Abstract
Self-cleaning fabrics can be developed based on introducing two mechanisms of superhydrophobicity and photocatalytic activity to conventional fabrics. However, there are some downsides such as inefficiency of superhydrophobic coatings against oily contaminants and low effectiveness of photocatalytic surface treatments under visible light which have restricted the widespread applications of these functional products. This research presents the development of self-cleaning cotton fabric with dual functionalities of superhydrophobicity and photocatalytic activity using microhierarchial TiO2-based particles. It is aimed to fabricate fluorine-free durable superhydrophobic coatings with simultaneous photocatalytic self-cleaning under simulated sunlight. Fluorine-free coating formulations composed of flower-like particles, either TiO2 or nitrogen-doped TiO2, and polydimethyl siloxane (PDMS) polymer were applied to cotton fabrics using a facile dip-coating method. The self-cleaning performance of fabrics was assessed based on their superhydrophobicity and effective removal of oil-based stains under simulated sunlight. Additionally, the impact of nitrogen doping on enhancing the photocatalytic activity of flower-like TiO2 particles was investigated. The obtained results demonstrated that the presence of both PDMS and hierarchical particles generated excellent superhydrophobicity on the cotton fabric with a water contact angle of 156.7° ± 1.9°. In addition, the coated fabric exhibited highly efficient photocatalytic activity, decomposing absorbed stains after 30 min of irradiation. Nitrogen doping process significantly boosted the photocatalytic activity of TiO2 particles in degrading food stains and Rhodamine B dye solution. The developed fabrics showed high robustness against chemical and physical durability tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fabrication of self-cleaning textiles has attracted considerable attention in recent years. The term of self-cleaning denotes two types of surfaces comprising either superhydrophobic or photocatalytic, each of which functions based on different mechanisms (Daoud 2013; Pakdel et al. 2020). On a superhydrophobic surface, water droplets form a spherical shape with a water contact angle (WCA) more than 150° and a sliding angle (SA) less than 10° (Geyer et al. 2020; Nguyen-Tri et al. 2019). This condition prevents any dirt adsorption onto the surface prompting water droplets to roll off the surface, and collecting impurities and cleaning it at the same time (Ghasemlou et al. 2019). To achieve the desired superhydrophobicity on a fabric, it is necessary to increase surface roughness by introducing hierarchical micro/nanostructures, and to decrease surface tension using low surface energy chemicals such as fluorinated compounds (Pakdel et al. 2020). Several strategies such as dip-coating, hydrothermal treatment, electrostatic layer by layer assembly, surface etching, UV-induced grafting, and cross-linking, to name but a few, have been used to obtain superhydrophobic coatings on textiles (Dalawai et al. 2020; Ghasemlou et al. 2019; Parvinzadeh Gashti et al. 2012, 2016). One of the widely reported strategies is treating fabrics surface with different types of nanomaterials to acquire the roughness, followed by applying low surface energy fluoroalkyl silanes (FAS) (Yang et al. 2018; Zhou et al. 2012). However, the resultant superhydrophobic effects suffer from main drawbacks such as the necessity of using fluorinated chemicals and poor durability (Dalawai et al. 2020; Pakdel et al. 2018b). In addition, the oxidation by-products of fluorinated compounds are potentially harmful to the environment and human health due to their low bio-elimination rate and potential accumulation in living organisms causing illnesses such as cancer, immune disorder and hormonal disturbance (Ju et al. 2017; Schellenberger et al. 2019; Zahid et al. 2019). The use of fluorine-free chemicals such as polydimethyl siloxane (PDMS) as a low surface energy polymeric binder is a promising approach to displace the banned FAS additives. The combination of PDMS with different types of nanomaterials has been reported to be effective at creating highly superhydrophobic surfaces (Zahid et al. 2019).
Another category of self-cleaning textiles refers to functionalised products with photocatalytic activity (Pakdel et al. 2014a; Talebi and Montazer 2020). The main function of these coatings is based on the elimination of stains or pollutions from fabrics using the photocatalytic activity of incorporated nanomaterials such as TiO2 and ZnO under a suitable light source (Montazer and Pakdel 2011; Pakdel et al. 2018b; Xu et al. 2016). However, low efficiency of the developed coatings and the necessity of using UV light to activate the self-cleaning function are limitations of this approach (Lu et al. 2020; Wu et al. 2014). To address these issues, different methods such as the addition of silica and metal oxides (Pakdel and Daoud 2013), doping with noble metals (gold, silver, etc.) (Luna et al. 2019; Pakdel et al. 2015), using non-metals such as nitrogen as dopants (Sun et al. 2020), loading with graphene (Anandan et al. 2013) and natural melanin (Liang et al. 2019; Xie et al. 2019, 2020), and dye-sensitisation (Afzal et al. 2012) have been employed to enhance the photocatalytic activity. These strategies can boost the photocatalytic activity of coatings and enable the use of visible light to activate the self-cleaning function of coatings. Other types of particles including zirconium oxide (ZrO2) (Parvinzadeh Gashti and Almasian 2013) and two dimensional nanosheets such as graphitic carbon nitride (g-C3N4) (Fan et al. 2018) and bismuth oxyiodide (Zhou et al. 2019) have also been used to develop self-cleaning fabrics.
