleotides, and was strongly suppressed by DNA methylation. In a word, MINT2 contains a potent promoter whose activity may be regulated by DNA methylation [24]. In the present study, we first assessed the status of MINT2 methylation by using tumor tissues, pairing PPLF and blood samples from gastric cancer patients, and then we clarified whether MINT2 methylation in PPLF/blood is feasible for predicting micrometastasis to the peritoneum and a poor prognosis in patients with gastric cancer.
Materials and Methods
Patients and Tissue Samples
A total of 92 sporadic gastric cancer patients were recruited at Zhejiang Province Cancer Hospital, Zhejiang Province People’s Hospital, and the First People's Hospital of Chunan County from January 2008 to December 2009. Detailed information on these patients is summarized in Table 1. The growth pattern of tumor cells was determined according to Ming’s classification [26]. None of these cases had undergone any medical treatment before surgery. Tumor specimens, as confirmed by histological identification, paired para-cancerous histological normal tissue (PCHNT) specimens at least 5 cm from the tumor border, and preoperative peritoneal lavage fluid (PPLF), were obtained in the operating room. PPLF samples from 92 GC patients were collected according to the previous report [17]. About 200 ml of warm, normal saline were introduced and manually dispersed in the Douglas cavity, the para-colic gutters, and in the right and left subphrenic cavity, when entering the abdominal cavity, prior to manipulating the tumor. At least 100 ml of fluid was subsequently recovered, after gentle stirring, from several regions of the abdominal cavity. The fluid was then centrifuged for 10 min at 1,500 rpm. The cells from PPLF samples were isolated and immediately stored at −80 °C until DNA was extracted. Meanwile, some sediment was smeared onto one or more glass slides and stained using the Papanicolaou’s method. All cytological examinations were performed by three cytopathologists independently. Cytological findings were classified as positive or negative according to the cell characteristics, as previously reported [26]. Meanwhile, paired blood samples from all recruited individuals were collected before surgery.
Antral mucosa biopsy specimens from 88 non-cancer volunteers were randomly collected by gastroscopy as controls within the same period, including 54 men and 34 women, with an average age of 52.9 years old. Among these volunteers, 48 patients were diagnosed with chronic, non-atrophic gastritis, and 40 were deemed healthy individuals without other digestive system diseases. Paired blood samples from the 88 non-cancer volunteers were also collected.
As a measure of prognosis, we analyzed the clinical data concerning disease-free survival (DFS), defined as the time from surgery data to first recurrence, or death by gastric cancer, or last contact. The cross-sectional imaging and serum tumor markers were used for the surveillance of tumor recurrence and metastasis. All recruited patients had been followed-up periodically until the due date. All patients provided written informed consent, and the study protocol was approved by the Institutional Review Board for Human Genetic and Genomic Research at Zhejiang Province Cancer Hospital, in accordance with the Declaration of Helsinki.
Analysis of H. pylori Infection
Biopsies were obtained from all recruited individuals who had endoscopic examination. H. pylori status was determined by rapid Urease test and Giemsa staining. H. pylori infection was confirmed when both tests were positive, and the samples with single positives were excluded from statistical analysis [27].
DNA Extraction, Sodium Bisulfite Modification, and Real-Time Methylation-Specific PCR (MSP)
The collected target cells were treated with 40 μl of 200 μg/ml proteinase K (Sigma–Aldrich) at 42 °C, for 72 h. DNA in blood samples was extracted using QIAamp DNA Blood Mini Kit (Qiagen Co., Germany). DNA was modified by sodium bisulfite using the EpiTect Bisulfite kit (Qiagen Inc.) following the kit’s instructions. Modified DNA samples were analyzed by real-time MSP on the ABI7500 PCR (ABI Co.) using the SYBR Premix Taq ExTaq Kit (TaKaRa Co. Ltd). MINT2 methylation and non-methylation specific primers were designed as reported previously [28]. The percentage of methylated DNA in the samples was calculated according to our previous description [19, 22], and methylated DNA samples were also scored according to previous reports [17, 22]. The cutoff threshold for DNA hypermethylation was set as 20 %, based on control normal samples and internal quality controls provided in the real-time MSP analysis.
