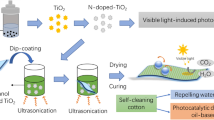
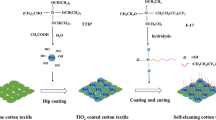
Abstract
This study aims to explore the function of hydrophilic features of natural SiO2 in supporting the TiO2 photocatalysis process for developing cotton fabric with high self-cleaning performance. SiO2 was produced by extracting natural materials such as Bengkulu beach sand with a high SiO2 content. The method used is dip-coating in its sol form with the help of ultrasonic waves to maximize the composite loading on the fabric. TiO2/SiO2 composite sols were synthesized at three distinct molar ratios, 2:1, 1:1, and 1:2. The self-cleaning activity was observed against methylene blue using the digital image colorimetry technique. SEM images reveal agglomerated TiO2 and SiO2 particles attached to the cotton fabric fibers with an average size of 14 nm. The FTIR investigations also demonstrate the existence of Ti \(-\) O \(-\) C and Si \(-\) O \(-\) C bonds, indicating TiO2 and SiO2 particles’ interaction with cotton fabric fibers. Contact angle measurements show that cotton fabric with TiO2 increases hydrophilicity to 17.8 \(^\circ\), whereas cotton fabric with SiO2 has a superhydrophilic surface (0 \(^\circ\)). This observation is consistent with the FTIR data, in which the O \(-\) H stretching band develops following the addition of TiO2 sol and is more obvious in samples containing SiO2. The O–H stretching band appears after adding TiO2 sol and is more pronounced in samples containing SiO2 The characterization results showed that TiO2/SiO2 composites were successfully added to cotton fabrics. Cotton fabrics with a ratio of TiO2/SiO2 (1:1) showed the best self-cleaning activity against methylene blue, reaching 95.25%. Modified cotton fabrics also showed particle adhesion stability with a slight decrease in self-cleaning activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile business is going through a revolutionary change in an environment where sustainability is becoming increasingly a concern (Gazzola et al. 2020). Conventional textiles made of cotton frequently struggle to remain clean and odor-free. Cotton fabrics are used as medical textiles, such as surgical gowns, sheets, patient pajamas, to health worker uniforms (Fijan et al. 2007). This sort of garment must have a high level of dirt protection. Frequent washing is required to remove stains and odors from daily life, which adds to excessive consumption of energy and water as well as detergent pollution (Goel & Kaur 2012). Recently, researchers have resorted to nanotechnology to solve these problems by utilizing its inherent ability to self-clean (Nozari et al. 2021).
Combining TiO2 nanoparticles with cellulose or cotton surfaces results in a self-cleaning effect (Diaa & Hassabo 2022). TiO2, as a semiconductor, can produce electron–hole pairs that will move to the surface of particles and undergo redox reactions that produce reactive oxygen species (ROS) (Kim et al. 2002). ROS such as hydroxyl radicals (•OH) and superoxide anions produced are the reason TiO2 can degrade organic pollutants (Liu et al. 2019). TiO2 decomposes organic molecules rapidly and promotes the spread of water on the surface to complete its self-cleaning routine (Lukong et al. 2022). However, electron–hole pairs in TiO2 can undergo rapid recombination, which is a big challenge for practical TiO2 applications (Yang et al. 2022). To improve the photocatalytic activity of TiO2, hybrid or composite materials are made (Le et al. 2021).
SiO2 can be added to create composite materials that have improved electron–hole pair separation and charge transport (Babyszko et al. 2022). Adding SiO2 contributes to surface acidity, which boosts the hydroxyl content of the composite and improves the self-cleaning performance (Guan 2005). Hydrophilic properties are also essential to ensure the comfort of the fabric when used (Das et al. 2009). Natural silica can be extracted from natural resources by a simple alkali treatment (Borah et al. 2023). Firdaus et al. (2020) successfully extracted silica from the sand of Bengkulu Beach, Indonesia. Natural silica has advantages in the field of catalysts because it is easy to obtain and apply in chemical reaction processes (Elmaria & Jenie 2021). TiO2/SiO2 composites with natural silica perform well in reducing harmful pollutants (Eddy et al. 2020).
Methylene blue is often used as a photocatalyst target for determining self-cleaning activity in several studies (Hosseini et al. 2020; Wei et al. 2022). The color change of methylene blue can be seen and measured using spectroscopy or colorimetry (Karimi et al. 2020; Springer et al. 2020). Digital image colorimetry is economical because it does not require expensive instruments or reagents (Lima et al. 2020). Color analysis is performed using a smartphone built-in camera and freely accessible software (Fan et al. 2021). Methylene blue concentration can be determined utilizing a smartphone-based digital image colorimetry technique with an RGB color response (Permana et al. 2023).