Using particles with hierarchical surface morphologies referred to as flower-like particles in combination with low surface free energy materials is a promising approach to the development of robust superhydrophobic surfaces (Li et al. 2015; Pakdel et al. 2020; Xu et al. 2018). These particles can generate the required roughness due to having numerous nanosheets and nanopores in their hierarchical surface. The developed coatings usually display a low sliding angle enabling water droplets to roll across the created surface protrusions. A variety of flower-like particles have been used to coat textile surfaces to achieve superhydrophobic effect. For instance, Huang et al. (Huang et al. 2015) developed a superhydrophobic cotton through applying flower-like TiO2 particles using a hydrothermal method followed by surface modification with fluoroalkyl silanes and reported a WCA of 160°. However, their adopted method would be challenging to scale up to practical applications considering the use of hydrothermal method and fluorinated compounds. Zhang et al. (Zhang et al. 2017) applied flower-like ZnO particles to different substrates including cotton fabric and used an epoxy resin to improve the coating durability. The coated fabrics showed superhydrophobicity with a WCA of 150° and SA of 2°, but no further information on the photocatalytic self-cleaning was reported. Xu et al. (Xu et al. 2018) reported a superhydrophobic coating on cotton with WCA of 157° and SA of 7.2° based on the combination of copper sulfide (CuS) and PDMS. Similarly, Cao et al. (Cao et al. 2020) developed a superhydrophobic cotton through depositing cupric oxide (CuO) nanostructures on the fabric via a solution immersion process. The coated fabric showed a WCA of 151° and was employed as a superhydrophobic-superoleophilic filter for oil/water separation. However, the applied CuO coating changed the colour of cotton fabrics and despite having a visible-light-induced photocatalytic activity, no information was provided regarding the capability of the developed coatings on cleaning the stained fabrics.
Although there are several publications on using flower-like particles for developing superhydrophobic textiles as mentioned, several aspects of the discussed coatings have not fully been explored yet. The self-cleaning textiles developed so far, have mainly focused on using superhydrophobicity to protect the fabric against dust and water-borne contaminants as the main mechanism. However, repelling all types of stains including high- (water) and low-surface-tension (oils) liquids is challenging as it requires developing omniphobic coatings by creating sophisticated surface topographies and using fluorinated compounds with long perfluoroalkyl chains, which mainly results in low durability (Cai et al. 2019; Wu et al. 2017; Zhou et al. 2016). Therefore, new strategies are required to develop novel fluorine-free self-cleaning coatings with better performance for all types of impurities and stains. This can be achieved through combining the superhydrophobic and photocatalytic self-cleaning mechanisms. This study intends to develop a durable coating on cotton fabric with dual functionalities of superhydrophobicity and enhanced photocatalytic activity using hierarchical TiO2-based particles. The photocatalytic activity of TiO2 particles is enhanced through nitrogen-doping method. To the best of our knowledge, investigations on using nitrogen-doped flower-like TiO2 particles in developing self-cleaning textiles based on the two mentioned mechanisms have rarely been reported. The developed self-cleaning fabric can repel water-based stains due to its superhydrophobic behaviour and at the same time photocatalytically decompose oil-based contaminants under simulated sunlight.
Experimental
Materials
A pure cotton woven fabric was used as the substrate in this study. Elemental metal powder of titanium (Ti) with a purity of 99.7 % and average particle size of 45 μm (Atlantic Equipment Engineers, USA) was used as the precursor for the flower-like TiO2 particles. Polydimethylsiloxane (PDMS) pre-polymer (Sylgard 184 Industrial Elastomer Base) and the curing agent (Sylgard 184 Silicone Elastomer Curing Agent) were purchased from Dow Corning (USA).
Synthesis of flower-like TiO2 particles
The synthesis of TiO2 particles was carried out based on the hydrothermal method reported previously (Pakdel et al. 2016). In brief, 60 mg of Ti powder was mixed with 60 ml of sodium hydroxide (NaOH) solution (10 M) for 10 min followed by the addition of 0.5 ml of 30 % hydrogen peroxide (H2O2) to the mixture and stirring for 3 min. The resultant mixture was transferred into a Teflon-sealed autoclave and heated at 150 °C for 90 min. After cooling down to room temperature, the TiO2 particles were separated by vacuum-filtering, followed by washing with HCl solution (0.2 M) then washing 5 times with water. The synthesised particles were calcinated at 550 °C for 2 h to obtain the white TiO2 powder. Nitrogen doping was carried out through exposing the samples to a flow of 2 % NH3 in N2 gas for 4 h at 550 °C where greyish N-doped TiO2 (N-TiO2) powders were obtained.
Preparation of PDMS/flower-like TiO2 coatings
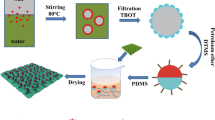
The cotton fabrics were scoured in a solution containing 2 g/L of nonionic detergent at 50 °C based on a liquid to good ratio of 50:1 for 20 min to remove any impurities. The prepared cotton fabrics were then coated using a dip-coating method in an ultrasonic bath. PDMS prepolymer (1 g) and curing agent (0.1 g) were added to 50 ml of isopropanol and mixed for 10 min under ultrasonication. The cotton fabrics were treated with pure PDMS, TiO2/PDMS, and N-doped-TiO2/PDMS formulations for 30 min in an ultrasonic bath. 0.2 g of the synthesised powders were used in each coating formulation containing either TiO2 or N-doped-TiO2. The samples were dried at room temperature followed by curing at 140 ºC for 1 h. Figure 1 shows different steps of preparing self-cleaning cotton fabrics.
