Abstract
In this study, Stöber method was utilized to synthesize silica NPs on the denim fabric via sodium silicate solution under alkali solution applying Keliab (Seidlitzia rosmarinus), as a natural alkali source, at pH 12 and also ethanol/Keliab. X-ray diffraction (XRD), Fourier transform infrared (FT-IR), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), and ultraviolet–visible (UV–Vis) analysis verified the formation of silica NPs on the denim fabric. Further, self-cleaning features, air permeability, hydrophilicity, flame retardant, shrinkage, bending rigidity and tensile strength of the fabrics were studied. The final denim fabric indicated boosted features along with novel properties such as higher heat resistance, strength, water absorption rate, air permeability, and self-cleaning properties. These traits are making the fabric appropriate for application in diverse environmental conditions due to the silica NPs.
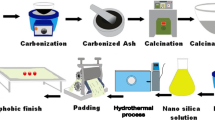
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human beings have always tried to increase safety of textiles such as fabric, furniture, carpet, curtain, and flooring in the fire accidents since they are recognized as one of the main combustion sources due to their high burning speed and the flame spread (Norouzi et al. 2015). Denim, among the fabrics used since 1800, has been popular due to characteristics such as perceived beauty, warmth, comfort, durability, biodegradation, coloring speed, and low-cost (El-Hady et al. 2013; Eryuruk 2019; Ghaani Farashahi et al. 2018; Haghighat et al. 2013; Kan and Wong 2010; Li et al. 2010; Marsh et al. 2005; Miller 2015; Miller and Woodward 2011; Morris and Prato 1981; Rahman 2012). Manufacturers have used a variety of finishes to enhance the efficiency, durability, color stability and quality of denim fabric, and also textile researchers have used nanofinishing on the denim fabrics (Kan et al. 2011; Nallathambi et al. 2011).
Cotton denim fabrics are flammable and burn quickly (Grancaric et al. 2016) and need to be fire retardant while still having suitable handle and drape (Nelson 2008). Horrocks et al. (1996) reported the flame retardant cotton fabric by using chemical softeners. Silica nanoparticles (NPs) have widespread applications in various industries with some known effects on textiles. Fanglong et al. (2016) caused enhanced flame retardance of cotton fabric by applying nano-silica. In fact, an optimal nano-silica amount increased the LOI and reduced the thermal decomposition of the cellulosic materials. Also, a mixture of nano-silica and traditional intumescent flame retardant showed synergistic effects on the flame resistance of cellulosic textiles (Fanglong et al. 2016). In addition, Carosio et al. (2011) applied silica NPs through the layer-by-layer coating as a flame-retardant and found impressive changes in the principle factors of flammability. Further, silica NP films reduced burning time, delayed rate of heat release ignition and eliminated melt dripping of polyester fabric.
The ignition of fabrics plays a key role at the start of the fire therefore, there are numerous reports on the combustion of textiles (Khattab et al. 1992). Early studies were accomplished by heating the fabrics to different temperatures using a hot plate (Brewster and Barker 1983; Nakagawa et al. 1989; Sayers 1965). Other investigators have tried to study the fabrics to ignite in the conventional thermo-gravimetric analyzers (Miller et al. 1983). Moreover, the ignition behavior of fabrics was studied by different methods such as placing the fabrics in hot air (Torvi et al. 1999; Vantelon and Breillat 1982), in different oxygen concentrations (Khattab et al. 1990), and in the furnace under defined time and temperature (Khattab et al. 1992).
The study of Norouzi et al. (2015) showed the effects of nanoparticles such as nano-clay, carbon nanotubes, and silica NPs on the flame retardant properties of various textile fibers. Silica NPs and cross-linking agents were applied to cotton to make a hydrophobic surface. The thermal properties and flame retardation of the coated fabrics increased due to the high heat resistance, heat protection effect, and mass transport limitation of the silica NPs in the coating. In addition, they confirmed the flame retardant effect of silica NPs on PET by reducing the heat release rate. Moreover, they compared thermal stability and flame retardation of the treated cotton fabrics with diverse metal alkoxide coatings showed the protective efficiency arrangement of: TiO2 > SiO2 > Al2O3 > ZrO2.
Recently, the nanoparticles were applied on the cellulosic fabrics in many studies. For instance, Xu and Cai (2008) reported formation of the chemical bonds between hydroxyl groups of cellulose and silica NPs through heat treatment. Moreover, cellulosic fabrics were treated through an eco-friendly synthesis with nano cupric oxide by Seidlitzia Rosmarinus ashes as a natural and non-toxic source of alkali (Rezaie et al. 2017b). Furthermore, silica NPs were synthesized on cellulosic fabric by treating with tetraethylorthosilicate (TEOS) under alkaline conditions (ethanol and 3-aminopropyl) to achieve flame retardant (Aksit et al. 2016). In addition, silica nanoparticles and polydimethylsiloxane were applied on cotton fabric to produce a cloth with multifunctional features (Liu et al. 2018).
The woody plant of S. Rosmarinus, a species of Chenopodiaceae from Salsoleaes tribe, is growing in Middle East and Central Asia that can be used as an alkaline source (Aladpoosh et al. 2014). This was previously used for laundry and feeding of animals however it is using for soap, pottery, glasswork, degumming of silk yarns and pharmaceutical industry (Aladpoosh et al. 2014; Rezaie et al. 2017a). The plant was burnt in the special ponds cooled the melt and collected the final solids. The solid material is called Keliab as an alkaline source that is rich in sodium carbonate (Aladpoosh et al. 2014; Rezaie et al. 2017a). Lately, it has been used for biosynthesis of Ag (Aladpoosh et al. 2014), ZnO (Aladpoosh et al. 2014), and CuO nanoparticles on various textile substrates (Rezaie et al. 2017b).