Wu et al. (2009) successfully prepared self-cleaning fabrics against methyl orange by depositing TiO2 nanoparticles on cotton fabrics via an aqueous sol process at low temperatures. TiO2-coated cotton fabrics were prepared under different processing temperatures by Doganli et al. (2016) and showed a self-cleaning activity against hot tea solution. Saleem et al. (2021) synthesized titanium dioxide nanoparticles and coated them over cotton fabrics initiated by atmospheric pressure dielectric barrier (DBD) discharge. The plasma-TiO2 fabric demonstrated a strong ability to self-clean against methylene blue. Pakdel & Daoud (2013) functionalized cotton fabric with TiO2/SiO2 using the dip-pad-dry cure technique. The samples coated with TiO2/SiO2 had a better ability to remove coffee stains and degrade methylene blue than samples functionalized with TiO2 only, indicating an improved self-cleaning effect. (Li et al. 2017) used ultrasonic irradiation to synthesize TiO2/SiO2 at low temperatures and deposit nanoparticles onto a polyester-cotton fabric. The TiO2/SiO2 nanoparticles had better photocatalytic activity on treated textiles than pure TiO2 nanoparticles. Our previous study has demonstrated that polyester textiles coated with TiO2 and SiO2 also possess excellent self-cleaning activity (Eddy et al. 2023).
The current work investigates a simple method of preparation of self-cleaning cotton fabrics by depositing TiO2 and natural SiO2 on the cotton fabrics. The self-cleaning effect of the modified cotton fabrics originates from the photocatalytic activity of TiO2 and is enhanced by the hydrophilic properties of SiO2. Here, SiO2 was extracted from Bengkulu Beach sand and combined with TiO2 to improve self-cleaning activity. The modified cotton fabrics were characterized for their superhydrophilic properties from natural SiO2. Using smartphone-based digital image colorimetry (DIC) techniques and methylene blue as the target, this study investigates the self-cleaning activity of cotton fabrics modified with TiO2 and SiO2. The utilization of TiO2 and natural SiO2 composites on cotton fabrics in the form of sols in this study has never been done before.
Materials and methods
Materials
The primary material utilized in this study was white cotton poplin fabric, which weighed 161 g/m2, had a density of 35 threads/cm, and had an entirely cotton composition. Bengkulu Beach sand, Indonesia, used as the main source of natural SiO2. The chemical reagents used were hydrochloric acid (HCl, 37%), acetone (CH3COCH3, 99%), distilled water, ethanol (CH3CH2OH, 99%), isopropanol (CH3CH(OH)CH3, 99%), potassium hydroxide (KOH, 98%), methylene blue (MB), titanium tetraisopropoxide (TTIP, 97%). All chemical reagents used were purchased from Merck & Co. (Rahway, USA).
Preparation of TiO2 and SiO2 Sols
The process of extracting silica from beach sand refers to previous research by Firdaus et al. (2020). The Bengkulu Beach sand was crushed with a grinder and then sifted with a 325-mesh sieve. Beach sand that passes through the 325-mesh then soaked in HCl 10 mol L-1 for 20 h to remove minerals other than silica. The residue from the mixture was washed with distilled water until neutral. Furthermore, an alkaline fusion reaction was carried out by mixing 30 g of cleaned silica sand with KOH 10 mol L-1, and the mixture was heated at 360 °C for 4 h, K2SiO3 was the product of this process. Next, K2SiO3 dissolved in 500 mL of distilled water, stirred, and left for 24 h. The mixture was filtered, and the filtrate was added with HCl 10 mol L-1 drop by drop until the pH of the mixture reached 1–2 and accompanied by the observation of white gel formation. The formed gel was filtered and washed with aquadest until neutral. The neutral formed gel was used to make SiO2 sols by dilution using ethanol and water.
The preparation of TiO2 sols tends to be simpler because it uses alkoxide precursors. TTIP was added to 20 mL of isopropanol and stirred quickly for 15 min for hydrolysis. Furthermore, distilled water was added to TTIP in isopropanol for the condensation process into a sol and stirred for 15 min. TiO2 sols are ready to use for fabric modification.
TiO2 and SiO2 coating on cotton fabrics
To remove soluble dust and dirt, cotton fabrics were cleaned with acetone under sonication for 10 min, and then ethanol was used for another 10 min of the ultrasonic cleaning process to remove wax and grease. Then, the cotton fabric was washed to remove water-soluble contaminants with distilled water and dried at 50 °C. After that, cleaned cotton fabric is rinsed with aquadest to pH = 7 and dried at 80 °C for 15 min.
Each TiO2 and SiO2 sols mixed to produce TiO2/SiO2 sols. Cotton fabric with a size of 6 cm × 6 cm is immersed in TiO2/SiO2 sols with variations in TiO2 and SiO2 composition according to Table 1. The fabric soaking process was carried out for 5 min with the help of a sonication process. This process was carried out three times to maximize the composite load on the fabric. Then, the fabric was removed and dried in the oven at 80 \(^\circ\) C for 10 min. Next, the curing process was carried out in the oven at 120 \(^\circ\) C for 5 min.
