Abstract
Cellulose is the most widely used biopolymer on Earth. Its large-scale production is mainly from lignocellulosic material (plant origin), however, this plant material is not the only source of this valuable polymer, since microorganisms, like bacteria, naturally produce cellulose, especially those of the genus Komagateibacter (formerly Gluconacetobacter). This type of cellulose is of great interest because of its unique properties such as high purity and resistance, nevertheless, it has not been produced in a large-scale industrial process to date using low-cost substrates, one of the key aspects that should be considered for the industrial obtaining of any biotechnological product. As a main finding we found that the majority of low-cost culture media discussed could have the potential to produce bacterial cellulose on an industrial scale, since in most cases they yield more cellulose (with similar physical chemical characteristics) to those obtained in standard media. However, for an appropriate large-scale production, a specific knowledge about these by-products (since their composition and characteristics, which have a direct impact on the productivity of this biopolymer, are quite heterogeneous) and a proper standardization of them would also be required. Research staff of many industries could use the information presented here to help design a process to use their respective byproducts as substrate to obtain a product with a high added value as bacterial cellulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most widely used biopolymer on Earth. In its industrial use, the main sources for cellulose are plants and their residues (cotton, the wood of pine and other trees, etc.), which are recycled into final products like paper (Arioli et al. 1998). In recent decades, however, research has been done into obtaining cellulose from microorganisms, particularly bacteria of the genus Komagataeibacter (formerly Acetobacter and Gluconacetobacter) (Nguyen et al. 2008; Yamada et al. 2012). This bacterial cellulose has unique properties which make it an ideal candidate for industrial-scale production, such as purity, high tensile strength, excellent water- holding capacity, biocompatibility and biodegradability. In fact, there are reports that support it has been used not only to produce desserts (which have a high content of dietary fiber) (Jagannath et al. 2008), artificial skin used in medical treatments (Yamanaka et al. 1989), acoustic diaphragms (Vandamme et al. 1998), artificial blood vessels (Klemm et al. 2001; Scherner et al. 2014) and electric conductors (Muller et al. 2012) among other products, but also to be incorporated as a reinforcement of other polymeric materials or paper (Miao and Hamad 2013; Zimmermann et al. 2010), or as a food stabilizer (Shi et al. 2014).
To date there are no reports of large-scale cellulose production using low-cost substrates, and the few companies worldwide that produce this biopolymer, such as Bowil Biotech (Poland), do so in order to obtain medical products such as masks, goggle shaped dressing and wound dressing that meet the requirements for medical devices of class IIb and III (http://bowil.pl/en/bowil-biotech-en/). From the information available regarding the production process of this company and the type of products, it is known that BC is obtained in small fermenters to avoid its contamination and it can be deduced that standard culture media are used to avoid the presence of toxic compounds.
For this lack of studies, it is not clear what would be an optimal system of production using these low cost substrates, but it could include bioreactors as plates, air lift or impeller systems, rotating disk, stirred tanks with a spin filter, biofilm systems, spherical type bubble columns, trickling bed systems, etc. (Vazquez et al. 2013; Goelzer et al. 2009; Shigematsu et al. 2005; Lin et al. 2014a, b; Jung et al. 2007; Cheng et al. 2011; Song et al. 2009; Lu and Jiang 2014), an optimal culture process, if static or submerged in batch, fed-batch or intermittent fed-batch mode and an optimal standardization of factors such as temperature, dissolved oxygen (DO) and pH principally (Campano et al. 2016). Also the uses that can be given to the cellulose obtained with these low-cost media are unclear, since in many cases the cellulose would have to undergo a purification process (mainly washing, since as a matrix it can absorb the color and some components of the substrate) and therefore could hardly be used in medical, cosmetic or food products.
To obtain cellulose (at laboratory scale), experiments with various unconventional (low cost) substrates have been made for the past 20 years. They include fruit juices (coconut, pineapple) (Budhiono et al. 1999); vegetal extracts (from black tea, green tea, litchi) (Nguyen et al. 2008; Yang et al. 2016); molasses (from sugar beet and sugar cane) (Bae et al. 2004; Bae and Shoda 2005; Keshk and Sameshima 2006; Çoban and Biyik 2011; Vazquez et al. 2013; Khattak et al. 2015); syrups (Zeng et al. 2011); fermentation wastewaters and sludge (vinasse, thin stillage, Makgeolli Sludge, waste bear yeast, glycerol from biodiesel and lipid fermentation wastewater) (Ha et al. 2008; Velásquez-Riaño and Lombana-Sánchez 2009; Velásquez-Riaño et al. 2013; Wu and Liu 2013; Hyun et al. 2014; Lin et al. 2014a, b; Huang et al. 2015a; Vazquez et al. 2013; Huang et al. 2016) and plant biomass hydrolysates (corn cob, elephant grass, spruce, wheat straws, fiber waste sludge, hot water extracted-wood, wastes of cotton-based textiles, sweet potato pulp, corn starch) (Shigematsu et al. 2005; Hong et al. 2012; Al-Abdallah and Dahman 2013; Cavka et al. 2013; Chen et al. 2013; Guo et al. 2013; Yang et al. 2013; Huang et al. 2015b; Kiziltas et al. 2015; Neera et al. 2015).
Most of the substrates described above are industrial by-products and they have characteristics that make them suitable industrial substrates for the production of large-scale BC, since they are inexpensive, good sources of carbon (such as sugars, alcohols, etc.) and nitrogen, and in some cases they provide the necessary cofactors for the optimal development of microorganisms (Table 1). Their use could also significantly reduce the organic load of industrial wastewaters and the amount of contaminating materials, so this could contribute to make other processes to be harmonic with the ideal of clean production.
Therefore, in this review it will be summarized the aspects (temperature, pH, time and type of culturing) that can influence the production of bacterial cellulose (BC) from different low-cost substrates, the types of strains used and the physical and chemical characteristics of the biopolymer obtained. It was also included as focus and as a differentiating element on this document the general characteristics of those by-products that have been used to obtain cellulose (initial sugar concentration, cofactors, COD, etc.). Finally, the general purpose was to determine what could be the best BC industrial production system, which low-cost carbon substrates have the greatest potential for that and what could be the possible commercial uses of this biopolymer.
Molasses and Syrups
General characteristics
Molasses are a viscous by-product of the final stage of the crystallization of sugar from sugar cane or beet (Çakar et al. 2014). Different kinds of molasses have been used as substrates in the processing of such industrial products as ethanol (Sheoran et al. 1998), lactic acid (Kotzamanidis et al. 2002), polyhydroxybutyrate (PHB) (Beaulieu et al. 1995), xanthan gum (Kalogiannis et al. 2003), and at the experimental level, microbial cellulose (Bae and Shoda 2005; Keshk and Sameshima 2006; Çakar et al. 2014). The composition of molasses may be quite heterogeneous depending on the type of sugar cane or beet used to refine sugar (Table 1), but in general terms, molasses have a high concentration of carbohydrates (51%, consisting of 35% sucrose and 16% reducing sugars) and total suspended solids (TSS, 88%). They also have a low concentration of cysteine (below 0.05 g/100 g), total nitrogen (0.35%), and phosphorus (0.04%) (Keshk and Sameshima 2006).