Characterisation methods
The superhydrophobicity of coated fabrics was measured using a water contact angle meter (KSV CAM101). The contact angles were measured at five different spots on each fabric and the average values were obtained. The crystalline structure of the synthesised particles was investigated using X-ray diffractometer (X’Pert Powder, PANalytical, Netherlands) with a Cu Kα radiation over 2θ range of 20–80° operating at 40 kV and 30 mA. The Brunauer–Emmett–Teller (BET) specific surface area of powders was measured at 77 K according to the N2 adsorption using a Quantachrome Autosorb Automated Gas Sorption System (USA). Scanning electron microscopy (SEM) images were taken using Zeiss Supra 55VP (Germany). X-ray photoelectron spectroscopy (XPS) was performed by a Thermo Fisher Scientific 250Xi X-ray photoelectron spectrometer with Al Kα X-ray source.
The photocatalytic activities of TiO2 and N-doped TiO2 powders were assessed based on the degradation of Rhodamine B (RhB) dye solution (6 g/L) under simulated sunlight (350 W/m2). To this end, 1.0 mg of the synthesised powders was added to 40 ml of the dye solution and exposed to the light source. At certain time intervals, 5 ml of the dye solution was taken out and the dye concentration was measured at 510 nm using a UV-vis spectrophotometer. The photocatalytic self-cleaning property of coatings was also assessed based on monitoring the discolouration rate of soybean oil stains (25 µl per square cm of fabric) after exposure to simulated sunlight.
To assess the durability of coatings, the samples were immersed into the acidic and alkali aqueous solutions with pH levels of 1, 3, 5, 7, 9, 11, and 14 for 24 h at room temperature followed by drying and measuring their WCA. The pH levels were adjusted using hydrochloric acid and sodium hydroxide. The physical durability of coatings was assessed based on measuring WCA of fabrics after 50 cycles of sandpaper abrasion test and adhesive tape peeling. The fabrics were located onto sandpaper under constant pressure of 100 g and pulled for 20 cm horizontally. For tape-peeling test, the sticky tape was located on fabrics under a maximum finger pressure and then was removed. The washing test was conducted through immersing the samples into the water/detergent (5 g/L) mixture for 1 h under agitation followed by drying.
Results and discussion
Characterisation of the synthesised particles
The surface morphology of the synthesised particles was observed using SEM images (Fig. 2a) where the obtained particles displayed a three-dimensional hierarchical structure with a size of around 1 μm. The particles consist of self-centred radial nanoflakes creating a porous structure, which is consistent with previously reported results (Wang et al. 2010). The XRD patterns indicated the dominant presence of anatase crystalline phase in the structure of the calcinated powders (Fig. 2b). The diffraction peaks that appeared at 25.2°, 37.5° and 48° were characteristic of anatase TiO2 corresponding to the lattice parameters of 3.5 (101), 2.4 (004), 1.9 (200), respectively (Pakdel et al. 2018a). The peak at 40.07° is related to the Ti phase left in the core of the synthesised particles (Wang et al. 2010). There is a slight shift to lower angles for the N-doped TiO2 diffraction peak at 25.2°, which is due to the incorporation of nitrogen atoms into the lattice of anatase TiO2, indicating the distortion and strain in the synthesised crystals after nitrogen doping. This is in good agreement with the findings reported by Boningari et al. (Boningari et al. 2018). The BET surface areas of pure TiO2 and N-doped TiO2 powders calculated based on their N2-adsorption-desorption isotherms were 70.305 and 76.590 m2/g, respectively (Fig. 2c and d). The pore radiuses for pure and doped TiO2 were 16.573 and 14.862 Å, respectively, showing a slight decrease after the doping process.
Figure 3 shows the XPS spectra of the synthesised N-doped TiO2 particles where the peaks related to Ti, O, and N elements were detected. The peak appeared at 528.5 eV was related to O1s which was attributed to Ti-O in the TiO2 crystalline lattice (Cheng et al. 2012). The peaks at binding energies of 457.3 eV and 463 eV belong to Ti2p3/2 and Ti2p1/2, respectively (Chen et al. 2019; Huang et al. 2021). The N1s XPS peak in the range of 396–404 eV is typical for N-doped TiO2 (Cong et al. 2007). The main peak appeared at binding energy of 398.48 eV is related to O-Ti-N and is the characteristic of interstitial N atoms (Cheng et al. 2012; Di Valentin et al. 2007). The small peak at around 396–397 eV is attributed to the substitutional nitrogen (Cheng et al. 2012; Di Valentin et al. 2007). These observations can indicate the incorporation of N atoms in the TiO2 crystalline lattice (Chen et al. 2019).
The photocatalytic activities of pure TiO2 and N-doped TiO2 powders for degradation of RhB dye under simulated sunlight were evaluated (Fig. 4). The results confirmed the positive role of nitrogen doping on enhancing the photocatalytic activity of TiO2 particles. For instance, pure TiO2 particles degraded the dye solution within 4.5 h, while N-doped TiO2 showed much faster pace where they successfully decomposed the RhB molecules after 2 h of irradiation. According to related studies, higher photocatalytic activity of the N-doped TiO2 is attributed to embedding the nitrogen atoms into the lattice of TiO2 (Liu et al. 2017). This narrows the band-gap of TiO2 particles through introducing nitrogen 2p states into the bandgap of anatase nanocrystals above the valence band, shifting the photocatalytic activation threshold to the visible-region and thus enhancing photocatalytic activity (Liu et al. 2017; Pakdel et al. 2014b). The results obtained here confirmed that N-doped TiO2 particles were more active under the incident light. This demonstrates that the modified TiO2 particles were more responsive to a larger portion of the incident simulated sunlight due to their narrowed bandgap which resulted in a higher dye degradation (Boningari et al. 2018). In general, photocatalytic activity of the TiO2-based photocatalysts is activated through absorbing the incident light energy by the electrons existing in the valence band of TiO2 and promoting them to the conduction band. This phenomenon produces electrons and positive holes on TiO2 surface where they can react with oxygen and water molecules in their surroundings producing active species such as hydroxyl radicals (·OH) and superoxide anions (·O2−) (Boningari et al. 2018; Zhao et al. 2017). These species can actively react with organic molecules, breaking them down into harmless products such as water and carbon dioxide (Boningari et al. 2018; Zhao et al. 2017).