Statistical Analysis
SPSS 17.0 (SPSS, Chicago, IL) statistical software was adopted for data analysis. Counting data comparisons between groups were subjected to the χ 2 test and Fisher’s exact test. Survival analysis was performed by means of the Kaplan–Meier method and significant levels were assessed by means of the log-rank test. A univariate analysis with the Cox regression model was used to determine identified prognostic factors, multivariate analysis with the Cox regression model was used to estimate the prognostic effect of methylated genes, and significant levels were assessed by means of the Wald test. For all statistical analyses, P values <0.05 were considered to represent statistical significance.
Results
Frequency of MINT2 Methylation in Primary Gastric Cancer Tissues, Paired Blood, and PPLF Samples
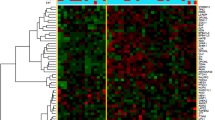
We first examined the methylation status of MINT2 promoter in gastric cancer tumors and corresponding normal tissues using the real-time MSP technique. Forty-one of 92 primary tumors (44.6 %) exhibited aberrant MINT2 promoter methylation, while 3/92 (3.3 %) paired PCHNT specimens exhibited it. There was a significant difference (P < 0.0001) between them for the methylation frequency of MINT2. We also examined MINT2 promoter methylation in non-cancer controls. Three of 48 (6.3 %) patients with chronic, non-atrophic gastritis exhibited MINT2 promoter methylation, while the methylation of MINT2 was not found in 40 healthy individuals. The frequency of MINT2 promoter methylation in gastric cancer tissues was significantly higher than that in non-cancer controls (P < 0.0001), and there was no significant difference in MINT2 methylation between the PCHNT and control groups (P > 0.05) (Fig. 1). These results indicated that MINT2 methylation may be a good marker to detect gastric cancer DNAs in paired PPLF and blood samples because of its high methylation frequency in tumors.
Subsequently, we examined MINT2 promoter methylation in DNA from the paired PPLF and blood samples of patients with a MINT2 alteration in their primary tumors. Thirty-seven of 41 (90.2 %) patients exhibited the same alteration in their PPLF DNA, and thirty-six of 41 (87.8 %) patients also demonstrated the same alteration in paired blood DNA. Of the 37 patients with a positive MINT2 methylation in PPLF, 24 (64.9 %) patients showed a positive PPLF cytology. And 24 (92.3 %) of 26 patients with both a positive PPLF cytology and MINT2 methylation in tumor tissues demonstrated a MINT2 methylation in PPLF. The results showed that the MINT2 methylation in blood (γ = 0.965, P < 0.0001) was closely correlated with the MINT2 methylation in tumor tissues (Fig. 2a); the methylated MINT2 DNA in PPLF (γ = 0.963, P < 0.001) was also significantly correlated with the MINT2 methylation in tumor tissues (Fig. 2b).
Using the results of methylated MINT2 in gastric cancer tissues as the golden standard, the diagnostic value of PPLF or blood was determined by means of receiver-operating characteristic (ROC) curves, of which the Aζ value of the ROC curve was 0.710 for PPLF and 0.696 for blood when compared to that in gastric cancer tissues (Fig. 3).
The diagnostic value of MINT2 methylation in PPLF or blood was determined by means of receiver-operating characteristic (ROC) curves, of which the Aζ value of ROC curve was 0.710 for PPLF and 0.696 for blood when compared to that in GC tissues. The value of MINT2 methylation in both PPLF and in blood to the diagnosis of GC progression was high
Methylation of MINT2 CpG Island Associated with Malignant Progression of Gastric Cancer
The methylated status of MINT2 in gastric cancer tissues or PPLF or blood significantly correlated with tumor size, growth manner, histological differentiation, lymphatic invasion, venous invasion, invasive depth, lymph node metastasis, distant metastasis, and clinical stage, respectively (all P < 0.05). In particular, aberrant MINT2 methylation in PPLF/blood was associated with the positive results in peritoneal lavage cytology (PLC), which predicts peritoneal micrometastasis. A total of 42.4 % (39/92) of GC patients showed a positive PPLF cytology, which was significantly related to the pathological findings. Overall, 94.9 % of patients with a positive PPLF cytology had a T3/T4 tumor and 100 % of the patients with a positive PPLF cytology had an N-positive tumor (P < 0.0001); in 76.9 % of patients with a positive PPLF cytology, the tumor grade was low (P < 0.0001). It indicated that the rate of positive PPLF cytology samples increases proportionally when the tumor invades the deeper layers of the gastric wall or the lymph nodes, and when the tumor has lost differentiation. However, aberrant MINT2 methylation in tumor tissues or PPLF/blood has no relevance to gender, age at diagnosis, H. pylori infection, or tumor site (all P > 0.05) (Table 1).