On the other hand, syrups are liquid sweeteners produced from starches, corn starch and the sap of trees like maple. They are characterized by a high concentration of sugar, which reaches 67% (w/w) in maple syrup: 89% of such sugars is sucrose, and the remaining carbohydrates are fructose and glucose (Morselli 1975), and unlike molasses they have been less used for microbial culture. For example, in the case of the syrup form maple, due to Canada is responsible for 84% of world syrup production (Kurosumi et al. 2009) it sets the standard for its commercialization on an international level, so the use of this substrate for the cultivation of microorganisms has been quite restricted. There are only two studies of its use for this purpose have been found: one for obtaining poly-β-hydroxybutyrate from Alcaligenes latus (Kurosumi et al. 2009) and another for the production of bacterial cellulose (Zeng et al. 2011).
Used strains and culturing processes
For the production of microbial cellulose from molasses and syrups different strains of G. xylinus (formerly A. xylinum) have been used under different conditions as presented in Table 2. These organisms were principally grown in static batch processes (~53% of the experiments), continuous fed-batch processes with the use of a jar fermenter (~20%), intermittent (~13%), and fed-batch processes with the use of an orbital shaker (~13%) (Zeng et al. 2011). Almost all cultures were incubated at temperatures between 28 and 30 °C (~93%) which is a temperature range regarded as standard for G. xylinus (Delmer and Amor 1995; El-Saied et al. 2008). And the pH, which is a limiting factor in the growth of this microorganism, was considered in most of the studies with molasses. It was worked with an initial pH of 5.0–6.5; however, only in the study by Keshk and Sameshima (2006) the pH was monitored throughout the process, which showed that there is a trend towards acidification (the pH fell from 6.0 to 3.95) and just in the study of Bae et al. (2004) the pH was maintained constant. The studies also showed that the optimal culture time was very heterogeneous, ranging from 3 days (Bae and Shoda 2005) to 28 days (Çakar et al. 2014), however, for ~80% of the experiments the optimal time was less than 10 days.
BC production
Unlike syrups, molasses proved to be an optimal medium in terms of BC production. The best results were reported by Bae et al. (2004), who obtained a yield of 0.7 gBC/g sugar using an intermittent fed-batch jar fermentor for cultivating Acetobacter xylinum subsp, sucrofermentans BPR2001, in only three days. Similar results (0.6 gBC/g sugar) were presented by Vásquez et al. (2013), but it was used a static culture and the time were 14 days. For some other experiments the yield was less than 0.3 gBC/g sugar and in the rest it was impossible to compare because the initial sugar concentrations of the substrates used were not mentioned (Table 2). It is to note that, in general terms, molasses have best results than the standard mediums, however since both substrates have a high viscosity and density, these byproducts should have a pretreatment for the production of microbial cellulose. Several dilutions and other types of pretreatments have been proposed in order to optimize the process whereby certain sugars in these substrates are broken down into simpler forms (Bae et al. 2004; Bae and Shoda 2005).
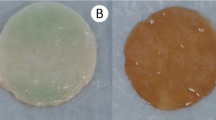
In addition, when the physical characteristics of the microbial cellulose obtained from molasses were analyzed by means of viscosity, Fourier-transform infrared spectroscopy (FT-IR), wide-angle X-ray diffractometry (XRD), solid-state 13C nuclear magnetic resonance spectroscopy (CP/MAS 13C NMR) (Keshk and Sameshima 2006; Çakar et al. 2014), or scanning electron microscopy (SEM) (Vazquez et al. 2013; Çakar et al. 2014; Khattak et al. 2015), it was concluded that the produced biopolymer was cellulose (type I principally), and, in turn, that it has very similar characteristics to the cellulose produced form standard media. However, some analysis like the FT-IR showed a slight difference in the region of the intermolecular hydrogen bonds and a lower viscosity (Keshk and Sameshima 2006) in molasses medium. Likewise, the SEM analysis revealed that the fibrils produced from molasses in the batch mode were denser than those produced from the standard HS medium (Çakar et al. 2014).
Fermentation by-products
General characteristics
In this category there are reported substrates such as: (a) vinasse and thin stillage, industrial byproducts of the distillation of ethanol from the fermentation of molasses (made from sugar cane or sugar beet) or cornstarch (Kretzschmar 1961) which generally have a brown color, high turbidity, low pH (ranging from 4.0-5.0), high content of suspended and dissolved organic matter (carbohydrates and organic acids) (Yang and Lin 1998; Hsieh et al. 2005), an appreciable amount of inorganic salts, composed of sulfates and phosphates of calcium, potassium, sodium and magnesium; (b) Acetone-Butanol-Ethanol (ABE) wastewaters, which are generated as a byproduct of a mixed fermentation, using Clostridium acetobutylicum bacteria, of media containing glucose and xylose to produce additives to fuels like gasoline, and contains residual sugars (glucose and xylose), organic acids (acetic and butyric), and alcohols (ethanol and butanol) (Chen et al. 2013; Huang et al. 2015a); (c) Beer yeast waste (WBY), a by-product of the fermentation of cereals rich in starch and malt (used to produce alcohol), which is mainly composed of protein (48–55%), carbohydrates (23–28%), RNA (6–8%), glutathione (1%) and vitamin B (2%), and are also rich in elements like P, K, Ca, Fe and Mg (Liu et al. 2012), characteristics that make them excellent candidates for the production of compounds of interest by microorganisms like bacterial cellulose (Ha et al. 2008; Lin et al. 2014a, b), (d) lipid fermentation wastes, such as glycerol and residual waters rich in lipids, residual sugars (glucose, xylose and arabinose) and exopolysaccharides generated as by-products during biodiesel production which are profiled as good substrates for BC production (Vazquez et al. 2013; Huang et al. 2015a, 2016), and (e) sludges, like Makgeolli sludge (MS) which is produced in traditional rice wine distilleries throughout South Korea and have proved to be a good source of carbon and nutrients in the bioremediation of acid mine drainage (Costa et al. 2009), and the production of bacterial cellulose (Hyun et al. 2014).