Multifunctional fabrics with self-cleaning (photocatalytic), flame retardant, hydrophilic, and air permeability properties, have attracted more attention. The photocatalytic properties of fabrics were broadly studied in recent years since some users may give up from washing their clothes for months due to the negative effects of the household laundering on the shade and mechanical properties. Many researchers coated titanium dioxide (TiO2) on textiles to chemically break down the color stains through exposing to the sunlight (Uğur et al. 2017; Onar et al. 2011; Sobczyk-Guzenda et al. 2013). Similarly, Guan (2005) reported the self-cleaning and hydrophilic properties of TiO2/SiO2 composite films.
Recently most published papers were concentrated on various finishing on textiles such as synthesis of nanoparticles on cotton fabric (Aladpoosh et al. 2014), industrial washing of denim garment (Jucienė et al. 2006) and changing the color of fabrics (Ding et al. 2010; Jucienė et al. 2006). The color psychology is a critical issue for the consumers because of the importance of the color in choosing of a product by the costumers (Dobilaitė and Jucienė 2005; Fan et al. 2009; Militky and Bajzik 1997; Thompson et al. 1992). Therefore color change can be a useful way to attract the costumers for buying a cloth.
The hydrophilic or hydrophobic nature of fabric can be strongly affected on the textile finishing, dyeing and printing. Hence, most researchers worked on various methods to enhance the hydrophilicity of fabrics. Nithya et al. (2011) treated the cotton fabrics by DC air plasma and cellulase to improve the hydrophilicity, wettability, and dye-ability without substantial fiber deterioration.
In this study, sodium silicate solution was used in an alkaline solution of Keliab as a friendly compound to synthesize silica NPs on the denim fabric. Also, the synthesis was performed in two different alkaline solutions and the results compared. The better thermal behavior of denim fabrics with minimum effect on the handle and low color difference were explored. Besides, the hydrophilic, photocatalytic, and mechanical properties of the treated samples were investigated.
Materials and methods
Materials
A 100% cellulose with 1/3 twill indigo-dyed denim fabric, weight of 411 g/m2, yarn count of 54/54 (warp/filling), thickness of 0.8 mm, warp density of 38/cm and weft density of 22/cm was used. A commercial nonionic detergent was purchased from a local market and Keliab obtained from Urmia, Iran. Sodium silicate solution [NaO2 (7.5–8.5%), SiO2 (25.5–28.5%)], having a pH 11.0–11.5, was provided from Merck Co., Germany. Ethanol (C2H5OH, 96%) was bought from Golriz Co., Iran. Methylene blue was obtained from Uhao Co., China.
Preparation of Keliab solution
In order to prepare an alkali solution, 5 g Keliab powder was mixed with 100 mL distilled water to obtain a heterogeneous mixture. After 24 h, some components of Keliab powder were dissolved in water and the rest precipitate at the bottom of the solution. The top transparent liquid having the pH of 13 was used.
In situ synthesis of silica NPs on denim fabric
First, denim fabric was washed with 1 g/L nonionic detergent at 60 °C for 30 min in liquor-to-goods ratio (L:G) of 40:1. Two different mixtures, a mixture of 20 mL distilled water and 10 mL Keliab solution (M1) and another mixture of 20 mL ethanol and 10 mL Keliab solution (M2) were made. Two denim samples (3 cm × 3 cm) were immersed in the each mixture. A 10 mL sodium silicate solution was added to 7 mL distilled water at room temperature. The diluted sodium silicate solution was added dropwise to M1 and M2 solutions, separately. Magnetic stirrer was used to stir. After 1 h, samples were washed with distilled water then dried at 100 °C for 1 h. A solution containing 10 mL Keliab and 20 mL distilled water (M3) without sodium silicate solution was made and the two samples were placed in the solution remained for 1 h and then washed with distilled water followed by drying at 100 °C for 1 h.
Methods
FESEM images and EDS patterns
Field emission scanning electron microscopy (FESEM, MIRA#TESCAN-XMU) pictures were used to observe the surface morphology and synthesized particle size. The weight percentage of the elements on the samples was obtained using energy dispersive spectroscopy (EDS) and the loading of the elements showed in the maps.
TEM analysis
Transmission electron microscopy was accomplished according to standard 3001-1503-7 by using Zeiss EM900. To prepare samples for TEM analysis, the synthesized effluents of M1 and M2 were placed on a copper grid coated with carbon. The solution was evaporated at room temperature left the nanosized silica particles on the grid.
X-ray diffractometer
An X-ray diffractometer (XRD, EQuniox 3000, INEL, France) was used to investigate the crystalline structure of synthesized silica nanoparticles on the treated fabrics. The wavelength of the Cu Kα radiation used in this study was 1.54190 Å.
FT-IR analysis
Fourier transform infrared spectrometry (FT-IR, NEXUS 670, Nicolet) was applied to recognize and analyze the new bonding of the treated fabrics from 500 to 4000 cm−1 at 4 cm−1 with 40 scans.
UV–Vis spectrophotometer
The UV–Vis absorbance (model 2100, Unico, China) was used in various wavelengths (200 to 800 nm) for the synthesized effluents according to Table 1.