The cross-sectional imaging and serum tumor markers were used for the surveillance of tumor recurrence and metastasis. Studies showed good correlation between computed tomography (CT) quantitative parameters for post-operative development of peritoneal carcinomatosis and/or ascites on surveillance imaging, and positive PPLF cytology or positive MINT2 methylation in PPLF samples (data not shown).
Effect of Methylated MINT2 on Prognosis of Gastric Cancer Patients
We performed a survival analysis using the Kaplan–Meier method. At first we examined the effect of methylated MINT2 in tumor tissues on the prognosis of gastric cancer patients. There was a very significant difference between preoperatively detectable and non-detectable MINT2 promoter DNA (P < 0.0001, hazard ratio, 3.259; CI 1.749–6.073) when detectable MINT2 was used as an unfavorable prognostic factor (Fig. 4a). Subsequently, we analyzed the effect of methylated MINT2 in preoperative blood samples on the prognosis of gastric cancer patients. Results revealed a significant difference in the median survival time of more than 32 months between patients with and without preoperatively detectable methylated MINT2 (P < 0.0001; hazard ratio, 3.262; CI 1.779–5.981) (Fig. 4b). Finally, we found that in PPLF specimens, methylated MINT2 showed significant association with survival (P < 0.0001; hazard ratio, 4.115; CI 2.223–7.618) (Fig. 4c). Moreover, we found that the patients with negative PPLF cytology, but positive PPLF methylation, were generally associated with poorer prognosis and increased death when compared with patients with positive PPLF cytology and negative PPLF methylation; however, there was no significant difference between them (P > 0.05).
MINT2 methylation in GC tissues and blood or PPLF correlated with patient prognosis. Cumulative, disease-free survival (Cum DFS) curves are plotted against MINT2 DNAs methylation level in GC tissues (a), in the blood (b) and in PPLF (c). In a, b and c, Kaplan–Meier analyses were used and all P < 0.0001, respectively
Disease-free survival (DFS), analyzed by a multivariate Cox regression model, revealed that patients with methylated MINT2 DNA in their PPLF/blood had an independent survival disadvantage (PPLF: P < 0.05; RR: 3.262, 95 % CI 1.779–5.981, blood: P < 0.05; RR: 4.115, 95 % CI 2.223–7.618), only when the effect of TNM stages was eliminated. In addition, TNM stage could be considered as an independent influencing factor of prognosis in gastric cancer (P < 0.0001; RR: 303.708, 95 % CI 20.801–4434.355). The results showed that increases in MINT2 promoter methylation had a greater impact than tumor clinical stage on the prognosis. Using preoperative circulating methylated MINT2 detection as a marker, we were able to discriminate between short- (<2.5 years) and long-term survivors with significant sensitivity and specificity. Preoperative detection levels of methylated MINT2 DNA in PPLF/blood were significantly higher in patients with peritoneal metastasis than in those patients without peritoneal metastasis (P < 0.0001).
Discussion
Postoperative recurrence of gastric cancer usually occurs in the peritoneum. Peritoneal recurrence is highly resistant to various chemotherapies, which leads to a poor prognosis in these patients. Therefore, the detection of micrometastasis in peritoneal lavage is essential, not only to make an accurate diagnosis, but also to start chemotherapy before the metastatic nodule is grossly formed in the peritoneum [4, 5]. Peritoneal dissemination is detected in patients with negative cytological results, indicating that conventional cytological analysis lacks appropriate sensitivity. Previously, some of the conventional molecular markers such as CEA, keratin 19, alpha-fetoprotein (AFP), and RegIV have been used to detect peritoneal micrometastases in RT-PCR-based assays of PPLF samples from patients with gastric cancer [8–16]. However, any assay of PPLF samples using real-time RT-PCR in detecting specific mRNA is inferior in sensitivity and specificity to real-time MSP in detecting methylated DNAs, as described by Hiraki et al [29]. The unstable nature of mRNA results in false-negative results using specific mRNA as a marker [30]. To reduce the frequency of missed diagnoses, markers with greater sensitivity and specificity are needed.