Used strains and culturing processes
For the production of microbial cellulose from the substrates mentioned above, different strains of the genus Gluconacetobacter have been used and grown under different conditions (Table 2). These bacteria have been (mostly) grown under static batch conditions (~87% of the experiments) except in the case of Velásquez-Riaño and Lombana Sánchez (2009), who used a semi-continuous static process, and Velásquez-Riaño et al. (2013), whose study employed an orbital shaker at 250 rpm to grow G. kakiaceti GM5. The best results were reported by Wu and Liu (2013), using Gluconacetobacter xylinus BCRC 12334 (2.7 gBC/g sugar) and pure thin stillage at 30 °C during 7 days. Analyzing variables such as time, temperature and pH, it was found that in most of the studies the optimal production conditions were between 5 and 7 days (~ 80%), 28-30 °C and 5.5 pH units. However, unlike the molasses, the pH tended to increase during the time when it was monitored (Velásquez-Riaño et al. 2013; Wu and Liu 2013). Related to this change, some authors have suggested that this increase is caused by the release of ammonium ion when the microorganisms degrade the amino acids present in these substrates (Wu and Liu 2012; Velásquez-Riaño et al. 2013; Huang et al. 2015a).
BC production
Vinasse and TS can be used in a pure form as substrate for the BC production, without any pretreatment (Velásquez-Riaño and Lombana-Sánchez 2009; Velásquez-Riaño et al. 2013). In the case of the MS medium, it must be previously dissolved (because it is solid), so that the study of Hyun et al. (2014) worked with a concentration of 20%. Otherwise, a prior hydrolysis is required for WBY, to degrade the proteins and carbohydrates found in the cell walls of yeasts in the form of large polymers make them available (Lin et al. 2014a, b). In all the studies of these low-cost carbon substrates, which have a total sugar content of about 1–3%, the standard HS medium was used as a control, and similar to the case of molasses, the total yield of BC was less than the experimental mediums. For example, in the case of Ha et al. (2008), the experiments yielded 4.5 g/L of bacterial cellulose, compared to the 0.5 g/L obtained from the HS medium with the use of G. hansenii PJK after 5 days.
Evaluations of the physical characteristics of the microbial cellulose obtained from distillery wastewaters confirmed that such byproducts might be optimal for producing BC. These characteristics include water-holding capacity (WHC) (Wu and Liu 2013); water release rate (WRR); and water absorption rate (WAR) (Lin et al. 2014a, b). The evaluations were undertaken with SEM, XRD (Vazquez et al. 2013; Wu and Liu 2013; Hyun et al. 2014; Lin et al. 2014a, b; Huang et al. 2015a, 2016); field emission scanning electron microscopy (FE-SEM) and FT-IR (Vazquez et al. 2013; Huang et al. 2015a, 2016). These studies also confirmed that this biopolymer was cellulose and it had very similar characteristics to those found in the BC produced using the standard media (Lin et al. 2014a, b). By Scanning Electron Microscopy for example, it was found that the structural nanofibrils in the TS, MS and HS media were of a similar size (Wu and Liu 2013; Hyun et al. 2014), but the lattice structure was a little denser in the TS medium. The presence of lattice structure might be directly related to the lowest WHC values (20%), since there would be less space to catch water, this contrasts with the microfibrils found in WBY medium with a high WHC values (Lin et al. 2014a, b). In the case of the diffraction X-rays of the glycerol from biodiesel (Vazquez et al. 2013); TS (Wu and Liu 2013), MS (Hyun et al. 2014), ABE (Huang et al. 2015a) and LFW (Huang et al. 2016) media revealed three characteristic peaks, which correspond to the cellulose Iα and at a wavelength of 400 to 4000 cm−1, the FT-IR analysis of the ABE medium found the typical bands of cellulose I.
Worldwide are produced large amounts of fermentation wastewaters, reaching for example 10 L/L ethanol and 45 ton/ton of bio-butanol. If these wastewaters are not disposed properly, they might have a significant negative environmental impact due to their values for Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD), between 20 and 100 g/L respectively (Yeşilada 1999; Wu and Liu 2012; Huang et al. 2015a, 2016). On the other hand, these byproducts have been extensively used as a substrate for the production of such substances of interest like biogas, single cell protein (biomass), enzymes, ethanol, and bacterial cellulose (Yang and Tung 1996; Yang and Lin 1998; Ha et al. 2008; Velásquez-Riaño and Lombana-Sánchez 2009; González et al. 2010; Vazquez et al. 2013; Velásquez-Riaño et al. 2013; Wu and Liu 2013; Moraes et al. 2015; Huang et al. 2015a, 2016).
Juices and extracts
General characteristics
A wide variety and large amount of fruits are consumed on a global level, due both to their attractive flavor and their high contents of vitamins and fibers. The fruits which are sold, either domestically or internationally, must meet certain quality standards related to their size, color and undamaged (non-bruised) state. Instead of being directly consumed, some fruits are used to produce jams, desserts, sauces, etc. Fruits are characterized by their high concentration of sugars like glucose and fructose and their low pH, qualities which enable them to be used (especially juices and extracts) for growing microorganisms, like the acetic bacteria which produce cellulose (Budhiono et al. 1999; Kurosumi et al. 2009; Neera et al. 2015; Yang et al. 2016). For the production of BC, experiments have been made with juices and extracts from oranges, pineapples, apples, Japanese pears and litchis.
Used strains and culturing processes
In Southeast Asia, for several decades by now, bacteria of the Gluconacetobacter genus have been cultured in coconut water for the handmade production of a fermented dessert known as nata-de-coco (coconut cream). The A. xilinum, A. xylinum NBRC 13693, G. xylinus CH001, and Gluconacetobacter xylinus DFBT strains have been used in the static batch process (Budhiono et al. 1999; Kurosumi et al. 2009; Yang et al. 2016; Neera et al. 2015).
The incubation temperature and pH for BC production using fruits and their extracts was quite homogeneous: 28–30 °C and 30 °C (Table 2), and with an initial acid pH (4.5 to 6.0). Nevertheless, monitoring the changes in pH is a key factor in growing microorganisms, as evidenced by the metabolic changes which usually occur in the culture. Since some substrates have a marked tendency to become alkalized and others to become acidified, this factor may affect the production of BC.
BC production
All of the tested juices and extracts show a strong potential for the large-scale production of BC, but pineapple extract clearly stood out among all the culture media (Table 2). First, because it yielded 1.6 g BC/g sugar after 7 days of processing, which is a low culture time considering other experiments needed 12 to 14 days to reach a maximum of 0.5 g BC/g sugar. Second because it does not need to be supplemented, a factor that would lower production costs.
The initial concentration of sugar is a parameter that must be taken into account in assessing the methods to produce BC. Although these substrates can be used pure, without any pre-treatment, they may have a very high concentration of sugars (up to 183.5 g/L for litchi, Yang et al. 2016), which means they have to be diluted beforehand. Although the carbohydrates content of fruit juices and extracts may be sufficient for the production of bacterial cellulose; in the case of coconut water, the medium have to be supplemented with sugars like sucrose; other compounds as a nitrogen sources, like peptone, yeast extract ammonium sulfate (NH4)2SO4 and diammonium hydrogen phosphate (NH4)2HPO4 (Kurosumi et al. 2009); or vitamins like B1, B2, B3, B5, B6, B12 and D (+) biotin (Budhiono et al. 1999).