Photocatalytic actions and color changes
The raw and treated samples were placed in a methylene blue solution (0.001%) for 10 min and then exposed to the sunlight for 48 h. The color difference of the stained fabrics before and after the experiment was determined using a spectrophotometer (Varian Carry 5000) based on CIELAB color coordinates. The total color difference, ΔE, was calculated via Eq. 1:
where L* stands for lightness and darkness, a* shows redness and greenness, and b* indicates yellowness and blueness.
The percentage of self-cleaning was determined by Eq. 2:
\(\varDelta {E}_{C}\,\text{a}\text{n}\text{d}\, \varDelta {E}_{T}\) are the color difference of the stained untreated and treated samples after sunlight irradiation (Rezaie et al. 2017a). Also, the color change of treated fabric was measured in CIELab color space comparing with untreated fabric using Eq. 2.
Strength and bending
The strength of the treated denim fabrics was investigated using an Iranian CRE instrument named Kardo Tec. The test speed was 75 mm/min and the relative ambient humidity was 32% and the ambient temperature was about 28 °C. The test was repeated in warp direction three times for each sample.
To assess the bending resistance, Shirley instrument was used according to ASTM D 1388. The bending rigidity is affected by fabric drape that can be measured by Eq. 3:
where B stands for bending rigidity, W is the fabric mass per unit area (g/m2) and C is half of the bending length (mm).
Air permeability and handle
Air permeability of the fabric was measured using the Shirley instrument according to ISO 9237 method, 1995 with constant pressure (100 Pa) at relative ambient humidity of 40% and the ambient temperature of 26 °C.
In this study, the hand-feel of treated fabrics was compared to the raw via expert panel and qualitatively reported.
The equipment can determine no feelings when someone touches a fabric (handle), therefore, a group of 50 people were used in this experiment for evaluating the handle property.
For the handle of fabrics, a 5-point scale was used. The meanings and the frequency of the rating numbers are shown in Table 3. Before the evaluation, the group members were asked to wash their hands with the non-moisturizing soap and dry by the paper towel.
In the evaluation process, the team members held the untreated sample in their most-used hand and tried to grade the fabric according to the designed 5-point scale. Then, they appraised the treated samples by their most-used hand. The data was statistically analyzed.
Water droplet absorption and fabric shrinkage
The time for the water absorbed was used to measure the fabric hydrophilicity after the treatment. For this purpose, a drop of water was trickled down on the surface of the samples from 2 cm using a dropping-tube. The time of the full absorption of the water droplet was measured for 10 times and the average value was reported.
The shrinkage percentage (S%) was also measured by Eq. 4:
A is the initial size and B is the secondary size.
Flame testing
The samples were placed at the distance of 4 cm from the 2 cm long flame for 12 s according to AATCC 6941 and the burning speed was measured using Eq. 5:
where x is burning length (cm), t is time, and v is burning speed.
Another important method to study the thermal properties of textiles is thermo-gravimetric analysis (TGA) that was conducted by using a SDT Q600 V20.9 Build 20. TGA showed weight losses of fabrics under different temperatures from 25 to 800 °C at a heating rate of 10 °C/min under N2 using derivative TGA (dTGA) plots, also the temperature of maximum weight loss was acquired. The amount of samples was about 4 mg. Also, the loss in mass of the samples was determined by using a furnace in 300 °C for 3 h.
Results and discussion
Mechanism of in-situ synthesis of silica NPs
The mechanism of synthesis and deposition of silica NPs on cotton denim fabric by using Keliab solution was considered as follows:
When sodium silicate solution was diluted in distilled water, SiO2 is formed (Reaction 1) (Nozari et al. 2018).
The Keliab solution contains sodium alkali-metals prepares alkali media through hydroxyl ions (OH−) in the synthesis bath (Reactions 2–4) (Aladpoosh et al. 2014; Rezaie et al. 2017a).
Also, cellulosate anion was produced due to alkali medium (Reaction 5) (Aladpoosh et al. 2014; Nozari et al. 2018; Rezaie et al. 2017a). This was then reacted with sodium cation created the sodium salt of cellulose (Reaction 6) (Aladpoosh et al. 2014; Rezaie et al. 2017a).
By adding diluted sodium silicate solution to the synthesis bath, SiO2 was replaced with Na+ owing to the higher electronegativity of SiO2 (1.90) comparing with sodium (0.93) (Reaction 7) (Aladpoosh et al. 2014).

Morphology and structure
Figure 1a–c indicates the surface of the untreated samples. Figure 1a clearly demonstrates the ribbon-shaped and size distribution of the cotton fibers in denim fabrics. Fibers are regular with a relatively smooth surface at low magnification. Figure 1b shows the fibrils in the individual cotton fiber at large magnification. This indicates the pure cotton surface without any additives (Fig. 1).
The rod-shaped silica NPs were synthesized using M1. Figure 1d–f illustrates the synthesized silica NPs on the cotton surface covered fibrils. The ethanol in M2 leads to the synthesis of spherical-shaped NPs. Figure 1g–i displays both the rod and spherical-shaped silica NPs wrapped on fibrils. Nozari et al. (2018) reported regular hexagonal silica NPs on PET fabric synthesized under alkali conditions with ammonia and ethanol. The average sizes of the rod and spherical-shaped silica NPs were 78.9 and 311.43 nm, respectively (Fig. 2).