Self-cleaning coating on cotton fabric
The synthesised particles combined with PDMS were applied to the surface of cotton fabrics. SEM images showed that both PDMS and the flower-like particles were coated evenly on the fibres surface (Fig. 5). The pure PDMS coating did not lead to any surface roughness; however, the presence of well-dispersed flower-like TiO2 particles, significantly increased surface roughness contributing to the superhydrophobicity of the fabrics. Fibres coated with N-doped TiO2 particles showed a similar morphology to pure TiO2. EDX analysis confirmed the presence of Ti and Si elements on fibres surface (Fig. 5e, f). The N element could not be detected via this technique due to its low concentration. The ATR-FTIR spectra of the cotton fabrics before and after the coating process showed characteristic peaks related to cotton and PDMS (Fig. 6). The broad peaks at 3100–3600 cm− 1 were assigned to -OH groups located at the outer surface of fabrics (Abidi and Manike 2018). Pristine fabric showed the peaks at 2900 cm− 1, 1311 cm− 1, and 1053 cm− 1, related to the stretching vibration of C-H, symmetric bending of CH2 and stretching vibrations of C-O present in the cellulose structure, respectively (Abidi and Manike 2018; Noralian et al. 2021). In addition, the small peak at 890 cm− 1 was associated with β-linkages between monosaccharides (Abidi and Manike 2018). Applying pure PDMS to cotton fabric resulted in some peaks located at 2960 cm− 1 and 1260 cm− 1 which were ascribed to stretching and bending modes of -CH3 existing in the structure of PDMS (Zahid et al. 2017). The sharp peaks at 1020 cm− 1 and 800 cm− 1 were attributed to the asymmetrical stretching vibration of Si―O (Hu et al. 2019; Wang et al. 2019). For samples coated with TiO2/PDMS formulations, the sharp peaks located at 800 cm− 1 and 1010 cm− 1 were related to Ti–O, and Si–O–Si bonds, respectively (Foorginezhad and Zerafat 2019; Tavares et al. 2014).
The presence of flower-like particles on the surface of cotton fabrics has resulted in self-cleaning functionality based on two mechanisms of photocatalytic stain removal and superhydrophobicity. Both TiO2 and N-doped TiO2 particles were stabilised on cotton fabrics using the PDMS binder which was crucial, as a fluorine free polymer, to provide low surface free tension on coated cotton. The thickness of PDMS layer on top of the applied particles on fabrics surface can determine the resultant features. The coated fabrics showed suitable photocatalytic activity in decomposition of stains, implying the adequate contact between stains and photocatalysts’ surface. Figure 7 shows that the pristine cotton does not have any photocatalytic self-cleaning in the absence of TiO2 particles. Comparing the performances of TiO2 and N-doped TiO2, the latter one provided higher photocatalytic activity over the same period of irradiation. As mentioned earlier, this is related to the modification of the bandgap of TiO2 particles after nitrogen doping and the resultant higher activation potential under the light source. Under this condition, an increased number of negative electrons are excited to the conduction band from the valence band, leading to producing higher contents of active species of hydroxyl radicals and superoxide anions. This promoted more intense reactions with the stains on the fabrics surface. This explains the reason of having a better self-cleaning performance on cotton fabrics coated with N-doped TiO2 particles. It seems that the presence of a thin PDMS layer on flower-like particles did not inhibit the photocatalytic activity after exposure to the light source. Moreover, testing the re-useability of coated samples with modified N-doped TiO2 showed that it can maintain its high performance even after 5 cycles of consecutive photocatalytic self-cleaning test.
In addition to the photocatalytic self-cleaning mechanism, the developed coating of N-doped TiO2/PDMS showed excellent performance for superhydrophobic self-cleaning effect. The presence of three-dimensional particles with a hierarchical surface morphology created the required roughness on fabrics surface which in combination with PDMS produced a superhydrophobic surface. Indeed, Fig. 8a shows that the surface of the pristine cotton fabric was hydrophilic in nature as the water droplet was able to spread rapidly on the surface. After applying the PDMS coating, the WCA increased to around 131°, indicative of the hydrophobic nature of the resultant surface. It is known however, that a pure PDMS coating using conventional coating methods will not lead to a superhydrophobic surface (Pakdel et al. 2020), complementing our observations. Introducing the flower-like particles to the coating formulation, significantly boosted the WCA to around 157°, indicating the superhydrophobicity of the coated surface. There were no differences between the superhydrophobic performance of coatings developed from pure TiO2 and N-doped TiO2 particles. Figure 8b displays the self-cleaning property on coated fabric in removing the dust and soil impurities from the fabric surface using a stream of water. Also, the superhydrophobic performance of the coated fabric was tested for different types of droplets including water in different pH levels, water (dyed with methylene blue), red wine, coffee, milk, and black tea. These droplets spread rapidly on the surface of pristine cotton while maintaining their spherical shape on the superhydrophobic fabric. Both samples showed superoleophilicity, resulting in a rapid spreading of oil-based droplets (coloured with Red Oil O dye for better visualisation).