Characterization techniques
Scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM–EDS, Tabletop Microscope-1000, Hitachi, Tokyo, Japan) with an acceleration voltage of 15.0 kV and magnification of 100–5000 times was carried out to determine the surface morphology and mapping of each atom of the fabric. X-ray diffraction (XRD, Rigaku/MiniFlex 600, Tokyo, Japan) was used to examine the crystal structure of the modified using Cu K \(\alpha\) emission (\(\lambda\) = 0.15418 nm), and scans were done in the range of 20–80° (2 \(\theta\)). The crystallite size of the sample was calculated with Eq. (1) (Permana et al. 2022).
where D is the crystallite size in nm, K is the Scherrer constant (0.9), λ is the wavelength of X-ray radiation (0.15418 nm), B is the value of the full width at half maximum (FWHM) peak (radians), and θ is the diffraction angle (radians).
Attenuated Total Reflection Fourier transform infrared spectroscopy (ATR-FTIR, Jasco FT/IR-4700, Tokyo, Japan) was used to observe the functional groups that appear on the modified cotton fabric. A micropipette was used to drip 5 \(\mu\) L of distilled water at a distance of 1 cm from the cotton fabric’s surface, and the contact angle on the cotton fabric was measured.
Self-cleaning activity test
The self-cleaning activity of TiO2/SiO2 composites on fabrics was measured by determining MB color change. Fabric samples before and after dipping in MB solution were photographed on a flat surface to determine RGB blanks and RGB before degradation. The fabric was irradiated with a 125 W UV–Vis Phillips HPL-N lamp. The color change of the MB was observed by comparing images taken in a span of 2 h over 6 h of radiation using the Samsung M21 (Samsung Electronics Co., Ltd., South Korea) smartphone. The characteristics of the Samsung M21 camera are included in Table 2.
The observed color changes were processed with digital image colorimetry (DIC). ImageJ 1.52a, Excel 2021 (Microsoft Office Home and Student) and Minitab 19 (Minitab Inc.) were used for digital image data processing. The method for determining dye concentration is by immersing the fabric in a series of standard solutions and placing it under constant light and photographic conditions. Image capture was done with three repetitions.
ImageJ software was used to determine the R, G, and B color values of each sample. The RGB color values were converted to a logarithmic scale to obtain color intensity, following the equation of the Lambert–Beer law as follows, Eq. (2).
IR,G,B is the effective intensity of each color red, green and blue, while A0 R,G,B and AS R,G,B are the red, green, and blue color values of the blank and sample respectively. The digital camera works as a spectrophotometer, analyzing light emitted by methylene blue. The intensity displayed is the sum of the digital image's R, G, and B color values. Further analysis of RGB data was performed by Excel and Minitab for simple linear regression and partial least squares, respectively. These calculations were needed to get the equation used for determining the self-cleaning activity.
Particle adhesion stability test
This procedure was carried out to test the stability of modified fabrics according to research by Eddy et al. (2023). The modified fabric is soaked in distilled water, shaken at of 30 rpm for 15 min, and dried at 40 \(^\circ\) C. This process is repeated five times to achieve five cycles.
Result and discussion
Surface analysis
The surface morphology of cotton fabric after treatment can be monitored by SEM measurements shown in Fig. 1. From the SEM results, it can be seen that SiO2 appears as a layer covering the cotton fabric fibers while TiO2 appears as agglomerated white dots. These particles tend to form agglomerations on the surface of cotton fabrics, caused by the adhesion of TiO2/SiO2 composite sols with cotton fabric fibers formed after saturation of the precursors within the cotton fabric fibers (Shaheen et al. 2019). The average particle size is 14 nm.
The EDS measurement results in Table 3 also show the composition of elements following the ratio of TiO2 and SiO2 applied to cotton fabrics. Further observations to determine the elements contained in each sample were carried out by mapping shown in Fig. 2. The mapping results show carbon and oxygen elements with green and blue colors along the cotton fabric fibers, because these carbon and oxygen elements are the constituents of cotton fabric fibers. At the same time, the elements titanium (yellow color) and silicon (red color) appear in the form of small dots with an even distribution. When viewed from the surface, the dots that appear tend to be rarely seen. While the mapping results show that small spots appear more, because TiO2 and SiO2 are trapped in the pores of cotton fabric fibers. The SEM–EDS analysis and mapping results show that TiO2/SiO2 has been successfully applied to fabrics with agglomerated round shapes, and the ratio between TiO2 and SiO2 is quite appropriate.
XRD analysis
XRD data is usually used to determine the atomic structure and crystal structure of materials. In this study, the XRD diffraction pattern of cotton fabric with and without modification is shown in Fig. 3. Figure 3 shows the diffraction pattern of the entire sample at 2 \(\theta\) = 15 \(^\circ\), 16.9 \(^\circ\), 22.9 \(^\circ\), and 34 \(^\circ\). These peaks correspond with the typical cellulose I \(\beta\) crystal structures (ICSD no. 00–056-1718), with planes (1–10), (110), (200) and (004) respectively (French 2014). Despite the modifications, the structure of the crystal has not changed, there have only been a few shifts in the planes (110) and (200). The crystallite size for all samples is 3.84 nm from calculation of 2 \(\theta\) and FWHM of (200) peak using the Debye–Scherrer equation. XRD measurements show that TiO2/SiO2 does not change the structure of the crystal of cellulose in fabrics.