Only in the study by Yang et al. (2016), which used SEM, FT-IR and XRD, were the characteristics of the biopolymer obtained from litchi extract tested. The SEM analysis of the morphology of the bacterial cellulose in the litchi extract and HS medium showed that this biopolymer had a cross-linked structure of ultrafine fibrils. The fibrils of the cellulose produced from litchi proved to be more “voluble”, with a tridimensional structure more clearly defined than in those produced from the standard medium, even though they were seen to be very similar in size and length. The functional groups of BC obtained from both media (evaluated by means of FT-IR) had the characteristic bands of cellulose polymer. However, the patterns revealed by the XRD analysis showed some slight differences between the BC obtained from the standard medium and the BC from the litchi extract. Compared with the other low-cost media, juices and extracts are the least studied in terms of their physical and chemical characteristics, which is one of the decisive factors in scaling a process and defining if these are useful or not. By the results obtained by Yuang et al. (2016), litchi extract could be a potential candidate for industrial scale production of bacterial cellulose.
Plant biomass hydrolysate
General characteristics
Plant biomass is a byproduct of various industries, like a) agriculture (sweet potato pulp, bark rice, wheat straw, dry olive mill, corncob, etc.) (Shigematsu et al. 2005; Goelzer et al. 2009; Al-Abdallah and Dahman 2013; Gomes et al. 2013; Huang et al. 2015b); b) textiles (cotton waste) (Hong et al. 2012); and c) pulp, paper and timber (wood residues, waste fiber sludge) (Cavka et al. 2013; Kiziltas et al. 2015), among others. These products are low-cost and made from renewable and worldwide available resources (Rubin 2008). They are mainly composed of cellulose, hemicellulose and, in some cases, lignin (Cavka et al. 2013).
Used strains and culturing processes
During the past 15 years, researchers have worked with different strains of the Gluconacetobacter genus to obtain BC from plant biomass hydrolysate. The most widely used strains are: a) Gluconacetobacter xylinus 23770, with cotton-based textile wastes (Hong et al. 2012), waste fiber sludge (Cavka et al. 2013), wheat straw (Chen et al. 2013.) and spruce wood (Guo et al. 2013); b) G. xylinus CH001, with elephant grass (Yang et al. 2013) and corncob (Huang et al. 2015b); and c) Acetobacter xylinum ATCC 23769, with bark rice (Goelzer et al. 2009) and hot water extracted-wood sugars (Kiziltas et al. 2015). There are also works with wheat straw and Gluconacetobacter xylinus ATCC 700178 (Al-Abdallah and Dahman 2013); dry olive mill residue and Gluconacetobacter sacchari (Gomes et al. 2013); waste water of candied jujube and Gluconacetobacter xylinum CGMCC 2955 (Li et al. 2015); and sweet potato pulp and G. xylinus BPR2001 and Gluconacetobacter xylinus BPR2001 GD-I (a mutant obtained from the above strain) (Shigematsu et al. 2005). In most cases (more than 60% of the experiments), a static batch process was used to cultivate these strains (Table 2). However, other methods of culture have been also tested: a) orbital shakers at 150 rpm (Huang et al. 2015b) and 250 rpm (Al-Abdallah and Dahman 2013) and b) aerated bioreactors with stirring (Goelzer et al. 2009) and without stirring (Shigematsu et al. 2005).
Various standard media were used to evaluate the yield of bacterial cellulose from different types of plant biomass. For example, the HS medium (Goelzer et al. 2009; Gomes et al. 2013; Kiziltas et al. 2015) and simple culture media. Simple culture media had glucose as a carbon source and peptone and yeast extract as nitrogen sources (Hong et al. 2012; Cavka et al. 2013; Chen et al. 2013; Guo et al. 2013) or corn steep liquor (CSL) as a nitrogen source (Shigematsu et al. 2005). It is important to note that these studies used a reference substrate, which is necessary for a proper comparison of such variables as the productivity, effectiveness and physical characteristics of the polymer which is obtained, whereas other studies did not (Al-Abdallah and Dahman 2013; Yang et al. 2013; Huang et al. 2015b; and Li et al. 2015). The incubation temperatures ranged from 28 to 30 °C, with the latter the most common (Shigematsu et al. 2005; Hong et al. 2012; Cavka et al. 2013; Chen et al. 2013; Guo et al. 2013; Li et al. 2015). Most studies started with an acidic pH in the range of 4.5 to 6.0, with 5.0 the preferred one: (Shigematsu et al. 2005; Goelzer et al. 2009; Al-Abdallah and Dahman 2013; Cavka et al. 2013; Guo et al. 2013); Kiziltas et al. (2015), was the only study with an alkaline pH. It regarded 8.0 as the optimum pH for the cultivation A. xylinus 23769 in hot water extracted-wood at an incubation temperature of 28 °C. Most studies where the pH was evaluated (Goelzer et al. 2009; Al-Abdallah and Dahman 2013; Cavka et al. 2013; Guo et al. 2013) showed that there is a marked trend towards acidification. Al-Abdallah and Dahman (2013) even reported a pH of 2.0, with a strain of G. xylinus ATCC 700178 and wheat straw as the culture medium. By contrast, Yang et al. (2013) reported that the pH rose from 6.0 to 7.3, with G. xylinus CH001 grown in a hydrolyzate of elephant grass. Finally, the time of cultivation ranged from 2.9 to 28 days, although the optimal period for most studies was 7 days (Al-Abdullah and Dahman 2013; Chen et al. 2013) and 14 days (Hong et al. 2012; Cavka et al. 2013; Guo et al. 2013; Yang et al. 2013; Huang et al. 2015b).
BC production
In recent decades, these byproducts have become important in the industrial production of second-generation ethanol, a process that consists of 3 stages: first, hydrolyzing cellulose and hemicellulose to fermentable sugars like glucose; second, fermenting with yeasts like S. cerevisiae, and finally, distilling ethanol from the mixture (Jeihanipour and Taherzadeh 2009; Cavka et al. 2013). Since the past decade, it has been proposed to use this biomass to obtain BC, in line with the principle of the above process (Shigematsu et al. 2005; Goelzer et al. 2009; Hong et al. 2012; Al-Abdallah and Dahman 2013; Cavka et al. 2013; Chen et al. 2013; Gomes et al. 2013; Guo et al. 2013; Yang et al. 2013; Huang et al. 2015b; Kiziltas et al. 2015; Li et al. 2015). This means that according to the type of pretreatment and hydrolysis method applied to them, they could also be used to obtain a variety of carbohydrates, acids and various sources of nitrogen, so that their use (alone or with supplements) as media for the culture of products of interest with microorganisms is feasible.