Self-cleaning mechanisms
The mechanism of superhydrophobic self-cleaning can be explained based on two models of Wenzel and Cassie–Baxter which are basically determined by the morphology and topography of the surface structure (Fig. 9a) (Darmanin and Guittard 2015). In the Wenzel state, the water droplet penetrates the created micro/nanostructures and is in contact with the substrate. Under this condition, the surface shows superhydrophobic property, high water adhesion force and resultantly a high sliding angle. This mechanism has been found on different natural surfaces such as rose petal with WCA of 152.4° (Zhu et al. 2014). However, the Cassie–Baxter defines the superhydrophobic surface with a low adhesive force for water. In this case, water droplets are suspended on top of the created protrusions on the coated surface. This mechanism is observed on the Lotus leaf surface where water droplets showed WCA of 160° and SA < 3° (Zhu et al. 2014). Therefore, it is plausible to claim that the existing superhydrophobic coating on cotton fabric consisting of flower-like particles and PDMS behaves similarly to the Cassie–Baxter model due to the low water adhesion (Abid et al. 2017). Therefore, the dirt and impurities were cleaned easily from the fabrics surface with a stream of water. In addition, the developed coating possessed photocatalytic self-cleaning functionality as well, where oily stains were eliminated after exposure to a light source. Due to the presence of PDMS, a superoleophilic behaviour was also observed on the coated fabric surface enabling oil droplets to rapidly spread on the fabric surface and remain in close contact with the photocatalytic TiO2 particles surfaces. Since the applied PDMS coating was thin and transparent, it did not block the penetration of light through the polymer coating for activation of photocatalytic behaviour of TiO2 particles. Therefore, food stains on the surface of coated fabrics efficiently broke down into colourless compounds. Figure 9b, c illustrate the proposed mechanisms for the self-cleaning mechanisms of the applied coatings.
Durability of coatings
The chemical stability of the applied coatings was assessed after immersing the fabrics into different water containers with different pH levels (Fig. 10a). It was observed that the strong acidic situations (pH = 1 and 3) slightly damaged the superhydrophobicity of the coated fabrics. However, the tested samples maintained a WCA of just below 150°. The fabrics immersed into water with 5 ≤ pH ≤ 11 did not show any tangible sign of reduction in their superhydrophobicity, implying the suitable durability of the applied coatings. Figure 10b compares the WCA of fabrics after washing, sandpaper abrasion resistance, and tape peeling tests. The results demonstrated suitable durability of coatings applied to fabrics where the tested samples maintained a WCA of around 150°.
Conclusions
This research successfully developed fluorine-free self-cleaning coatings on cotton fabrics with dual functionalities of superhydrophobicity and photocatalytic activity. Flower-like particles were synthesised through a hydrothermal method and applied to fabrics through a dip-coating method using the PDMS binder. The synthesised flower-like TiO2 and N-doped TiO2 particles displayed hierarchical surface morphologies. It was demonstrated that the nitrogen doping process enhanced the photocatalytic activity of TiO2 particles under simulated sunlight and the developed coating showed more efficient self-cleaning property. The cotton fabrics prevented the attachment of water-based stains and at the same time decomposed oily absorbed stains under light irradiation. The fabric coated with N-doped TiO2/PDMS formulation effectively decomposed the oily stains within 30 min of exposure to simulated sunlight, and this performance was repeatable after frequent usages. Applying pure PDMS coating to cotton fabric led to a hydrophobic surface with water contact angle around 131° while incorporating particles with hierarchical structures generated a superhydrophobic surface with water contact angle of 156.7° ± 1.9°. The developed superhydrophobic coating possessed nonwetting property to various types of liquids. The resultant superhydrophobicity was maintained after washing test with detergent, 50 cycles of abrasion test with sandpaper, and adhesive tape peeling. In addition, samples showed high chemical stability against different pH of water, implying suitable applicability of the developed coatings.
Code availability
There is no code availability for software application or custom code.