Functional group analysis
The interaction between TiO2/SiO2 soles and cotton fabric fibers results in changes in intermolecular bonds in the structure of cotton fabrics. These changes can be detected by characterization using FTIR spectroscopy as shown in Fig. 4a, The widened peak at wavenumber 33238 cm−1 which appears after the cotton fabric was modified is the vibration of the O–H stretching (Ahmad et al. 2019). In this region, samples containing SiO2 or having higher levels of SiO2 (CS, CT1S1 and CT1S2) display a peak intensity increase. The peaks that appear at wavenumbers 3028 and 2950 cm−1 are peaks that arise due to stretching vibrations of the Csp2–H and group Csp3–H. While the peak at wavenumber 1250 cm−1 is the peak of the stretching vibration of C–H bond (Rosales et al. 2019). A peak at 1160 cm−1 indicates the presence of a C–O–C group from the \(\beta\)(1–4) glycoside bond in the cellulose polymer that makes up cotton fabric fibers (Seeharaj et al. 2018). There are peaks of C − O stretching and C − H bending vibrations at wave numbers 1108 and 908 cm−1. Furthermore, there is a peak of C − OH bending vibrations in 660 cm−1. These peaks correspond to the functional groups of cellulose, the material that makes up cotton fabric fibers.
Si–O–Si groups are indicated by peaks appearing in the region of 1108 cm−1 and Si–O–C in 1053 cm−1. This peak pattern is similar with previous study by Paryab et al. (2021). From what can be seen in Fig. 4b samples with TiO2 or a higher proportion of TiO2 (CT, CT2S1) show increased intensity in this region. Meanwhile, in samples with SiO2 there is a slight decrease in intensity in this area. This indicates that the Ti–O–C bond has a peak at 908 cm−1, overlapping with the C–H bending vibrations. The Ti–O–C bond is obeserved 900–1000 in several studies (Wang et al. 2017; Zhu et al. 2020). While Ti–O stretching was observed around 665 cm−1 (Al-Amin et al. 2016). This band is also overlap with C − OH bending vibrations. Sample with a higher proportion of TiO2 (CT2S1) show similar FTIR spectrum to CT sample and vice versa. FTIR test results show that functional group peaks corresponding to TiO2/SiO2 are appeared, and TiO2/SiO2 are succesfully deposited on cotton fabrics. The findings of the FTIR analysis demonstrate the presence of functional group peaks corresponding to TiO2/SiO2, and the successful deposition of TiO2/SiO2 on cotton fabrics.
Contact angle analysis
The interaction between the fabric sample before and after modification with water was observed by measurement of the contact angle. The contact angle of each sample can be seen in Fig. 5. Cotton fabric has the highest contact angle of 62.4 \(^\circ\). Adding TiO2 to the cotton fabric can decrease the contact angle by up to 17.8 \(^\circ\) (CT samples) due to the Ti–OH sols deposited on the cotton fabric. This contact angle result is consistent with the previously stated FTIR data, which showed the presence of a peak of O–H stretching vibrations after adding TiO2 to the fabric. Meanwhile, after SiO2 addition, the fabric sample has the smallest contact angle, which is 0 \(^\circ\), causing the CS sample to enter the superhydrophilic surface range. The increased hydrophilic properties after SiO2 addition can be due to the abundance of O–H groups adsorbed on the surface. This result is aligns with earlier FTIR data that revealed a more obvious O–H stretching peak after adding SiO2 to the cotton fabric. This increased number of O–H groups can increase the hydrophilicity of cotton fabric samples.
CT2S1 samples with more TiO2 have a contact angle like CT samples. Meanwhile, samples with a larger SiO2 composition have a contact angle like CS samples. Different amounts of SiO2 in TiO2/SiO2 composites can produce different surface properties, a sample with more SiO2 has a surface with superhydrophilic properties. This result is similar to the prior study by Pakdel & Daoud (2013), which found that increasing the amount of silica gives the coated fabric hydrophilic properties, resulting in the total dispersion of water droplets. Since the hydrophilicity of TiO2 is not high enough to allow for self-cleaning, it is challenging to remove pollutants from the photocatalyst surface (Cha et al. 2019). When there is SiO2 present with the hydrophilicity performance, there might be more interaction between TiO2 and water. This interaction may increase the production of reactive oxygen species (ROS), which can lead to self-cleaning activity.