Ethanol leads to the increase in the number of synthesized nanoparticles (Zulfiqar et al. 2016). EDS was employed to recognize C, N, O elements on the raw fabric and C, N, O, Na, Si on the treated samples along with the weight percentages. Figure 3 illustrated that the weight percentages of Si and Na increased for the treated samples due to synthesis in alkaline condition. The percentage of O increased for the treated samples, because of the formation of hydroxyl groups also C and N decreased by addition of Si and Na. Further, Fig. 4 shows the uniform distribution of Si on the specific area of the fabric.
Figure 5 shows the TEM micrographs of the various shapes of silica NPs. Figure 5a, b, is related to the rod-shaped silica NPs synthesized by Keliab solution (M1). The ethanol in M2 leads to the synthesis of spherical-shaped silica NPs however in Fig. 5c, d, both the rod and spherical-shaped NPs can be observed.
The XRD patterns of different samples are revealed in Fig. 6. The peaks at 2θ = 14.9°, 16.8°, 22.8°, and 34.7° are related to the crystalline structure of cellulose Iβ (French 2014). Silica NPs are synthesized in amorphous and crystalline forms (Nozari et al. 2018). By stimulating the synthesis condition on the fabric, the silica nanoparticles are synthesized and the powder of SiO2 obtained. The XRD pattern of SiO2 powder shows the amorphous peak in the range of 10°–30° (Nozari et al. 2018) and the strongest peak at 2θ = 22.5° can be matched with SiO2 JCPDS Card No. 39-1425. However here, X-ray diffraction data cannot clearly prove the synthesis of silica NPs on the samples due to the overlapping of SiO2 and cellulose peaks in the treated samples.
Figure 7a illustrated the FT-IR spectrum of the untreated sample indicated the characteristic peaks of denim fabric due to the cellulose macromolecule and indigo dye at 3267 cm−1 (O–H and N–H stretching), 2915 cm−1 (C–H aromatics), 1560 cm−1 (N–H stretching), 1313 cm−1 (C–N stretching), and 1026 cm−1 (C–O stretching) (El-Shishtawy et al. 2011).
According to Fig. 7b, c, the stretching of Si–O–Si appears at 1000 cm−1 (Al-Oweini and El-Rassy 2009; Bertoluzza et al. 1986). Mendoza-Castillo et al. (2016) claimed that the intense stretching vibrations of Si–O–Si groups locate at 1040 cm−1. Moreover, the stretching of O–H and N–H is positioned at 3264 and 3271 cm−1 for treated sample with M1 and M2, respectively. El Nahrawy et al. (2018) showed a very weak peak at 3103 and 3473 cm−1for stretching of N–H and OH respectively. The peaks at 1629 and 1312 cm−1 are corresponded to the absorbance of N–H and C–N stretching in treated sample with M1. The peaks at 1606 and 1313 cm−1 are related to N–H and C–N stretching in the treated sample with M2.
Keliab solution, rich in sodium carbonate, has the peak around 250 cm−1 for M3 (Fig. 8) effluent corresponds to Na2CO3 (Jin et al. 2013). The peaks related to the M1 and M2 effluents (Fig. 8) are shifted toward 300 cm−1 with the intense absorbance due to the SiO2 in mixtures (Kukovecz et al. 2001). Moreover, the width of the peaks demonstrates the aggregation of NPs which is higher for M2 due to the ethanol as similarly reported by Aladpoosh et al. (2014).
Photocatalytic properties
The fabrics treated with SiO2 semiconductor NPs have self-cleaning properties decomposing the color stain of methylene blue (Nozari et al. 2018; Rezaie et al. 2017a).
The valence electrons of SiO2 available on the treated fabrics are affected by ultraviolet radiation lead to positive holes. Further, the electrons react with oxygen and water molecules produced superoxide ions (\({\text{O}}_{2}^{-}\)) and hydrogen peroxide (H2O2). Moreover, hydroxyl radicals are formed as a result of a reaction between positive holes with water molecules. The hydroxyl radicals are known as the main factor in the decomposition of the harmful compounds. Hence, the blue stain of the methylene can be decomposed to non-toxic products such as CO2, H2O, and minerals (Nozari et al. 2018; Rezaie et al. 2017a).
The color differences and self-cleaning enhancement of different samples are reported in Table 2 indicating a higher level of self-cleaning property for the treated fabrics with M2 comparing with M1. As a result, the treated fabrics designated the higher color differences than raw due to silica NPs (Nozari et al. 2018). The self-cleaning enhancement was measured nearby 12 and 153% for the treated samples with M1 and M2 comparing with the untreated.
Color change measurement
The lower ΔE indicates the less color difference (Zhang et al. 2003). Table 2 shows the color difference (ΔE) of treated samples as M3 with no silica NPs displays less color change and M1 confirms a higher color difference. However, the results confirm no significant changes after synthesis in alkaline condition. Hence, this method is very efficient for in-situ silica NPs synthesis without striking color changes.
Moreover, the reflections of the samples were investigated in the visible wavelength since more reflection confirms less absorption (Montazer et al. 2012). Figure 9 provides the closest reflection curve for sample M3 to untreated because of the less color change (ΔE = 1.3). Sample M1 has the highest reflection and the greatest difference was recorded at 380 to 520 nm related to the violet, blue, and cyan colors corresponded to the blue jeans dyed with indigo.
Physico-mechanical properties
The bending properties of fabrics determine different characteristics such as handle, softness, drape, thickness and crease recovery of cloth (Lee et al. 2015). The bending length and stiffness of the treated samples increased (Table 2). In addition, the shrinkage in Table 2 exhibited no-shrinkage since the broken cellulose bonds under alkaline conditions reacted with SiO2 and the newly formed bonds left no space for the fabric to shrink.