Among the pretreatments used to obtain bacterial cellulose from lignocellulosic material, there is hydrolysis with: a) acids (Huang et al. 2015b); b) enzymes (Shigematsu et al. 2005; Goelzer et al. 2009; Cavka et al. 2013; Guo et al. 2013); c) high temperatures (Li et al. 2015); and d) different pressures. However, these processes tend to be aggressive and may alter the type of monosaccharides produced (Kiziltas et al. 2015). These processes can be undertaken individually or in combination, depending on the type of substrate (Al-Abdallah and Dahman 2013; Chen et al. 2013; Gomes et al. 2013, Yang et al. 2013). Despite acid hydrolysis is economic, it produces small quantities of glucose, because cellulose is not completely hydrolyzed or other non-fermentable products are formed. On the other hand, despite enzymatic hydrolysis has a high glucose yield; it requires expensive enzymes (Cavka et al. 2013). Regardless of which method is used, hydrolysis may produce metabolites that inhibit fermentation, like furan aldehydes (furfural and 5-hydroxymethyl-furfural (HMF), aliphatic acids (such as acetic acid, formic acid and levulinic acid), and phenolic compounds (Jönsson et al. 2013). Therefore, these substances must be eliminated (detoxified) before the fermentation starts, using physical and chemical mechanisms like alkali treatment, ion exchange, reducing agents, activated charcoal and enzymes like laccase and peroxidase (Guo et al. 2013).
Most of the evaluated plant byproducts proved to be quite optimal for the production of BC, because a larger amount of biopolymer was obtained from them than from the reference medium (when one was used). Except in the study of Gomes et al. (2013), where G. sacchari using dry olive mill residue as a substrate yielded a larger amount of BC than using the reference medium HS. The study in which it was reported the highest bacterial cellulose productivity was that of Hong et al. (2012), who obtained 10.8 g/L cellulose from cotton-based textile waste with G. xylinus 23770, employing a static process for a period of 7 to 14 days. However, when other variables are taken into account, such as the initial concentration of sugar and the optimal culture time, the most efficient process was that of Chen et al. (2013). Chen et al. (2013) obtained 8.3 g/L of BC from a hydrolyzate of wheat straw with an initial sugar concentration of 12 g/L s within 7 days, using a static process. These results show that such byproducts could be used for the large-scale production of this biopolymer.
The physical characteristics of the microbial cellulose obtained from plant byproducts were evaluated, as follows: a) an analysis of their tensile strength (Hong et al. 2012; Cavka et al. 2013); b) WHC (Huang et al. 2015b); c) thermogravimetric analysis (TGA) (Kiziltas et al. 2015); d) XRDM; e) SEM (Goelzer et al. 2009; Gomes et al. 2013; Yang et al. 2013; Huang et al. 2015b; Kiziltas et al. 2015; Li et al. 2015); f) FT-IR (Goelzer et al. 2009; Yang et al. 2013; Huang et al. 2015b; Kiziltas et al. 2015; Li et al. 2015); g) FESEM (Cavka et al. 2013); h) transmission electron microscopy (TEM); i) atomic force microscopy (AFM) (Goelzer et al. 2009) and j) Fourier-transform infrared spectroscopy attenuated with full reflection (FTIR-ATR) (Gomes et al. 2013). The results of these analyses confirmed that the product obtained from these alternative media was cellulose, also that in most cases this biopolymer had similar characteristics to those found in the reference media. For example, the BC obtained from dry olive mill residue had the typical uniform three-dimensional network of nano- and micro-fibrils (evidenced by SEM), and the typical spectrum (FT-IR) of cellulosic substrates. Furthermore, this biopolymer showed the characteristic profile (XRD) of cellulose I. The degree of crystallinity was about 80% (Gomes et al. 2013; Kiziltas et al. 2015). However, Goelzer et al. (2009) obtained strongly intertwined sheets of BC (semicrystalline) of type II and type I celullose, using a static process with, respectively, a bark rice plus glucose medium and a standard one. Moreover, Hong et al. (2012) reported that although the thickness of the bacterial cellulose obtained from the hydrolyzate of cotton-based textile waste was lower than that obtained from the reference medium (1.48 mm and 1.63 mm, respectively), its tensile strength was significantly higher (79%) with the hydrolyzate.
Gaps, challenges and trends
In microbial fermentations, the cost of substrates normally accounts for up to 50–65% of the total cost of production. BC production is not the exception, and thus in recent years much work has been devoted to find new low cost carbon sources (Vazquez et al. 2013). All the low-cost culture media discussed in this review could have the potential to be used for large-scale BC production, not only by the quantities that can be obtained with this type of substrates (Table 2), but also because the physical chemical characteristics of the BC obtained in most of them are very similar to those obtained in standard media (Table 3). However, some obstacles would have to be overcome. For example, a further research of the use of submerged cultures would be required, since most of the studies (85%) used static culture techniques. The outlook for the submerged cultures could be good, since Al-Abdallah and Dahman (2013) obtained up to 10.6 g/L of bacterial cellulose from a submerged culture using G. xylinus ATCC 700178 with wheat straw, which is fairly close to the maximum productivity of reference mediums and static cultures. Work with strains with high yields of cellulose is also important. In most cases, this will depend on the alternative culture media which is used, since, depending on the medium one strain may be more or less productive. Nevertheless, there are strains which clearly produce as much cellulose in reference mediums as in alternative ones. Among them, there is G. kakiaceti GM5, which showed the best results in the reviewed articles: it can produce up to 16.4 g/L of biopolymer in the HS medium (Velásquez-Riaño and Lombana-Sánchez 2009). There is also G. xilynus BCRC 12334, which can produce up 10.4 g/L when grown in thin stillage (Wu and Liu 2012) and G. xylinus 23770, with which more than 8 g/L of biopolymer can be obtained using 4 types of lignocellulosic hydrolysates (cotton-based textile waste, fiber waste sludge, wheat straw and spruce wood) (Hong et al. 2012; Cavka et al. 2013; Chen et al. 2013; Guo et al. 2013). The strain which proved to be the most versatile was G. xylinus CH001, because it can be grown in 4 different culture media (ABE, litchi extract, elephant grass and corncob) (Huang et al. 2015a; Yang et al. 2013, 2016; Huang et al. 2015b) (Table 1).