Self-cleaning activity test
Standard curve
The self-cleaning ability of MB stains owned by TiO2 and SiO2-modified fabrics is determined by the digital image colorimetry (DIC) technique. A standard curve is required to determine the concentration of MB during the degradation process of RGB values recorded in each time range. The standard curve is a plot of concentration on the recorded signal (in this study, it is the RGB value). However, the recorded signal is never perfectly proportional to the sample concentration, so a standard curve is used to correct this deficiency. The accuracy of the proposed method is assessed by repeating the same sample shooting analysis and RGB calculations. Three tests were also performed to evaluate the accuracy of MB degradation measurements. From the simple linear regression calculated with Excel 2021 (Microsoft Inc.), the effect of each R, G and B color on the concentration of MB in cotton fabric has been known. This method treats RGB data as univariate data. The average RGB color values and the logarithmic conversion (color intensity) of RGB to various concentrations of methylene blue plotted on the standard curve are shown in Table 4.
Standard curves and image series of fabrics soaked in various concentrations can be seen in Fig. 6. The slope of the calibration curve is 8 × 10–3, 2.2 × 10–3, and 1.7 × 10–3 for R, G, and B, respectively. The intensity of the color with the highest slope is red because the most noticeable change as the concentration increases are the decrease in red as one of the color constituents compared to the other two colors. The limit of detection of each red, green, and blue are 2.6987, 0.4842, and 0.5575 ppm, respectively.
Partial least squares (PLS) regression was calculated using Minitab 19 software (Minitab Inc.). PLS regression is a better calculation approach because it creates a new linear regression by considering RGB data as a multivariate set. Previous research mentioned that calculations with this model are more sophisticated calculation methods that have some relationship with regression of major components (Firdaus et al. 2014). This calculation results in a good correlation coefficient (R2) of 0.9891. This calculation yields Eq. (3):
Methylene blue degradation
Methylene blue (MB) is widely employed in various applications, including determining self-cleaning activity, owing to its ability to work as a visible, quantitative signal. The gradual removal of the MB colors indicates that a substance with a self-cleaning ability breaks down impurities or contaminants when exposed to light. The color of MB is recorded and quantified with smartphone-based digital image colorimetry, resulting in the graph shown in Fig. 7.
All samples, including the raw cotton fabric show decreased MB concentration. The raw cotton fabric shows the least self-cleaning activity, with a degradation efficiency of only 47.86%, because no material can interact more with the methylene blue except the cotton fabric itself. It is followed by the cotton fabric coated with only one component of SiO2 (72.88%) and TiO2 (77.14%). More degradation efficiency is obtained when TiO2 and SiO2 are combined, as shown in CT2S1, CT1S1, and CT1S2 samples. This finding is because there is a synergistic effect of TiO2 and SiO2 to work together as self-cleaning material. Cotton fabrics coated with composite with more TiO2 (CT2S1) and more SiO2 (CT1S2) showed a degradation efficiency of 81.79% and 78,03%, respectively. The degradation efficiency values of these samples are not optimal because the proportion of each component cannot initiate a maximum self-cleaning effect. Meanwhile, cotton fabric with a composite of TiO2/SiO2 (1:1) with code CT1S1 showed the highest degradation efficiency, reaching 95.25%.
During irradiation, TiO2 can produce electron–hole pairs, which then react with oxygen (O2) and water (H2O), producing reactive oxygen species (ROS) such as anions superoxide (O2•−) and hydroxyl radicals (•OH) (Guo et al. 2019). ROS is unstable and can react and break the structure from contaminants, so contaminants can be degraded. Uniquely, holes produced during the hydrophilic photocatalysis process can be captured by − OH groups adsorbed on the surface to increase electron–hole separation (Ollis et al. 1991). The photocatalytic activity can increase because more − OH groups can be adsorbed on the surface due to of hydrophilicity (Guan 2005). This phenomenon is known as the synergetic effect of photocatalysis and hydrophilicity. The color change of methylene blue during irradiation of each sample is shown in Fig. 8.
Particles adhesion stability test result
This test is carried out on the fabric to determine the stability of SiO2 and TiO2 attachment on cotton fabric. This test is performed because the modified cotton fabric has a high hydrophilicity. This property might let the particles be carried away by water when they come into contact with it. So, this investigation is done to determine how stable particle adhesion to the fabric was when soaked in a large volume of water. This test is a preliminary study, and we plan to develop it in the future. This particle adhesion stability was observed by determining the degradation efficiency of methylene blue before and after water immersion, shown by Fig. 9. These results demonstrate that the modified cotton fabric has particle adhesion stability. However, there is a slight reduction in self-cleaning activity after five water immersion cycles. It is important to note that the self-cleaning performance decreases get larger gradually.
The use of sol forms of TiO2 and SiO2 during the coating process can increase the adhesion of these composites to the cotton fabric fibers. SiO2 can also increase particle adhesion stability, enhancing its resistance to various stresses and conditions, including light, moisture, and mechanical stress (Zambrano-Mera et al. 2022). This particle adhesion stability guarantees that the self-cleaning process lasts for an extended period.