The alkaline condition of synthesis possibly leads to the scission of cellulosic chains reduces the tensile strength of the fabric (Aladpoosh et al. 2014; Maryan et al. 2013a; Rezaie et al. 2017a). Moreover, the sample treated with M3 (Keliab without sodium silicate solution) has the lowest tensile strength (Fig. 10).
The bonds formation between SiO2 and cellulose compensate the decrease in the tensile strength to some extent. As a result, the samples treated with silica NPs are stronger than the untreated (Fig. 10). The treated sample with M2 has the highest tensile strength among the others (Fig. 10) due to the more synthesis in ethanol and formation of more bonds between SiO2 and cellulosic chains. The Young’s modulus of various samples is also reported in Table 2. It was shown that he synthesis of silver NPs on the cotton fabric under alkaline conditions reduced the tensile strength (Aladpoosh et al. 2014; Maryan et al. 2013a). In addition, (Rezaie et al. 2017a) similar results reported with the biosynthesis of cupric oxide on cotton fabric under alkaline conditions. However, other researchers reported the increase in tensile strength after synthesis of silica NPs on PET in alkali media (Nozari et al. 2018).
Air permeability is an important comfort factor of garments (Maryan et al. 2013b; Yuen 2009). According to Table 2, the fabric air permeability increases for M1 higher than M2 and M3. The alkaline condition can be a reason for the higher air permeability of treated samples. In addition, the loose fibers can be removed from the surface of the fabric during the synthesis of silica NPs causing the air easily pass through the pores of the fabric.
The finishing can affect the fabric handle that is influencing on the product and the application (Ünal 2010). Here, the synthesis of silica NPs on denim fabric negatively influenced on the handle though improved other properties. The participants were evaluated the untreated fabric with an average scale of 2.88 arranging the untreated fabric handle as medium (Table 3). They also rated the treated samples as M3 with a medium rate (3.1) showed the closest handle to the untreated fabric. The silica NPs on surface of treated samples led to the rough (4.04) and harsh (4.8) handle for M1 and M2, respectively. Moreover, according to the bending rigidity results, it is obvious that the handle of the treated samples with M1 and M2 is harsh, hard, and rough hence the samples treated with M3 are not different from the raw.
Hydrophilic properties
The time needed for the entire spreading of water drop on the sample is a way to evaluate hydrophilic properties. The full absorption on untreated fabric required 10.36 s whereas M3 showed an inconsiderable longer time of 13.16 s. The treated samples are more hydrophilic than the raw. The silica NPs synthesized on the fabric led to the higher surface roughness (Zhu et al. 2011) however, the alkaline conditions of synthesis caused to the formation of the hydroxyl groups. Furthermore, SiO2 NPs under the light and humidity hydroxylated forming silanol groups (Si–OH) initiated the higher hydrophilicity (Nozari et al. 2018). Therefore, the required time for the full absorption of water drop on the treated sample with M1 and M2 was 1 and 6.6 s, respectively.
Thermal behavior
One of the important factors of the silica NPs is the heat resistance which leads to slow burning and shorter burning length (Alongi and Malucelli 2012). A special high level of silica is the main factor in increasing fabric stability at high temperature hence the silica NPs on treated fabrics decreases the burning speed (Rezaei et al. 2013). Burning length and speed of treated samples are lower than raw. Moreover, the size of SiO2 NPs on the treated samples with M2 is bigger with more agglomeration thus its burn length and speed was lower compared to M1. Table 4 indicates the lowest burning length and speed for the sample treated with M3.
Figures 11 and 12 show the TGA thermograms of untreated and treated samples, derivative TGA (dTGA) plots and temperatures of maximum weight loss (\({T}_{max}\)). The three main stages of weight loss can be observed for both untreated and treated samples with M2. The first stage for the both samples displays the loss of water near 100 °C (Nam et al. 2014). The second stage occurs between 300–380 °C and 250–350 °C for untreated and treated with M2 verifying a higher rate of weight loss due to the decomposition of cellulose (Gaan and Sun 2007). A lower rate of weight loss happens at 380 to 500 °C for the decomposition of residue or char with no residue or char for the untreated at 500 °C. Moreover, the amount of residue was about 28% at the highest temperature (500 °C) for the treated sample with M2. In fact, the silica enhances the heat resistance and ethanol expands the NPs’ aggregation and size.
The treated samples with M1 and M3 demonstrate four main stages. The first stage is similar to the untreated however the treated samples confirm two stages of decomposition for cellulose due to the silica NPs and Keliab. The first stage happens around 250–350 °C and the second between 400 and 450 °C. The stage of decomposition of residue occurs between 450 and 500 °C however there is no residue for sample M3 at 500 °C. A 4.2% weight of sample treated with M1 was remained as a result of silica NPs. Hence, the silica NPs synthesis under the alkaline condition improved the heat resistance caused to the good thermal stability of the treated denim fabrics.
The derivative TGA curves in Fig. 12 and the temperature of the maximum weight loss in Table 5 related to the untreated and treated samples confirm the TGA curve findings.