References
Abid M, Bouattour S, Ferraria AM, Conceição DS, Carapeto AP, Ferreira V, Botelho do Rego LF, Chehimi AM, Rei Vilar MM, M., & Boufi S (2017) Facile functionalization of cotton with nanostructured silver/titania for visible-light plasmonic photocatalysis. J Colloid Interface Sci 507:83–94. https://doi.org/10.1016/j.jcis.2017.07.109
Abidi N, Manike M (2018) X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Tex Res J 88(7):719–730. https://doi.org/10.1177/0040517516688634
Afzal S, Daoud Wa, Langford SJ (2012) Self-cleaning cotton by porphyrin-sensitized visible-light photocatalysis. J Mater Chem 22:4083–4088. https://doi.org/10.1039/C2JM15146D
Anandan S, Narasinga Rao T, Sathish M, Rangappa D, Honma I, Miyauchi M (2013) Superhydrophilic graphene-loaded TiO2 thin film for self-cleaning applications. ACS Appl Mater Interfaces 5(1):207–212. https://doi.org/10.1021/am302557z
Boningari T, Inturi SNR, Suidan M, Smirniotis PG (2018) Novel one-step synthesis of nitrogen-doped TiO2 by flame aerosol technique for visible-light photocatalysis: effect of synthesis parameters and secondary nitrogen (N) source. Chem Eng J 350:324–334. https://doi.org/10.1016/j.cej.2018.05.122
Cai R, De Smet D, Vanneste M, Nysten B, Glinel K, Jonas AM (2019) One-step aqueous spraying process for the fabrication of omniphobic fabrics free of long perfluoroalkyl chains. ACS Omega 4(15):16660–16666. https://doi.org/10.1021/acsomega.9b02583
Cao C, Wang F, Lu M (2020) Superhydrophobic CuO coating fabricated on cotton fabric for oil/water separation and photocatalytic degradation. Colloids Surf A 601:125033. https://doi.org/10.1016/j.colsurfa.2020.125033
Chen Q, Ozkan A, Chattopadhyay B, Baert K, Poleunis C, Tromont A, Snyders R, Delcorte A, Terryn H, Delplancke-Ogletree M-P, Geerts YH, Reniers F (2019) N-doped TiO2 photocatalyst coatings synthesized by a cold atmospheric plasma. Langmuir 35(22):7161–7168. https://doi.org/10.1021/acs.langmuir.9b00784
Cheng X, Yu X, Xing Z (2012) Characterization and mechanism analysis of N doped TiO2 with visible light response and its enhanced visible activity. Appl Surf Sci 258(7):3244–3248. https://doi.org/10.1016/j.apsusc.2011.11.072
Cong Y, Zhang J, Chen F, Anpo M (2007) Synthesis and characterization of nitrogen-doped TiO2 nanophotocatalyst with high visible light activity. J Phys Chem C 111(19):6976–6982. https://doi.org/10.1021/jp0685030
Dalawai SP, Saad Aly MA, Latthe SS, Xing R, Sutar RS, Nagappan S, Ha C-S, Sadasivuni K, K., & Liu S (2020) Recent advances in durability of superhydrophobic self-cleaning technology: a critical review. Prog Org Coat 138:105381. https://doi.org/10.1016/j.porgcoat.2019.105381
Daoud WA (ed) (2013) Self-cleaning materials and surfaces: a nanotechnology approach. Wiley, Chichester
Darmanin T, Guittard F (2015) Superhydrophobic and superoleophobic properties in nature. Mater Today 18(5):273–285. https://doi.org/10.1016/j.mattod.2015.01.001
Di Valentin C, Finazzi E, Pacchioni G, Selloni A, Livraghi S, Paganini MC, Giamello E (2007) N-doped TiO2: theory and experiment. Chem Phys 339(1):44–56. https://doi.org/10.1016/j.chemphys.2007.07.020
Fan Y, Zhou J, Zhang J, Lou Y, Huang Z, Ye Y, Jia L, Tang B (2018) Photocatalysis and self-cleaning from g-C3N4 coated cotton fabrics under sunlight irradiation. Chem Phys Lett 699:146–154. https://doi.org/10.1016/j.cplett.2018.03.048
Foorginezhad S, Zerafat MM (2019) Fabrication of stable fluorine-free superhydrophobic fabrics for anti-adhesion and self-cleaning properties. Appl Surf Sci 464:458–471. https://doi.org/10.1016/j.apsusc.2018.09.058
Geyer F, D’Acunzi M, Sharifi-Aghili A, Saal A, Gao N, Kaltbeitzel A, Sloot T-F, Berger R, Butt H-J, Vollmer D (2020) When and how self-cleaning of superhydrophobic surfaces works. Sci Adv 6(3):9727. https://doi.org/10.1126/sciadv.aaw9727
Ghasemlou M, Daver F, Ivanova EP, Adhikari B (2019) Bio-inspired sustainable and durable superhydrophobic materials: from nature to market. J Mater Chem A 7(28):16643–16670. https://doi.org/10.1039/C9TA05185F
Hu M, Wu Z, Sun L, Guo S, Li H, Liao J, Huang C, Wang B (2019) Improving pervaporation performance of PDMS membranes by interpenetrating polymer network for recovery of bio-butanol. Sep Purif Technol 228:115690. https://doi.org/10.1016/j.seppur.2019.115690
Huang JY, Li SH, Ge MZ, Wang LN, Xing TL, Chen GQ, Liu XF, Al-Deyab SS, Zhang KQ, Chen T, Lai YK (2015) Robust superhydrophobic TiO2@fabrics for UV shielding, self-cleaning and oil–water separation. J Mater Chem A 3(6): 2825–2832. https://doi.org/10.1039/C4TA05332J
Huang J, Dou L, Li J, Zhong J, Li M, Wang T (2021) Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J Hazard Mater 403:123857. https://doi.org/10.1016/j.jhazmat.2020.123857
Ju J, Yao X, Hou X, Liu Q, Zhang YS, Khademhosseini A (2017) A highly stretchable and robust non-fluorinated superhydrophobic surface. J Mater Chem A 5(31):16273–16280. https://doi.org/10.1039/C6TA11133E