Conclusions
The results of the characterization revealed that TiO2/SiO2 composites were successfully incorporated into cotton textiles. Surface analysis showing that after modifications there are small particles on cotton fabrics corresponds to TiO2/SiO2 composite. The XRD data shows that the crystallite size for all synthesized TiO2/SiO2 is 3.84 nm. The FTIR investigations also demonstrate the existence of Ti \(-\) O \(-\) C and Si \(-\) O \(-\) C bonds, indicating that TiO2 and SiO2 particles’ have chemical bonding with cotton fabric fibers. The O–H stretching band appears after adding TiO2 sol and is more pronounced in samples containing SiO2, this finding is consistent with contact angle results. Cotton fabric containing more TiO2 (CT, CT2S1) has a contact angle decrease of 17.8°. Cotton fabric containing SiO2 (CS, CT1S1, CT1S2) has a superhydrophilic surface of 0°. Different levels of SiO2 in TiO2/SiO2 composites could result a variety of surface hydrophilicity properties. The optimal ratio between TiO2 as a photocatalyst and SiO2 as a supporting material in cotton fabrics is TiO2:SiO2 1:1 which shows the highest self-cleaning activity reaching 95.25% degradation to methylene blue through DIC (digital image colorimetry) determination. Cotton fabrics coated with TiO2/SiO2 (1:1) composite showed good stability of the particle adhesion to the cotton fabric after five water immersion cycles.
Data availability
All the required data has been mentioned in the study.
References
Ahmad I, Kan CW, Yao Z (2019) Photoactive cotton fabric for UV protection and self-cleaning. RSC Adv 9(32):18106–18114. https://doi.org/10.1039/c9ra02023c
Al-Amin M, Chandra Dey S, Rashid TU, Ashaduzzaman M, Shamsuddin SM (2016) Solar assisted photocatalytic degradation of reactive azo dyes in presence of anatase titanium dioxide. Int J Latest Res Eng Technol (IJLRET) 2(3):14–21
Babyszko A, Wanag A, Sadłowski M, Kusiak-Nejman E, Morawski AW (2022) Synthesis and characterization of SiO2/TiO2 as photocatalyst on methylene blue degradation. Catalysts 12(11). https://doi.org/10.3390/catal12111372
Borah MP, Kalita BB, Jose S, Baruah S (2023) Fabrication of hydrophobic surface on eri silk/wool fabric using nano silica extracted from rice husk. Silicon 15(16):7039–7046. https://doi.org/10.1007/s12633-023-02568-3
Cha BJ, Saqlain S, Seo HO, Kim YD (2019) Hydrophilic surface modification of TiO2 to produce a highly sustainable photocatalyst for outdoor air purification. Appl Surf Sci 479:31–38. https://doi.org/10.1016/j.apsusc.2019.01.261
Das B, Das A, Kothari V, Fanguiero R, Araujo MD (2009) Moisture flow through blended fabrics - effect of hydrophilicity. J Eng Fibers Fabr 4(4):20–28. https://doi.org/10.1177/155892500900400405
Diaa M, Hassabo AG (2022) Self-cleaning properties of cellulosic fabrics (A review). Biointerface Res Appl Chem 12(2):1847–1855. https://doi.org/10.33263/BRIAC122.18471855
Doganli G, Yuzer B, Aydin I, Gultekin T, Con AH, Selcuk H, Palamutcu S (2016) Functionalization of cotton fabric with nanosized TiO2 coating for self-cleaning and antibacterial property enhancement. J Coat Technol Res 13(2):257–265. https://doi.org/10.1007/s11998-015-9743-7
Eddy DR, Ishmah SN, Permana MD, Lutfi Firdaus M (2020) Synthesis of titanium dioxide/silicon dioxide from beach sand as photocatalyst for Cr and Pb remediation. Catalysts 10(11):1–11. https://doi.org/10.3390/catal10111248
Eddy DR, Luthfiah A, Permana MD, Deawati Y, Firdaus ML, Rahayu I, Izumi Y (2023) Rapid probing of self-cleaning activity on polyester coated by titania-natural silica nanocomposite using digital image-based colorimetry. ACS Omega 8(8):7858–7867. https://doi.org/10.1021/acsomega.2c07606
Elmaria FA, Jenie SNA (2021) Magnetic nanoparticles based on natural silica as a methyl ester forming acid catalyst. J Kim Terap Indones 23(2):49–54. https://doi.org/10.14203/inajac.v23i2.473
Fan Y, Li J, Guo Y, Xie L, Zhang G (2021) Digital image colorimetry on smartphone for chemical analysis: a review. Measurement 171:108829. https://doi.org/10.1016/j.measurement.2020.108829
Fijan S, Šostar Turk S, Neral B, Pušić T (2007) The influence of industrial laundering of hospital textiles on the properties of cotton fabrics. Text Res J 77(4):247–255. https://doi.org/10.1177/0040517507077476