Conclusions
In summary, S. Rosmarinus (Keliab) ash, as a source of alkali, can be used for in situ synthesis of silica NPs on denim fabric. This can be achieved by means of diluted sodium silicate solution under alkaline conditions. The FESEM images revealed the rod-shaped silica NPs by Keliab solution and spherical-shaped NPs by ethanol in alkaline conditions with the average size of 78.9 and 311.43 nm, respectively. FTIR spectra confirmed formation of the Si–O stretching at 1000 cm−1 on the treated fabric. The breakages in cellulosic chains due to alkaline treatment at high temperature along with ionic linkages of cellulose with SiO2 caused to the higher tensile strength and bending rigidity. The silica NPs on the fabrics made absorption of water faster and improved the hydrophilic properties. Besides, the silica NPs on the fabric surface significantly decreased the burning length and increased the residue of fabric at 500 °C providing the consumer protection. The handle of the fabrics turn to rigid, harsh, and hard, nevertheless the fabric air permeability improved. Overall, the applied synthesis method is environmentally friendly and low cost that can be applied on cellulose fabric to produce multifunctional properties such as flame retardant and self-cleaning. The final denim fabric can be used in various situations such as apparel manufacturing, protective clothing for humans and pets, mobile cases, insulation textiles, and bags and shoes industry. It can also be exploited in home textiles including curtains, furniture, seat covers, mattresses, and sleepwear.
References
Aksit A, Onar N, Kutlu B, Sergin E, Yakin I (2016) Synergistic effect of phosphorus, nitrogen and silicon on flame retardancy properties of cotton fabric treated by sol-gel process. Int J Cloth Sci Technol 28:319–327. https://doi.org/10.1108/IJCST-03-2016-0029
Al-Oweini R, El-Rassy H (2009) Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si (OR) 4 and R′′ Si (OR′) 3 precursors. J Mol Struct 919:140–145. https://doi.org/10.1016/j.molstruc.2008.08.025
Aladpoosh R, Montazer M, Samadi NJC (2014) In situ green synthesis of silver nanoparticles on cotton fabric using Seidlitzia rosmarinus ashes. Cellulose 21:3755–3766. https://doi.org/10.1007/s10570-014-0369-1
Alongi J, Malucelli G (2012) State of the art and perspectives on sol–gel derived hybrid architectures for flame retardancy of textiles. J Mater Chem 22:21805–21809. https://doi.org/10.1039/C2JM32513F
Bertoluzza A, Fagnano C, Morelli M, Gottardi V, Guglielmi M (1986) Raman defect peaks in the spectra of Na2O silica gels evolving toward glass. J Non-cryst Solids 82:127–136. https://doi.org/10.1016/0022-3093(86)90120-1
Brewster EP, Barker RL (1983) A summary of research on heat resistant fabrics for protective clothing. Am Ind Hyg Assoc J 44:123–130. https://doi.org/10.1080/15298668391404491
Carosio F, Laufer G, Alongi J, Camino G, Grunlan JC (2011) Layer-by-layer assembly of silica-based flame retardant thin film on PET fabric. Polym Degrad Stab 96:745–750. https://doi.org/10.1016/j.polymdegradstab.2011.02.019
Ding Y, Invernale MA, Sotzing GA (2010) Conductivity trends of PEDOT-PSS impregnated fabric and the effect of conductivity on electrochromic textile. ACS Appl Mater Interfaces 2:1588–1593. https://doi.org/10.1021/am100036n
Dobilaitė V, Jucienė M (2005) Influence of industrial washing on denim garment colours change light industry-fibrous materials. In: III International scientific conference Radom. Paper presented at the III international scientific conference Radom
El Nahrawy AM, Hammad ABA, Abdel-Aziz MS, Wassel AR (2018) Spectroscopic and antimicrobial activity of hybrid chitosan/silica membranes doped with Al2O3 nanoparticles. Silicon. https://doi.org/10.1007/s12633-018-9986-x
El-Hady MMA, Farouk A, Sharaf S (2013) Flame retardancy and UV protection of cotton based fabrics using nano ZnO and polycarboxylic acids. Carbohydr Polym 92:400–406. https://doi.org/10.1016/j.carbpol.2012.08.085
El-Shishtawy RM, Asiri AM, Abdelwahed NA, Al-Otaibi MM (2011) In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 18:75–82. https://doi.org/10.1007/s10570-010-9455-1
Eryuruk SH (2019) The effects of elastane and finishing processes on the performance properties of denim fabrics. Int J Cloth Sci Technol. https://doi.org/10.1108/IJCST-01-2018-0009
Fan J, Hunter L, Fan J (2009) Psychological comfort of fabrics and garments. In: Engineering apparel fabrics and garments. Woodhead, Oxford
Fanglong Z, Qun X, Qianqian F, Rangtong L, Kejing L (2016) Influence of nano-silica on flame resistance behavior of intumescent flame retardant cellulosic textiles: remarkable synergistic effect? Surf Coat Technol 294:90–94. https://doi.org/10.1016/j.surfcoat.2016.03.059
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cell 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Gaan S, Sun G (2007) Effect of phosphorus flame retardants on thermo-oxidative decomposition of cotton. Polym Degrad Stab 92:968–974. https://doi.org/10.1016/j.polymdegradstab.2007.03.009