Epigenetic gene silencing through DNA methylation occurs in various cancers. Recently, several reports have demonstrated aberrant gene methylation detected in various samples from cancer patients and have suggested the feasibility of methylation analysis in the evaluation of occult neoplastic cells or micrometastasis [17, 19, 21, 22, 29]. We previously reported that aberrant methylation of the CDH1 gene in the PPLF samples possibly predicts peritoneal micrometastasis in gastric cancer [17]. The study demonstrated that 39 (86.7 %) of the 45 patients with a positive CDH1 methylation in PPLF samples were detected a positive cytology, and 100 % of the patients with a positive PLC had a CDH1 methylation in PPLF. The results showed that the CDH1 methylation in PPLF (P < 0.0001, γ = 0.782) was closely correlated with the positive PLC [17].
In present study, we clarified whether MINT2 promoter methylation in the PPLF/blood is feasible for determining micrometastasis to the peritoneum in gastric cancer patients. We initially analyzed the promoter methylation of the MINT2 gene in gastric cancer tissues. The methylation analysis of MINT2 that used 92 pairs of cancer tissue and adjacent normal gastric mucosa was performed, and showed that MINT2 methylation was statistically significantly higher in tumor tissues than in PCHNT samples, suggesting that MINT2 represents cancer-specific methylation in gastric cancer. The methylation frequency of MINT2 in this study was revealed to be similar to that in the previous reports [29]. Our data showed that the methylated status of MINT2 significantly correlated with tumor size (≥5 cm), growth type (infiltration), histological differentiation (poor), PPFL cytology (+), lymphatic invasion (+), vein invasion (+), T stage (T3/T4), lymph node metastasis (+), distant metastasis (+) and clinical stage (III/IV stage), but had no correlation with gender, age at diagnosis, tumor site, and H. pylori infection, which is consistent with previous reports (Table 1) [29, 31, 32].
As for the methylation analysis using PPLF, it is important to detect cancer-specific gene methylation, leading to the diagnosis of occult cancer cells in PPLF. The analysis methylation status of MINT2 in PPLF demonstrated a significant difference between the PPFL cytology (+) and PPFL cytology (−) groups (Table 1) (P < 0.0001). The frequency of methylation of MINT2 increased in lymphatic invasion (+), vein invasion (+), T stage (T3/T4), lymph node metastasis (+), distant metastasis (+), and clinical stage (III/IV stage), respectively, reflecting the existence of tumor progression. As for the methylation status of the PPLF, we previously reported that the methylation status of CDH1 showed a very similar pattern between the primary tumor and PPLF DNA, in which the Aζ value of the ROC curve was 0.8 compared to that in gastric cancer tissues [17]. On the basis of previous studies, we expect that the methylation status of MINT2 in the PPLF originated from the primary cancer in this study.
In this study, we attempted to detect methylated MINT2 in the PPLF/blood of patients with GC and to evaluate whether there is an association between the preoperative detection of methylated MINT2 and the clinicopathological parameters. In particular, the detection of MINT2 methylation in circulating DNAs may be used as a non-invasive marker for the diagnosis of GC and prognostic treatment evaluation. Our results showed that, of the 41 patients with the methylated MINT2 in the primary tumors, 90.2 % (37/41) f paired PPLF and 87.8 % (36/41) of paired blood samples were found to have detectable methylated MINT2 DNA available for MSP evaluation. However, no abnormal methylation was found in circulating DNA if this alteration was not present in the primary gastric tumors. The presence of detectable methylated MINT2 in blood indicates the presence of circulating tumor DNA, which is closely related to peritoneal metastasis and TNM stage (all P < 0.0001).