Li S, Huang J, Ge M, Cao C, Deng S, Zhang S, Chen G, Zhang K, Al-Deyab SS, Lai Y (2015) Robust flower-like TiO2@cotton fabrics with special wettability for effective self-cleaning and versatile oil/water separation. Adv Mater Interfaces 2(14):1500220. https://doi.org/10.1002/admi.201500220
Liang Y, Pakdel E, Zhang M, Sun L, Wang X (2019) Photoprotective properties of alpaca fiber melanin reinforced by rutile TiO2 nanoparticles: a study on wool fabric. Polym Degrad Stab 160:80–88. https://doi.org/10.1016/j.polymdegradstab.2018.12.006
Liu X, Xing Z, Zhang Y, Li Z, Wu X, Tan S, Yu X, Zhu Q, Zhou W (2017) Fabrication of 3D flower-like black N-TiO2-x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl Catal B 201:119–127. https://doi.org/10.1016/j.apcatb.2016.08.031
Lu X, Li Z, Liu Ya, Tang B, Zhu Y, Razal JM, Pakdel E, Wang J, Wang X (2020) Titanium dioxide coated carbon foam as microreactor for improved sunlight driven treatment of cotton dyeing wastewater. J Cleaner Prod 246:118949. https://doi.org/10.1016/j.jclepro.2019.118949
Luna M, Mosquera MJ, Vidal H, Gatica JM (2019) Au-TiO2/SiO2 photocatalysts for building materials: self-cleaning and de-polluting performance. Build Environ 164:106347. https://doi.org/10.1016/j.buildenv.2019.106347
Montazer M, Pakdel E (2011) Functionality of nano titanium dioxide on textiles with future aspects: Focus on wool. J Photochem Photobiol C, 12(4):293–303. https://doi.org/10.1016/j.jphotochemrev.2011.08.005
Nguyen-Tri P, Tran HN, Plamondon CO, Tuduri L, Vo D-VN, Nanda S, Mishra A, Chao H-P, Bajpai AK (2019) Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog Org Coat 132:235–256. https://doi.org/10.1016/j.porgcoat.2019.03.042
Noralian Z, Gashti MP, Moghaddam MR, Tayyeb H, Erfanian I (2021) Ultrasonically developed silver/iota-carrageenan/cotton bionanocomposite as an efficient material for biomedical applications. Int J Biol Macromol 180:439–457. https://doi.org/10.1016/j.ijbiomac.2021.02.204
Pakdel E, Daoud W (2013) Self-cleaning cotton functionalized with TiO2/SiO2: focus on the role of silica. J Colloid Interface Sci 401:1–7. https://doi.org/10.1016/j.jcis.2013.03.016
Pakdel E, Daoud WA, Sun L, Wang X (2014a) Visible and UV functionality of TiO2 ternary nanocomposites on cotton. Appl Surf Sci 321:447–456. https://doi.org/10.1016/j.apsusc.2014.10.018
Pakdel E, Daoud WA, Wang X (2014b) Assimilating the photo-induced functions of TiO2-based compounds in textiles: emphasis on the sol-gel process. Text Res J 85(13):1404–1428. https://doi.org/10.1177/0040517514551462
Pakdel E, Daoud WA, Afrin T, Sun L, Wang X (2015) Self-cleaning wool: effect of noble metals and silica on visible-light-induced functionalities of nano TiO2 colloid. J Text Inst 106(12):1348–1361. https://doi.org/10.1080/00405000.2014.995461
Pakdel E, Wang J, Allardyce BJ, Rajkhowa R, Wang X (2016) Functionality of nano and 3D-microhierarchical TiO2 particles as coagulants for sericin extraction from the silk degumming wastewater. Sep Purif Technol 170:92–101. https://doi.org/10.1016/j.seppur.2016.06.025
Pakdel E, Daoud WA, Seyedin S, Wang J, Razal JM, Sun L, Wang X (2018a) Tunable photocatalytic selectivity of TiO2/SiO2 nanocomposites: effect of silica and isolation approach. Colloids Surf A 552:130–141. https://doi.org/10.1016/j.colsurfa.2018.04.070
Pakdel E, Fang J, Sun L, Wang X (2018b) Nanocoatings for smart textiles. In: Yilmaz ND (ed) Smart textiles: wearable nanotechnology. Wiley, Hoboken, pp 247–300
Pakdel E, Wang J, Kashi S, Sun L, Wang X (2020) Advances in photocatalytic self-cleaning, superhydrophobic and electromagnetic interference shielding textile treatments. Adv Colloid Interface Sci 277:102116. https://doi.org/10.1016/j.cis.2020.102116
Parvinzadeh Gashti M, Alimohammadi F, Shamei A (2012) Preparation of water-repellent cellulose fibers using a polycarboxylic acid/hydrophobic silica nanocomposite coating. Surf Coat Technol 206(14):3208–3215. https://doi.org/10.1016/j.surfcoat.2012.01.006
Parvinzadeh Gashti M, Almasian A (2013) Citric acid/ZrO2 nanocomposite inducing thermal barrier and self-cleaning properties on protein fibers. Compos Part B 52:340–349. https://doi.org/10.1016/j.compositesb.2013.04.037
Parvinzadeh Gashti M, Pakdel E, Alimohammadi F (2016) Nanotechnology-based coating techniques for smart textiles. In: Hu J (ed) Active coatings for smart textiles. Woodhead Publishing, Duxford, pp 243–268
Schellenberger S, Hill PJ, Levenstam O, Gillgard P, Cousins IT, Taylor M, Blackburn RS (2019) Highly fluorinated chemicals in functional textiles can be replaced by re-evaluating liquid repellency and end-user requirements. J Cleaner Prod 217:134–143. https://doi.org/10.1016/j.jclepro.2019.01.160