Ghaani Farashahi B, Easter E, Annett-Hitchcock K (2018) Price and perceived product quality: a comparison of denim jeans in three price categories. J Fash Mark Manag 22:369–386. https://doi.org/10.1108/JFMM-10-2017-0104
Grancaric AM, Botteri L, Alongi J, Tarbuk A (2016) Silica precursor as synergist for cotton flame retardancy. Int J Cloth Sci Technol 28:378–386. https://doi.org/10.1108/IJCST-03-2016-0036
Guan K (2005) Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf Coat Technol 191:155–160. https://doi.org/10.1016/j.surfcoat.2004.02.022
Haghighat E, Mohammad Etrati S, Shaikhzadeh Najar S (2013) Modeling of needle penetration force in denim fabric. Int J Cloth Sci Technol 25:361–379. https://doi.org/10.1108/IJCST-01-2012-0031
Horrocks A, Kandola B, Price D, Coleman G (1996) Flame-retardant treatments of cellulose and their influence on the mechanism of cellulose pyrolysis. J Macromol Sci Polym Rev 36:721–794. https://doi.org/10.1080/15321799608014859
Jin Y, Huang S, Zhang M, Jia M, Hu D (2013) A green and efficient method to produce graphene for electrochemical capacitors from graphene oxide using sodium carbonate as a reducing agent. Appl Surf Sci 268:541–546. https://doi.org/10.1016/j.apsusc.2013.01.004
Jucienė M, Dobilaitė V, Kazlauskaitė G (2006) Influence of industrial washing on denim properties. Mater Sci 12:355
Kan C-w, Wong W-y (2010) Color properties of cellulase-treated cotton denim fabric manufactured by torque-free ring spun yarn. Text Res J 81:875–882. https://doi.org/10.1177/0040517510391699
Kan CW, Yuen CWM (2009) Evaluation of the performance of stretch denim fabric under the effect of repeated home laundering processes. Int J Fash Des Technol Educ 2:2–3. https://doi.org/10.1080/17543260903302329
Kan C, Yuen C, Wong W (2011) Optimizing color fading effect of cotton denim fabric by enzyme treatment. J Appl Polym Sci 120:3596–3603. https://doi.org/10.1002/app.33561
Khattab M, Price D, Horrocks A (1990) The inhibition of spontaneous ignition by flame-retarding cotton fabrics. J Appl Polym Sci 41:3069–3078. https://doi.org/10.1002/app.1990.070411139
Khattab M, Kandil S, Gad A, El-Latif M, Morsi S (1992) Effect of condensed‐phase and gas‐phase flame retardants on the ignition behaviour of cotton fabric. Fire Mater 16:23–28
Kukovecz A, Kónya Z, Mönter D, Reschetilowski W, Kiricsi I (2001) UV–Vis investigations on Co, Fe and Ni incorporated into sol–gel SiO2–TiO2 matrices. J Mol Struct 563:403–407. https://doi.org/10.1016/S0022-2860(00)00797-3
Lee IY, Jeong GE, Kim SR, Bengelsdorff C, Kim SD (2015) Effects of biowashing and liquid ammonia treatment on the physical characteristics and hand of denim fabric. Color Technol 131:192–199. https://doi.org/10.1111/cote.12142
Li L, Frey M, Browning KJ (2010) Biodegradability study on cotton and polyester fabrics. J Eng Fibers Fabr 5:155892501000500400. https://doi.org/10.1177/155892501000500406
Liu Q et al (2018) Thermal, waterproof, breathable, and antibacterial cloth with a nanoporous structure . ACS Appl Mater Interfaces 10:2026–2032. https://doi.org/10.1021/acsami.7b16422
Marsh G, Trynka P, Marsh J (2005) Denim: from cowboys to catwalks: a history of the world’s most legendary fabric. Aurum
Maryan AS, Montazer M, Harifi T (2013) One step synthesis of silver nanoparticles and discoloration of blue cotton denim garment in alkali media. J Polym Res 20:189. https://doi.org/10.1007/s10965-013-0189-2
Maryan AS, Montazer M, Rashidi A (2013) Introducing old-look, soft handle, flame retardant, and anti-bacterial properties to denim garments using nano clay. J Eng Fibers Fabr 8:155892501300800420. https://doi.org/10.1177/155892501300800416
Mendoza-Castillo DI, Reynel-Ávila HE, Bonilla-Petriciolet A, Silvestre-Albero J (2016) Synthesis of denim waste-based adsorbents and their application in water defluoridation. J Mol Liq 221:469–478. https://doi.org/10.1016/j.molliq.2016.06.005
Militky J, Bajzik V (1997) Influence of washing/ironing cycles on selected properties of cotton type weaves. Int J Cloth Sci Technol 9:193–199. https://doi.org/10.1108/09556229710168306
Miller B, Martin JR, Turner R (1983) Studies on polymer ignition and development of a relative hazard ranking method. J Appl Polym Sci 28:45–56. https://doi.org/10.1002/app.1983.070280105
Miller D, Woodward S (2011) Global denim. Berg, Oxford
Miller D (2015) Denim Consum Mark Cult 18:298–300. https://doi.org/10.1080/10253866.2015.1008193
Montazer M, Alimohammadi F, Shamei A, Rahimi MK (2012) Durable antibacterial and cross-linking cotton with colloidal silver nanoparticles and butane tetracarboxylic acid without yellowing. Colloids Surf B 89:196–202. https://doi.org/10.1016/j.colsurfb.2011.09.015
Morris M, Prato H (1981) Consumer perception of comfort, fit and tactile characteristics of denim jeans. Text Chem Color 13:60–66