The present study finally evaluated whether MINT2 methylation in PPLF/blood predicts the prognosis of GC patients after surgery. We found that the patients with negative PPLF cytology but positive PPLF methylation were generally associated with poorer prognosis and increased death when compared with patients with positive PPLF cytology and negative PPLF methylation. However, there was no significant difference between them (P > 0.05), which may be the result of too few cases. The results were concordant with our hypothesis that methylation is a “better” marker for peritoneal micro-metastasis than conventional cytology. Here, multivariate Cox proportional hazards regression analysis revealed that preoperative methylated MINT2 detection in PPLF/blood is a significantly independent factor associated with the outcome of GC patients (PPLF: P < 0.05; RR: 3.262, 95 % CI 1.779–5.981, blood: P < 0.05; RR: 4.115, 95 % CI 2.223–7.618). Using preoperative circulating methylated MINT2 detection in PPLF/blood as a marker, we were able to discriminate between short- (<2.5 years) and long-term survivors with quite a high sensitivity and specificity. Preoperative detection levels of methylated MINT2 DNA in PPLF/blood were significantly different between patients with and without peritoneal metastasis (P < 0.0001), which can be taken as a marker for peritoneal metastasis in GC patients. We could show that the level of methylated MINT2 DNA in PPLF/blood was significantly associated with outcome, and that it significantly increases the sensitivity and specificity for the diagnosis of short- versus long-term overall survival in GC patients. All these evidences suggest that MINT2 methylation predicts a poor prognosis for GC patients.
In conclusion, the present study investigated the methylation status of MINT2 in PPFL/blood samples using real-time MSP analysis. The MINT2 methylation in PPFL/blood samples revealed the clinical feasibility of detecting occult neoplastic cells on the peritoneum. A methylation analysis along with a cytological examination might therefore increase the positive detection of cancer cells or free-circulating tumor nucleic acid in PPFL/blood samples.
References
Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673.
Khosravi Shahi P, Munoz Diaz, de la Espada VM, et al. Management of gastric adenocarcinoma. Clin Transl Oncol. 2007;9:438–442.
Wanebo HJ, Kennedy BJ, Chmiel J, Steele G, Winchester D Jr, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583–592.
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242.
Fujiwara Y, Doki Y, Taniguchi H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197–204.
Benevolo M, Mottolese M, Cosimelli M, et al. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol. 1998;16:3406–3411.
Mori K, Suzuki T, Uozaki H, et al. Detection of minimal gastric cancer cells in peritoneal washings by focused microarray analysis with multiple markers: clinical implications. Ann Surg Oncol. 2007;14:1694–1702.
Asao T, Fukuda T, Yazawa S, Nagamachi Y. Carcinoembryonic antigen levels in peritoneal washings can predict peritoneal recurrence after curative resection of gastric cancer. Cancer. 1991;68:44–47.
Yamamoto M, Baba H, Toh Y, Okamura T, Maehara Y. Peritoneal lavage CEA/CA125 is a prognostic factor for gastric cancer patients. J Cancer Res Clin Oncol. 2007;133:471–476.
Da MX, Wu XT, Guo TK, et al. Clinical significance of telomerase activity in peritoneal lavage fluid from patients with gastric cancer and its relationship with cellular proliferation. World J Gastroenterol. 2007;13:3122–3127.
Nakanishi H, Kodera Y, Yamamura Y, et al. Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer. 2000;89:411–417.
Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005;8:142–148.
Ito S, Nakanishi H, Kodera Y, Mochizuki Y, Tatematsu M, Yamamura Y. Prospective validation of quantitative CEA mRNA detection in peritoneal washes in gastric carcinoma patients. Br J Cancer. 2005;93:986–992.
Wang JY, Lin SR, Lu CY, et al. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett. 2005;223:129–135.
Katsuragi K, Yashiro M, Sawada T, Osaka H, Ohira M, Hirakawa K. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer. 2007;97:550–556.
Tamura N, Iinuma H, Takada T. Prospective study of the quantitative carcinoembryonic antigen and cytokeratin 20 mRNA detection in peritoneal washes to predict peritoneal recurrence in gastric carcinoma patients. Oncol Rep. 2007;17:667–672.
Yu QM, Wang XB, Luo J, et al. CDH1 methylation in preoperative peritoneal washes is an independent prognostic factor for gastric cancer. J Surg Oncol. 2012;106:765–771.
Peng DF, Razvi M, Chen H, et al. Glutathione peroxidase 7 protects against oxidative DNA damage in oesophageal cells. Gut. 2009;58:5–15.