Firdaus ML, Alwi W, Trinoveldi F, Rahayu I, Rahmidar L, Warsito K (2014) Determination of chromium and iron using digital image-based colorimetry. Procedia Environ Sci 20:298–304. https://doi.org/10.1016/j.proenv.2014.03.037
Firdaus ML, Madina FE, Sasti YF, Elvia R, Ishmah SN, Eddy DR, Cid-Andres AP (2020) Silica extraction from beach sand for dyes removal: Isotherms, kinetics and thermodynamics. Rasayan J Chem 13(1):249–254. https://doi.org/10.31788/RJC.2020.1315496
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21(2):885–896. https://doi.org/10.1007/s10570-013-0030-4
Gazzola P, Pavione E, Pezzetti R, Grechi D (2020) Trends in the fashion industry. The perception of sustainability and circular economy: a gender/generation quantitative approach. Sustainability 12(7):1–19. https://doi.org/10.3390/su12072809
Goel G, Kaur S (2012) A study on chemical contamination of water due to household laundry detergents. J Hum Ecol 38(1):65–69. https://doi.org/10.1080/09709274.2012.11906475
Guan K (2005) Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf Coat Technol 191(2–3):155–160. https://doi.org/10.1016/j.surfcoat.2004.02.022
Guo Q, Zhou C, Ma Z, Yang X (2019) Fundamentals of TiO2 photocatalysis: concepts, mechanisms, and challenges. Adv Mater 31(50):1–26. https://doi.org/10.1002/adma.201901997
Hosseini MS, Ebratkhahan M, Shayegan Z, Niaei A, Salari D, Rostami A, Raeisipour J (2020) Investigation of the effective operational parameters of self-cleaning glass surface coating to improve methylene blue removal efficiency; application in solar cells. Sol Energy 207:398–408. https://doi.org/10.1016/j.solener.2020.06.109
Karimi H, Heidari MA, Emrooz HBM, Shokouhimehr M (2020) Carbonization temperature effects on adsorption performance of metal-organic framework derived nanoporous carbon for removal of methylene blue from wastewater; experimental and spectrometry study. Diam Relat Mater 108:107999. https://doi.org/10.1016/j.diamond.2020.107999
Kim JW, Shim JW, Bae JH, Han SH, Kim HK, Chang IS, Kang HH, Suh KD (2002) Titanium dioxide/poly(methyl methacrylate) composite microspheres prepared by in situ suspension polymerization and their ability to protect against UV rays. Colloid Polym Sci 280(6):584–588. https://doi.org/10.1007/s00396-002-0655-6
Le MC, Le TH, Bui Thi TH, Nguyen QD, Do Thi TH, Tran Thi MN (2021) Synthesizing and evaluating the photocatalytic and antibacterial ability of TiO2/SiO2 nanocomposite for silicate coating. Front Chem 9:1–12. https://doi.org/10.3389/fchem.2021.738969
Li WD, Gao J, Wang L (2017) Enhancement of durable photocatalytic properties of cotton/polyester fabrics using TiO2/SiO2 via one step sonosynthesis. J Ind Text 46(8):1633–1655. https://doi.org/10.1177/1528083716629138
Lima MJA, Sasaki MK, Marinho OR, Freitas TA, Faria RC, Reis BF, Rocha FRP (2020) Spot test for fast determination of hydrogen peroxide as a milk adulterant by smartphone-based digital image colorimetry. Microchem J 157:105042. https://doi.org/10.1016/j.microc.2020.105042
Liu G, Zhang Y, Xu L, Xu B, Li F (2019) A PW12/Bi2WO6 composite photocatalyst for enhanced visible light photocatalytic degradation of organic dye pollutants. New J Chem 43(8):3469–3475. https://doi.org/10.1039/c8nj05862h
Lukong VT, Mouchou RT, Enebe GC, Ukoba K, Jen TC (2022) Deposition and characterization of self-cleaning TiO2 thin films for photovoltaic application. Mater Today: Proc 62, S63–S72.https://doi.org/10.1016/j.matpr.2022.02.089
Nozari B, Montazer M, Mahmoudi Rad M (2021) Stable ZnO/SiO2 nano coating on polyester for anti-bacterial, self-cleaning and flame retardant applications. Mater Chem Phys 267(March):124674. https://doi.org/10.1016/j.matchemphys.2021.124674
Ollis DF, Pelizzetti E, Serpone N (1991) Photocatalyzed destruction of water contaminants. Environ Sci Technol 25(9):1522–1529. https://doi.org/10.1021/es00021a001
Pakdel E, Daoud WA (2013) Self-cleaning cotton functionalized with TiO2/SiO2: Focus on the role of silica. J Colloid Interface Sci 401:1–7. https://doi.org/10.1016/j.jcis.2013.03.016
Paryab A, Godary T, Abdollahi S, Anousheh M, Khachatourian AM (2021) Manufacturing and structural evaluation of polymer derived SiOC/TiC and SiOC/TiC/mullite nanocomposites. Iran J Mater Sci Eng 18(3). https://doi.org/10.22068/ijmse.2093
Permana MD, Noviyanti AR, Lestari PR, Kumada N, Eddy DR, Rahayu I (2022) Enhancing the photocatalytic activity of TiO2/Na2Ti6O13 composites by gold for the photodegradation of phenol. ChemEngineering 6(5). https://doi.org/10.3390/chemengineering6050069