Another factor, which significantly affects the viability of the scaling process, is the pretreatment of these alternative substrates. Some treatments are quite costly and complex, especially those involving the enzymatic hydrolysis of lignocellulosic biomass, since those enzymes are expensive. Detoxification may also could be needed after the stages of pretreatment and hydrolysis in order to avoid substances which inhibit the production of the bacterial cellulose when there are large volumes of the culture medium. The alternative substrates must also be sterilized to prevent contamination, which is a costly and complex procedure. This point is very important, since only one of the studies worked with a non-sterile substrate (hot water extracted-wood), although it did not obtain BC (Kiziltas et al. 2015). That is, one would need to determine whether that process is indispensable for such culture media (if not, the costs would be less), also if the BC-producing strains might interact with the microbiota in these substrates (like the yeasts in vinasse) and if so, what kinds of interactions occur. To do that, researchers would have to test these non-sterile media with different concentrations of inoculum (to ensure that the concentration of BC-producing bacteria exceeds the native microbiota), evaluate the accompanying microbiota and measure the amounts of aerobic heterotrophic bacteria and molds and yeasts (Cruz-Morató et al. 2013; Gros et al. 2014). It is important to stress that research has already shown that G. xylinus has the ability to grow in mixed cultures with yeast and lactic acid bacteria (Marsh et al. 2014).
A proper standardization of the procedures, which employ these low cost alternative substrates, would also be required, since their compositions, which have a direct impact on the productivity of BC, are quite heterogeneous (Table 1). It would have to include such variables of control as the initial concentration of carbon and nitrogen sources, temperature, pH, degree of agitation and amount of dissolved oxygen, among others. The studies under review found that a large initial concentration of sugar (its easy absorption is more important) and concentrations of between 10 and 20 g/L would be sufficient to obtain an optimum productivity of BC. However, most of the studies had to supplement the culture media with alternative nitrogen sources, since those sources are scarce in juices, extracts and hydrolyzate substrates from lignocellulosic material (Budhiono et al. 1999; Kurosumi et al. 2009; Gomes et al. 2013). They likewise show that the temperature may be kept in a range of 28 to 30 °C and pH control is not essential during the process of cultivation since more BC was obtained when the pH was not constant. Nevertheless, in the media where was a tendency toward alkalinity, it would be necessary to measure the effect of a constant acidic pH.
With all the information obtained over the years about the production of bacterial cellulose from low cost substrates, research trends should now focus on the economic part of the process, to determine if it is commercially viable. Although the share of substrate costs can be recouped using by-products (which in most cases have no monetary value), none of the studies report that the cellulose produced will generally be colored and may absorb undesirable substances (even potentially toxic) from these by-products, which would make it necessary to undergo a subsequent purification process that could increase their production costs on an industrial scale. There is a constant trend towards the investigation of new applications for this type of bacterial cellulose produced from standard media: as explosives, sponges, conductive materials, magnetic materials, cellulose nanocrystals, nanocomposites, among others (Sun et al. 2010; Gao et al. 2011; Marins et al. 2014, De Salvi et al. 2014; Ren et al. 2014; Huang et al. 2014; Rosa et al. 2014). And although the characteristics of the cellulose obtained from low cost substrates are usually very similar to those obtained from the standard media (Table 3), the aforementioned purification and detoxification processes could limit the use of this polymer especially in the biomedical, food, pharmaceutical and cosmetic areas (since for such uses the regulatory requirements are highly restrictive). However, the above makes it necessary to start researching new applications of bacterial cellulose obtained from these low-cost substrates without purification, since this bacterial cellulose could be enriched with components that could be beneficial for the improvement of, for example, soils or could be used as feed for animals, etc.
Finally, although several studies suggest that the production of BC from these byproducts could be used to control the environmental pollution, only three studies evaluated the changes in COD values in the course of the process (Velásquez-Riaño et al. 2013; Huang et al. 2015b), and none dealt with the question of whether this process might yield novel byproducts that could be more contaminating than the original ones. Hence, it is needed toxicity testing, with indicators like Daphnia magna or Aliivibrio fischeri, among others (Botelho et al. 2012).
Conclusions
Even though the static batch process for obtaining microbial cellulose from different alternative low-cost media was the most widely used by the reviewed studies, more research needs to be done on the submerged and semi-continuous or continuous processes in order to find the most suitable one for large-scale production.
G. xilynus BCRC 12334 and G. xylinus 23770 strains were the ones that produced the largest amounts of BC in both the reference and the alternative media. G. xylinus CH001 proved to be the most versatile strain because it can grow in different types of non-conventional culture media. For an optimal production of BC in low-cost media, with bacteria of the Komagataeibacter genus (formerly Gluconacetobacter), the initial concentration of sugar should be between 1 and 2%, the temperature should be kept in a range of 28–30 °C, the initial pH between 4.5 and 5.5 (there is no need for it be constant), and the optimal culture time would be 7 days.
Using all the low-cost culture media discussed here, bacteria of the Komagataeibacter genus have the potential to produce BC on an industrial scale, since in most cases they yield more cellulose (with similar characteristics) when cultured in them than the reference media. Taking into account this unconventional media (cheap byproducts which do not require complex pre-treatments, detoxification and supplements) and variables like the time of culture and productivity, the media which have the greatest potential for large-scale use are beer yeast waste, vinasse and pineapple juice. Their use would naturally depend on their availability in a particular place and an economic feasibility study for the production of BC.
References
Al-Abdallah W, Dahman Y (2013) Production of green biocellulose nanofibers by Gluconacetobacter xylinus through utilizing the renewable resources of agricultura residues. Bioprocess Biosyst Eng 36:1735–1743
Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R, Cork A, Glover J, Redmond J, Williamson RE (1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279:717–720
Bae SO, Shoda M (2005) Production of bacterial cellulose by Acetobacter xylinum BPR2001 using molasses medium in a jar fermentor. Appl Microbiol Biotechnol 67:45–51
Bae S, Sugano Y, Shoda M (2004) Improvement of bacterial cellulose production by addition of agar in a jar fermentor. J Biosci Bioeng 97:33–38
Beaulieu M, Beaulieu Y, Mélinard J, Pandian S, Goulet J (1995) Influence of ammonium salts and cane molasses on growth of Alcaligenes eutrophus and production of polyhydroxybutyrate. Appl Environ Microbiol 61:165–169
Botelho RG, Tornisielo VL, Olinda RA, Maranho LA, Machado-Neto L (2012) Acute toxicity of sugarcane vinasse to aquatic organisms before and after pH adjustment. Toxicol Environ Chem 94:1–11
Bowil Biotech (2017). http://bowil.pl/en/bowil-biotech-en/. Accesed 30 Jan 2017
Budhiono A, Rosidi B, Taher H, Iguchi M (1999) Kinetic aspects of bacterial cellulose formation in nata-de-coco culture system. Carbohydr Polym 40:137–143
Çakar F, Özer I, Aytekin AO (2014) Improvement production of bacterial cellulose by semi-continuous process in molasses medium. Carbohydr Polym 106:7–13
Campano C, Balea A, Blanco A, Negro C (2016) Enhancement of the fermentation process and properties of bacterial cellulose: a review. Cellulose 23:57–91
Cavka A, Guo X, Tang S, Winestrand S, Jönsson LJ, Hong F (2013) Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol Biofuels 6:25
Chen L, Hong F, Yang XX, Han SF (2013) Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresource Technol. 135:464–468
Cheng KC, Catchmark JM, Demirci A (2011) Effects of CMC addition on bacterial cellulose production in a biofilm reactor and its paper sheets analysis. Biomacromol 12:730–736
Coban EP, Biyik H (2011) Evaluation of different pH and temperatures for bacterial cellulose production in HS (Hestrin-Scharmm) medium and beet molasses medium. Afr J Microbiol Res 5:1037–1045
Costa MC, Santos ES, Barros RJ, Pires C, Martins M (2009) Wine wastes as carbon source for biological treatment of acid mine drainage. Chemosphere 75:831–836
Cruz-Morató C, Ferrando-Climent L, Rodriguez-Mozaz S, Barceló D, Marco-Urrea E, Vicent T, Sarrà M (2013) Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidized bed bioreactor. Water Res 47:5200–5210
De Salvi DTB, Barud HS, Pawlicka A, Mattos RI, Raphael E, Messaddeq Y, Ribeiro SJL (2014) Bacterial cellulose/triethanolamine based ion-conducting membranes. Cellulose 21:1975–1985
Delmer DP, Amor Y (1995) Cellulose biosynthesis. Plant Cell. 7:987–1000
El-Saied H, El-Diwany AI, Basta AH, Atwa NA, El-Ghwas DE (2008) Production and characterization of economical bacterial cellulose. BioResources 3:1196–1217
Gao CA, Wan YZ, Yang CX, Dai KR, Tang TT, Luo HL, Wang JH (2011) Preparation and characterization of bacterial cellulose sponge with hierarchical pore structure as tissue engineering scaffold. J Porous Mat. 18:139–145
Goelzer FDE, Faria-Tischer PCS, Vitorino JC, Sierakowski MR, Tischer CA (2009) Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater Sci Eng. 29:546–551
Gomes FP, Silva NHCS, Trovatti E, Serafim LS, Duarte MF, Silvestre AJD, Neto CP, Freire CSR (2013) Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass Bioenerg 55:205–211
González R, Campbell P, Wong M (2010) Production of ethanol fromthin stillage bymetabolically engineered Escherichia coli. Biotechnol Lett 32:405–411
Gros M, Cruz-Morato C, Marco-Urrea E, Longrée P, Singer H, Sarrà M, Hollender J, Vicent T, Rodriguez-Mozaz S, Barceló D (2014) Biodegradation of the X-ray contrast agent iopromide and the fluoroquinolone antibiotic ofloxacin by the white rot fungus Trametes versicolor in hospital wastewaters and identification of degradation products. Water Res 60:228–241
Guo X, Cavka A, Jönsson LJ, Hong F (2013) Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb Cell Fact 12:93
Ha JH, Shehzad O, Khan S, Lee SY, Park JW, Khan T, Park JK (2008) Production of bacterial cellulose by a static cultivation using the waste from beer culture broth. Korean J Chem Eng 25:812–815
Hong F, Qiu KY (2008) An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohyd Polym 72:545–549
Hong F, Guo X, Zhang S, Han SF, Yang G, Jönsson LJ (2012) Bacterial cellulose production from cotton-based waste textiles: enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour Technol 104:503–508
Hsieh C, Hsu TH, Yang FC (2005) Production of polysaccharides of Ganoderma lucidum (CCRC36021) by reusing thin stillage. Process Biochem 40:909–916
Huang Y, Zhu CL, Yang JZ, Nie Y, Chen CT, Sun DP (2014) Recent advances in bacterial cellulose. Cellulose 21:1–30
Huang C, Yang XY, Xiong L, Guo HJ, Luo J, Wang B, Zhang HR, Lin XQ, Chen XD (2015a) Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett Appl Microbiol 60:491–496
Huang C, Yang X, Xiong L, Guo H, Luo J, Wang B, Zhang H, Lin XQ, Chen XD (2015b) Utilization of Corncob Acid Hydrolysate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Appl Biochem Biotechnol 175:1678–1688
Huang C, Guo HJ, Xiong L, Wang B, Shi SL, Chen XF, Lin XQ, Wang C, Luo J, Chen XD (2016) Using Wastewater After Lipid Fermentation as Substrate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Carbohydr Polym 136:198–202
Hyun JY, Mahanty B, Kim CG (2014) Utilization of Makgeolli Sludge Filtrate (MSF) as Low-Cost Substrate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Appl Biochem Biotechnol 172:3748–3760
Jagannath A, Kalaiselvan A, Manjunatha SS, Raju PS, Bawa AS (2008) The Effect of pH, Sucrose and Ammonium Sulphate Concentrations on The Production of Bacterial Cellulose (Nata-De-Coco) by Acetobacter xylinum. World J Microbiol Biotechnol 24:2593–2599
Jeihanipour A, Taherzadeh MJ (2009) Ethanol production from cotton-based waste textiles. Bioresour Technol 100:1007–1010
Jönsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16
Jung JY, Khan T, Park JK, Chang HN (2007) Production of bacterial cellulose by Gluconacetobacter hansenii using a novel bioreactor equipped with a spin filter. Korean J Chem Eng 24:265–271
Kalogiannis S, Iakovidou G, Liakopoulou-Kyriakides M, Kyriakidis DA, Skaracis GN (2003) Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process Biochem 39:249–256
Keshk S, Sameshima K (2006) The utilization of sugar cane molasses with/without the presence of lignosulfonate for the production of bacterial cellulose. Appl Microbiol Biotechnol 72:291–296
Khattak WA, Khan T, Ul-Islam M, Park JK (2015) Production, characterization and physico-mechanical properties of bacterial cellulose from industrial wastes. J Polym Environ 23:45–53
Kiziltas EE, Kiziltas A, Gardner DJ (2015) Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr Polym 124:131–138
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci 26:1561–1603
Kotzamanidis CH, Roukas T, Skaracis G (2002) Optimization of lactic acid production from beet molasses by Lactobacillus delbrueckii NCIMB 8130. World J Microbiol Biotechnol 18:441–448