Lu XX, Yu JL, Ying LS, et al. Stepwise cumulation of RUNX3 methylation mediated by Helicobacter pylori infection contributes to gastric carcinoma progression. Cancer. 2012;118:5507–5517.
Ge MH, Chen C, Xu JJ, Ling ZQ. Critical regions and spreading of runt-related transcription factor-3 C-phosphate-G (CpG) island methylation in human salivary gland adenoid cystic carcinoma. Hum Pathol. 2011;42:1862–1872.
Ling ZQ, Zhao Q, Zhou SL, Mao WM. MSH2 promoter hypermethylation in circulating tumor DNA is a valuable predictor of disease-free survival for patients with esophageal squamous cell carcinoma. Eur J Surg Oncol. 2012;38:326–332.
Ling ZQ, Lv P, Lu XX, et al. Circulating methylated XAF1 DNA indicates poor prognosis for gastric cancer. PLoS ONE. 2013;8:e67195.
Charlet J, Schnekenburger M, Brown KW, Diederich M. DNA demethylation increases sensitivity of neuroblastoma cells to chemotherapeutic drugs. Biochem Pharmacol. 2012;83:858–865.
Hao Y, Chai KH, McLoughlin DM, Chan HY, Lau KF. Promoter characterization and genomic organization of the human X11β gene APBA2. Neuroreport. 2012;23:146–151.
Miller CC, McLoughlin DM, Lau KF, Tennant ME, Rogelj B. The X11 proteins, Abeta production and Alzheimer’s disease. Trends Neurosci. 2006;29:280–285.
Luebke T, Baldus SE, Grass G, et al. Histological grading in gastric cancer by Ming classification: correlation with histopathological subtypes, metastasis, and prognosis. World J Surg. 2005;29:1422–1427.
Tian XY, Zhu H, Zhao J, She Q, Zhang GX. Diagnostic performance of urea breath test, rapid urea test, and histology for Helicobacter pylori infection in patients with partial gastrectomy: a meta-analysis. J Clin Gastroenterol. 2012;46:285–292.
Lee S, Kim WH, Jung HY, Yang MH, Kang GH. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol. 2002;161:1015–1022.
Hiraki M, Kitajima Y, Koga Y, et al. Aberrant gene methylation is a biomarker for the detection of cancer cells in peritoneal wash samples from advanced gastric cancer patients. Ann Surg Oncol. 2011;18:3013–3019.
Miyagawa K, Sakakura C, Nakashima S, et al. Overexpression of RegIV in peritoneal dissemination of gastric cancer and its potential as a novel marker for the detection of peritoneal micrometastasis. Anticancer Res. 2008;28:1169–1179.
Saito M, Nishikawa J, Okada T, et al. Role of DNA methylation in the development of Epstein-Barr virus-associated gastric carcinoma. J Med Virol. 2013;85:121–127.
Kondo T, Oka T, Sato H, et al. Accumulation of aberrant CpG hypermethylation by Helicobacter pylori infection promotes development and progression of gastric MALT lymphoma. Int J Oncol. 2009;35:547–557.
Acknowledgments
This research was supported by a grant from the program for New Century Excellent Talents in University, from the Ministry of Education, China (NCET-11-0949), a grant from the Science and Technology General Project of Zhejiang Province (No. 2009C33143), Key Research Projects of Medicine, from the Ministry of Health, China (No. WKJ2010-2-004), partly by a grant from the Backbone Talent of Zhejiang Provincial Medicine and Hygiene Platform Programs (No. 2011RCA009), a grant from the Scientific and Technological Innovations Fund of Henan Province Higher Education (No. 2009HAST1T001), and by a grant from the Science and Technology Key Project of the Ministry of Education, China (No. 210130).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Han and Ping Lv wish it to be known that, in their opinion, the first two authors should be regarded as joint-first authors.
Rights and permissions
About this article
Cite this article
Han, J., Lv, P., Yu, JL. et al. Circulating Methylated MINT2 Promoter DNA Is a Potential Poor Prognostic Factor in Gastric Cancer. Dig Dis Sci 59, 1160–1168 (2014). https://doi.org/10.1007/s10620-013-3007-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-3007-0