Sun Z, Pichugin VF, Evdokimov KE, Konishchev ME, Syrtanov MS, Kudiiarov VN, Li K, Tverdokhlebov SI (2020) Effect of nitrogen-doping and post annealing on wettability and band gap energy of TiO2 thin film. Appl Surf Sci 500:144048. https://doi.org/10.1016/j.apsusc.2019.144048
Talebi S, Montazer M (2020) Denim fabric with flame retardant, hydrophilic and self-cleaning properties conferring by in-situ synthesis of silica nanoparticles. Cellulose 27(11):6643–6661. https://doi.org/10.1007/s10570-020-03195-6
Tavares MTS, Santos ASF, Santos IMG, Silva MRS, Bomio MRD, Longo E, Paskocimas CA, Motta FV (2014) TiO2/PDMS nanocomposites for use on self-cleaning surfaces. Surf Coat Technol 239:16–19. https://doi.org/10.1016/j.surfcoat.2013.11.009
Wang C, Yin L, Zhang L, Qi Y, Lun N, Liu N (2010) Large scale synthesis and gas-sensing properties of anatase TiO2 three-dimensional hierarchical nanostructures. Langmuir 26(15):12841–12848. https://doi.org/10.1021/la100910u
Wang Y, Huang Z, Gurney RS, Liu D (2019) Superhydrophobic and photocatalytic PDMS/TiO2 coatings with environmental stability and multifunctionality. Colloids Surf A 561:101–108. https://doi.org/10.1016/j.colsurfa.2018.10.054
Wu D, Wang H, Li C, Xia J, Song X, Huang W (2014) Photocatalytic self-cleaning properties of cotton fabrics functionalized with p-BiOI/n-TiO2 heterojunction. Surf Coat Technol 258:672–676. https://doi.org/10.1016/j.surfcoat.2014.08.019
Wu Y, Zhou S, You B, Wu L (2017) Bioinspired design of three-dimensional ordered tribrachia-post arrays with re-entrant geometry for omniphobic and slippery surfaces. ACS Nano 11(8):8265–8272. https://doi.org/10.1021/acsnano.7b03433
Xie W, Pakdel E, Liu D, Sun L, Wang X (2019) Waste Hair-Derived Natural Melanin/TiO2 Hybrids as Highly Efficient and Stable UV-Shielding Fillers for Polyurethane Films. ACS Sustainable Chem Eng 8(3):1343–1352. https://doi.org/10.1021/acssuschemeng.9b03514
Xie W, Pakdel E, Liang Y, Liu D, Sun L, Wang X (2020) Natural melanin/TiO2 hybrids for simultaneous removal of dyes and heavy metal ions under visible light. J Photochem Photobiol A 389:112292. https://doi.org/10.1016/j.jphotochem.2019.112292
Xu Q, Fan Q, Ma J, Yan Z (2016) Facile synthesis of casein-based TiO2 nanocomposite for self-cleaning and high covering coatings: Insights from TiO2 dosage. Prog Org Coat 99:223–229. https://doi.org/10.1016/j.porgcoat.2016.05.024
Xu L, Zhang X, Shen Y, Ding Y, Wang L, Sheng Y (2018) Durable superhydrophobic cotton textiles with ultraviolet-blocking property and photocatalysis based on flower-like copper sulfide. Ind Eng Chem Res 57(19):6714–6725. https://doi.org/10.1021/acs.iecr.8b00254
Yang M, Liu W, Jiang C, He S, Xie Y, Wang Z (2018) Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohydr Polym 197:75–82. https://doi.org/10.1016/j.carbpol.2018.05.075
Zahid M, Heredia-Guerrero JA, Athanassiou A, Bayer IS (2017) Robust water repellent treatment for woven cotton fabrics with eco-friendly polymers. Chem Eng J 319:321–332. https://doi.org/10.1016/j.cej.2017.03.006
Zahid M, Mazzon G, Athanassiou A, Bayer IS (2019) Environmentally benign non-wettable textile treatments: a review of recent state-of-the-art. Adv Colloid Interface Sci 270:216–250. https://doi.org/10.1016/j.cis.2019.06.001
Zhang X, Si Y, Mo J, Guo Z (2017) Robust micro-nanoscale flowerlike ZnO/epoxy resin superhydrophobic coating with rapid healing ability. Chem Eng J 313:1152–1159. https://doi.org/10.1016/j.cej.2016.11.014
Zhao J, Wang J, Fan L, Pakdel E, Huang S, Wang X (2017) Immobilization of titanium dioxide on PAN fiber as a recyclable photocatalyst via co-dispersion solvent dip coating. Text Res J 87(5):570–581. https://doi.org/10.1177/0040517516632479
Zhou H, Wang H, Niu H, Gestos A, Wang X, Lin T (2012) Fluoroalkyl silane modified silicone rubber/nanoparticle composite: a super durable, robust superhydrophobic fabric coating. Adv Mater 24(18):2409–2412. https://doi.org/10.1002/adma.201200184
Zhou H, Zhao Y, Wang H, Lin T (2016) Recent development in durable super-liquid-repellent fabrics. Adv Mater Interfaces 3(23):1600402. https://doi.org/10.1002/admi.201600402
Zhou P, Lv J, Xu H, Wang X, Sui X, Zhong Y, Wang B, Chen Z, Feng X, Zhang L, Mao Z (2019) Functionalization of cotton fabric with bismuth oxyiodide nanosheets: applications for photodegrading organic pollutants, UV shielding and self-cleaning. Cellulose 26(4):2873–2884. https://doi.org/10.1007/s10570-019-02281-8
Zhu H, Guo Z, Liu W (2014) Adhesion behaviors on superhydrophobic surfaces. Chem Commun 50(30):3900–3913. https://doi.org/10.1039/C3CC47818A
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pakdel, E., Zhao, H., Wang, J. et al. Superhydrophobic and photocatalytic self-cleaning cotton fabric using flower-like N-doped TiO2/PDMS coating. Cellulose 28, 8807–8820 (2021). https://doi.org/10.1007/s10570-021-04075-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04075-3