Kretzschmar H (1961) Levaduras y alcoholes y otros productos de la fermentación (alcohols and yeasts and other fermentation products). Reverté, Zaragoza
Kurosumi A, Sasaki C, Yamashita Y, Nakamura Y (2009) Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr Polym 76:333–335
Li Z, Wang L, Hua L, Jia S, Zhang J, Liu H (2015) Production of nano bacterial cellulose from waste water of candiedjujube-processing industry using Acetobacter xylinum. Carbohydr Polym 120:115–119
Lin D, Lopez-Sanchez P, Li R, Li Z (2014a) Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresource Technol. 151:113–119
Lin SP, Hsieh SC, Chen KI, Demirci A, Cheng KC (2014b) Semicontinuous bacterial cellulose production in a rotating disk bioreactor and its materials properties analysis. Cellulose 21:835–844
Liu M, Zhang M, Lin S, Liu J, Yang Y, Jin Y (2012) Optimization of extraction parameters for protein from beer waste brewing yeast treated by pulsed electric fields (PEF). Afr J Microbiol Res. 6:4739–4746
Lu HM, Jiang XL (2014) Structure and properties of bacterial cellulose produced using a trickling bed reactor. Appl Biochem Biotechnol 172:3844–3861
Marins JA, Soares BG, Fraga M, Muller D, Barra GMO (2014) Self-supported bacterial cellulose polyaniline conducting membrane as electromagnetic interference shielding material: effect of the oxidizing agent. Cellulose 21:1409–1418
Marsh AJ, O’Sullivan O, Hill C, Ross RP, Cotter PD (2014) Sequence-based analysis of the bacterial and fungal Compositions of multiple kombucha (tea fungus) samples. Food Microbiol 38:171–178
Miao CW, Hamad WY (2013) Cellulose reinforced polymer composites and nanocomposites: a critical review. Cellulose 20:2221–2262
Moraes BS, Zaiat M, Bonomi A (2015) Anaerobic digestion of vinasse from sugarcane etanol production in Brazil: challenges and perspectives. Renew Sustain Energy Rev 44:888–903
Morselli MF (1975) Nutritional value of maple syrup. Natl Maple Syrup Dig 14:12
Muller D, Mandelli JS, Marins JA, Soares BG, Porto LM, Rambo CR, Barra GMO (2012) Electrically conducting nanocomposites: preparation and properties of polyaniline (PAni)-coated bacterial cellulose nanofibers (BC). Cellulose 19:1645–1654
Neera R, Ramana KV, Batra HV (2015) Occurrence of cellulose-producing Gluconacetobacter spp. in fruit samples and kombucha tea, and production of the biopolymer. Appl Biochem Biotechnol 176:1162–1173
Nguyen VT, Flanagan B, Gidley MJ, Dykes GA (2008) Characterization of cellulose production by a Gluconacetobacter xylinus strain from Kombucha. Curr Microbiol 57:449–453
Ren Y, Li SR, Dai B, Huang XH (2014) Microwave absorption properties of cobalt ferrite-modified carbonized bacterial cellulose. Appl Surf Sci 311:1–4
Rosa JR, da Silva ISV, de Lima CSM, Neto WPF, Silverio HA, dos Santos DB, Barud HD, Ribeiro SJL, Pasquini D (2014) New biphasic mono-component composite material obtained by partial oxypropylation of bacterial cellulose. Cellulose 21:1361–1368
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454:841–845
Scherner M, Reutter S, Klemm D, Sterner-Kock A, Guschlbauer M, Richter T, Langebartels G, Madershahian N, Wahlers T, Wippermann J (2014) In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: proof of concept? J Surg Res 189:340–347
Sheoran A, Yadav BS, Nigam P, Singh D (1998) Continuous etanol production from sugarcane molasses using a column reactor of immobilized Saccharomyces cerevisiae HAU-1. J Basic Microbiol 38:123–128
Shi ZJ, Zhang Y, Phillips GO, Yang G (2014) Utilization of bacterial cellulose in food. Food Hydrocoll 35:539–545
Shigematsu T, Takamine K, Kitazato M, Morita T, Naritomi T, Morimura S, Kida K (2005) Cellulose production from glucose using a glucose dehydrogenase gene (gdh)-deficient mutant of Gluconacetobacter xylinus and its use for bioconversion of sweet potato pulp. J Biosci Bioeng 99:415–422
Song HJ, Li HX, Seo JH, Kim MJ, Kim SJ (2009) Pilot-scale production of bacterial cellulose by a spherical type bubble column bioreactor using saccharified food wastes. Korean J Chem Eng 26:141–146
Sun DP, Ma B, Zhu CL, Liu CS, Yang JZ (2010) Novel Nitrocellulose Made from Bacterial Cellulose. J Energ Mater 28:85–97
Thompson DN, Hamilton MA (2001) Production of bacterial cellulose from alternate feedstocks. Twenty-second symposium on biotechnology for fuels and chemicals. Springer, Berlin, pp 503–513
Vandamme EJ, De Baets S, Vanbaelen A, Joris K, De Wulf P (1998) Improved production of bacterial cellulose and its application potential. Polym Degrad Stabil 59:93–99
Vazquez A, Foresti ML, Cerrutti P, Galvagno M (2013) Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. J Polym Environ 21:545–554
Velásquez-Riaño M, Lombana-Sánchez N (2009) Cellulose production by Gluconacetobacter sp. GM5 in a static semi-continuous fermentation process using vinasse as culture media. Water Sci Technol 59:1195–1200
Velásquez-Riaño M, Lombana-Sánchez N, Villa-Restrepo A, Fernández-Calle P (2013) Cellulose production by Gluconacetobacter kakiaceti sp. GM5 in two batch process using vinasse as culture media. Water Sci Technol 68:1079–1084
Wu JM, Liu RH (2012) Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr Polym 90:116–121
Wu JM, Liu RH (2013) Cost-effective production of bacterial cellulose in static cultures using distillery wastewater. J Biosci Bioeng 115:284–290
Yamada Y, Yukphan P, Lan Vu HT, Muramatsu Y, Ochaikul D, Tanasupawat S, Nakagawa Y (2012) Description of Komagataeibacter gen. nov., with proposals of new combinations (Acetobacteraceae). J Gen Appl Microbiol 58:397–404
Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S, Nishi Y, Uryu M (1989) The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 25:3141–3145
Yang FC, Lin IH (1998) Production of acid protease using thin stillage froma rice-spirit distillery by Aspergillus niger. Enzyme Microb Technol 23:397–402
Yang FC, Tung HL (1996) Reuse of thin stillage from rice spirit for the culture of the yeast Saccharomyces cerevisiae. Process Biochem 31:617–620
Yang XY, Huang C, Guo HJ, Xiong L, Li YY, Zhang HR, Chen XD (2013) Bioconversion of elephant grass (Pennisetum purpureum) acid hydrolysate to bacterial cellulose by Gluconacetobacter xylinus. J Appl Microbiol 115:995–1002
Yang XY, Huang C, Guo HJ, Xiong L, Luo J, Wang B, Lin XQ, Chen XF, Chen XD (2016) Bacterial cellulose production from the litchi extract by Gluconacetobacter xylinus. Prep Biochem Biotechnol 41:39–43
Yeşilada E (1999) Genotoxic activity of vinasse and its effect on fecundity and longevity of Drosophila melanogaster. Bull Environ Contam Toxicol 63:560–566
Zeng X, Small DP, Wan W (2011) Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr Polym 85:506–513
Zimmermann T, Bordeanu N, Strub E (2010) Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohyd Polym 79:1086–1093
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Velásquez-Riaño, M., Bojacá, V. Production of bacterial cellulose from alternative low-cost substrates. Cellulose 24, 2677–2698 (2017). https://doi.org/10.1007/s10570-017-1309-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1309-7