Permana MD, Sakti LK, Luthfiah A, Lutfi Firdaus M, Takei T, Eddy DR, Rahayu I (2023) A simple methods for determination of methylene blue using smartphone-based as digital image colorimetry. Trends Sci 20(4). https://doi.org/10.48048/tis.2023.5149
Rosales E, Escudero S, Pazos M, Sanromán MA (2019). Sustainable removal of Cr(VI) by lime peel and pineapple core wastes. Appl Sci (Switzerland) 9(10). https://doi.org/10.3390/app9101967
Saleem M, Naz MY, Shukrullah S, Ali S, Hamdani STA (2021) Ultrasonic biosynthesis of TiO2 nanoparticles for improved self-cleaning and wettability coating of DBD plasma pre-treated cotton fabric. Appl Phys A: Mater Sci Process 127(8). https://doi.org/10.1007/s00339-021-04767-4
Seeharaj P, Pasupong P, Detsri E, Damrongsak P (2018) Superhydrophobilization of SiO2 surface with two alkylsilanes for an application in oil/water separation. J Mater Sci 53(7):4828–4839. https://doi.org/10.1007/s10853-017-1925-5
Shaheen TI, Salem SS, Zaghloul S (2019) A new facile strategy for multifunctional textiles development through in situ deposition of SiO2/TiO2 nanosols hybrid. Ind Eng Chem Res 58(44):20203–20212. https://doi.org/10.1021/acs.iecr.9b04655
Springer V, Avila F, Avena M (2020) A simple strategy for methylene blue determination in human and veterinary dosage forms by digital imaging. J Anal Chem 75(7):958–964. https://doi.org/10.1134/S1061934820070151
Wang W, Xu D, Cheng B, Yu J, Jiang C (2017) Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J Mater Chem A 5(10):5020–5029. https://doi.org/10.1039/c6ta11121a
Wei B, Li X, Sun H, Song K, Wang L (2022) Comparative study on the corrosion and self-cleaning behavior of Fe-B-C and Fe-B-P amorphous alloys in methylene blue dye solution degradation. J Non-Cryst Solids 575:121212. https://doi.org/10.1016/j.jnoncrysol.2021.121212
Wu D, Long M, Zhou J, Cai W, Zhu X, Chen C, Wu Y (2009) Synthesis and characterization of self-cleaning cotton fabrics modified by TiO2 through a facile approach. Surf Coat Technol 203(24):3728–3733. https://doi.org/10.1016/j.surfcoat.2009.06.008
Yang H, Yang B, Chen W, Yang J (2022) Preparation and photocatalytic activities of tio2-based composite catalysts. Catalysts 12(10). https://doi.org/10.3390/catal12101263
Zambrano-Mera DF, Espinoza-González R, Villarroel R, Rosenkranz A, Carvajal N, Pintor-Monroy MI, Montaño-Figueroa AG, Arellano-Jiménez MJ, Quevedo-López M, Valenzuela P, Gacitúa W (2022) Optical and mechanical properties of Zr-oxide doped TiO2/SiO2 anti-reflective coatings for PV glass covers. Solar Energy Mater Solar Cells 243. https://doi.org/10.1016/j.solmat.2022.111784
Zhu X, Shen S, Tang Z, Yang J (2020) Ti3+-doped TiO2@C nanorods with enhanced photocatalytic performance under visible light. Compos Interfaces 27(3):263–275. https://doi.org/10.1080/09276440.2019.1623608
Acknowledgments
Authors are grateful for the facilities from Universitas Padjadjaran, Indonesia, by Academic Leadership Grant (ALG), Prof. Iman Rahayu (ID: 1549/UN6.3.1/PT.00/2023) and Universitas Bengkulu, Indonesia.
Funding
Indonesian Ministry of Research by Penelitian Tesis Magister (PTM 2023), Dr. Diana Rakhmawaty Eddy (ID: 3018/UN6.3.1/PT.00/2023) and Penelitian Kerjasama Dalam Negeri (PKDN 2023), Dr. Diana Rakhmawaty Eddy (ID: 3018/UN6.3.1/PT.00/2023).
Author information
Authors and Affiliations
Contributions
Conceptualization, D.R.E. and M.L.F.; methodology, L.K.S.; software, L.K.S.; validation, D.R.E., M.L.F. and M.D.P.; formal analysis, L.K.S.; investigation, L.K.S.; resources, L.K.S.; data curation, L.K.S.; writing original draft preparation, L.K.S.; writing, review, and editing, L.K.S, Y.D., D.R.E., M.L.F., M.D.P., and Y.I.; visualization, M.D.P.; supervision, D.R.E., Y.D., M.L.F., and M.D.P.; project administration, D.R.E. and Y.D.; funding acquisition, D.R.E., I.R.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publications
All the authors agreed to publish this manuscript on this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakti, L.K., Eddy, D.R., Permana, M.D. et al. Utilizing TiO2 photocatalysis and natural SiO2 superhydrophilicity synergy to improve self-cleaning activity in cotton fabric. Cellulose 31, 5885–5898 (2024). https://doi.org/10.1007/s10570-024-05938-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-05938-1