Nakagawa Y, Komai T, Kohno M (1989) Correlation between the hot plate ignition test and other laboratory-scale flammability tests on rubber conveyor belts with fabric skeletons. Fire Mater 14:159–162. https://doi.org/10.1002/fam.810140407
Nallathambi G, Ramachandran T, Rajendran V, Palanivelu R (2011) Effect of silica nanoparticles and BTCA on physical properties of cotton fabrics. Mater Res 14:552–559. https://doi.org/10.1590/S1516-14392011005000086
Nam S, Condon BD, Foston MB, Chang S (2014) Enhanced thermal and combustion resistance of cotton linked to natural inorganic salt components. Cellulose 21:791–802. https://doi.org/10.1007/s10570-013-0133-y
Nelson G (2008) Microencapsulation in textile finishing . Rev Prog Color Relat Top 31:57–64. https://doi.org/10.1111/j.1478-4408.2001.tb00138.x
Nithya E, Radhai R, Rajendran R, Shalini S, Rajendran V, Jayakumar S (2011) Synergetic effect of DC air plasma and cellulase enzyme treatment on the hydrophilicity of cotton fabric. Carbohydr Polym 83:1652–1658. https://doi.org/10.1016/j.carbpol.2010.10.027
Norouzi M, Zare Y, Kiany P (2015) Nanoparticles as effective flame retardants for natural and synthetic textile polymers: application, mechanism, and optimization. Polym Rev 55:531–560. https://doi.org/10.1080/15583724.2014.980427
Nozari B, Montazer M, Rad MM (2018) In-situ synthesis of SiO2 nanoparticles on polyester fabric as benign multi-purpose catalysts. Fibers Polym 19:2564–2573. https://doi.org/10.1007/s12221-018-8668-z
Onar N, Aksit A, Sen Y, Mutlu M (2011) Antimicrobial, UV-protective and self-cleaning properties of cotton fabrics coated by dip-coating and solvothermal coating methods. Fibers Polym 12:461–470. https://doi.org/10.1007/s12221-011-0461-1
Rahman O (2012) The influence of visual and tactile inputs on denim jeans evaluation. Int J Des 6:11–25
Rezaei M, Schaffie M, Ranjbar M (2013) Thermocatalytic in situ combustion: influence of nanoparticles on crude oil pyrolysis and oxidation. Fuel 113:516–521. https://doi.org/10.1016/j.fuel.2013.05.062
Rezaie AB, Montazer M, Rad MM (2017) Biosynthesis of nano cupric oxide on cotton using Seidlitzia rosmarinus ashes utilizing bio, photo, acid sensing and leaching properties. Carbohydr Polym 177:1–12. https://doi.org/10.1016/j.carbpol.2017.08.053
Rezaie AB, Montazer M, Rad MM (2017) A cleaner route for nanocolouration of wool fabric via green assembling of cupric oxide nanoparticles along with antibacterial and UV protection properties. J Clean Prod 166:221–231. https://doi.org/10.1016/j.jclepro.2017.08.046
Sayers L (1965) The flammability of fibers, fabrics and garments. Text Inst Ind 3:168–171
Sobczyk-Guzenda A, Szymanowski H, Jakubowski W, Błasińska A, Kowalski J, Gazicki-Lipman M (2013) Morphology, photocleaning and water wetting properties of cotton fabrics, modified with titanium dioxide coatings synthesized with plasma enhanced chemical vapor deposition technique. Surf Coat Technol 217:51–57. https://doi.org/10.1016/j.surfcoat.2012.11.071
Thompson R, Summers S, Rampey-Dobbs R, Wheeler T (1992) Color pressure garments versus traditional beige pressure garments. J Burn Care Rehabil 13:590–596. https://doi.org/10.1097/00004630-199209000-00016
Torvi DA, Douglas Dale J, Faulkner B (1999) Influence of air gaps on bench-top test results of flame resistant fabrics. J Fire Prot Eng 10:1–12. https://doi.org/10.1177/104239159901000101
Uğur ŞS, Sarıışık A, Çavuşlar E, Ertek M (2017) Development of self-cleaning denim fabrics. IOP Conf Ser Mater Sci Eng 12:122012. https://doi.org/10.1088/1757-899X/254/12/122012/meta
Ünal PG (2010) Investigation of some handle properties of fabrics woven with two folded yarns of different spinning systems. Text Res J 80:2007–2015
Vantelon J, Breillat C (1982) Autoignition limits of some polymeric materials. Eur Polym J 18:903–906. https://doi.org/10.1016/0014-3057(82)90025-8
Xu B, Cai Z (2008) Fabrication of a superhydrophobic ZnO nanorod array film on cotton fabrics via a wet chemical route and hydrophobic modification. Appl Surf Sci 254:5899–5904. https://doi.org/10.1016/j.apsusc.2008.03.160
Zhang J, France P, Radomyselskiy A, Datta S, Zhao J, Van Ooij W (2003) Hydrophobic cotton fabric coated by a thin nanoparticulate plasma film. J Appl Polym Sci 88:1473–1481. https://doi.org/10.1002/app.11831
Zhu Q, Gao Q, Guo Y, Yang CQ, Shen L (2011) Modified silica sol coatings for highly hydrophobic cotton and polyester fabrics using a one-step procedure. Ind Eng Chem Res 50:5881–5888. https://doi.org/10.1021/ie101825d
Zulfiqar U, Subhani T, Wilayat Husain S (2016) Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J Sol–Gel Sci Technol 77:753–758. https://doi.org/10.1007/s10971-015-3950-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Talebi, S., Montazer, M. Denim Fabric with Flame retardant, hydrophilic and self-cleaning properties conferring by in-situ synthesis of silica nanoparticles. Cellulose 27, 6643–6661 (2020). https://doi.org/10.1007/s10570-020-03195-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03195-6