Abstract
A fully bleached commercial acid dissolving pulp was treated with two endoglucanases, one obtained from Paenibacillus barcinonensis (B) and the other one produced from Cerrena unicolor (F) with the intention to improve cellulose reactivity and processability in the viscose process. B cellulase was tested under 120 U/g oven dry pulp (odp) and the F cellulase under two conditions, 12 and 60 U/g odp. In addition, a purification stage, consisting in a cold caustic extraction (CCE) of 9 % w/v NaOH, was applied before or after the enzymatic treatment in order to reduce the amount of hemicellulose and improve the action of enzymes. The treated pulps were evaluated in terms of brightness, viscosity, water retention value, fibre morphology, carbohydrate composition, Fock solubility and NMR. In general, results revealed that both endoglucanases improved cellulose reactivity, albeit in a different way; thus, B caused no scissions in the cellulose chain and no significant reduction in fibre length, whereas F strongly decreased viscosity, shortened fibre length and increased considerably the amount of fines. The result of applying two different doses of F cellulase was reflected on Fock solubility and fibre morphology. F60 treatment was found to give the highest value of Fock solubility and the biggest reduction of fibre length. The effect of both endoglucanases on Fock solubility was increased by introducing an earlier CCE stage. Finally, a CCE_B120 pulp with 3 % of hemicellulose and 69 % of Fock solubility was obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, pulps for chemical processing (so-called “dissolving pulps”) constitute only a small share of the global pulp production (about 3 %); however, this share is growing and dissolving pulps are playing an increasing important role in the pulp market by effect of ongoing restrictions on cotton cultivation and the growth of regenerated cellulose fibre production, mainly, in Asian countries. Also, the targeted gradual replacement of petroleum-based products with biomass-based equivalents, and the rise of speciality cellulose markets in emerging economies, are further increasing the importance of dissolving pulps (Patrick 2011; Testova et al. 2014). Most wood-based dissolving grade pulp is obtained with the acid sulphite or pre-hydrolysis kraft processes (Sixta 2006); however, the search for new technological procedures for efficient production of dissolving-grade pulps has aroused much interest in recent years.
Cellulose presents an extremely compact structure due to its supramolecular structure (viz., its intermolecular and intramolecular hydrogen bonds). The structure of cellulose consists of crystalline and non-crystalline domains (Krässig 1993; Köpcke et al. 2008). This non uniform structure is responsible for the low accessibility and reactivity of cellulose, and hence for its limited solubility in some special solvents. The crystalline domain of cellulose is highly ordered and exhibits extensive hydrogen bonding between molecular chains. As a result, cellulose can crystallize into various polymorphs. Natural cellulose (cellulose I) is the form found in nature and occurs in two allomorphs: Iα and Iβ (Nishiyama et al. 2002, 2003; Ciolacu et al. 2011). Cellulose II is the crystalline form that emerges after regeneration from different media or mercerization with aqueous sodium hydroxide (Langan et al. 2001; Nishiyama et al. 2003; Langan et al. 2005). Cellulose IIII and IIIII are obtained by treating cellulose I and II, respectively, with liquid ammonia or an organic amine (Wada et al. 2004).
Strongly alkaline pre-treatments are used in the production of carboxymethyl cellulose and viscose for textile fibres (Mozdyniewicz et al. 2013). Such treatments cause native cellulose (cellulose I) to swell and after washing shrink back to another allomorphs, cellulose II, through Na-cellulose (Klemm et al. 1998; Van de Weyenberg et al. 2006; Pönni et al. 2014). The conversion of cellulose I into Na-cellulose is fast. Cellulose II is unfavourable for the production of cellulose derivatives or regenerated cellulose such as viscose for textile fibres because it has an antiparallel orientation relative to cellulose I and a more entangled and complex hydrogen-bonding network than the natural form (Ibarra et al. 2010a); as a result, cellulose II has higher difficulty of dissolution than cellulose I (Krässig 1993).
The presence of cellulose II considerably affects the accessibility of cellulose. Thus, the reactivity of pulp is defined as the accessibility of hydroxyl groups at C6 and C2/C3 of the glucose monomer units to reactants, and dictates the processability of dissolving pulps for the viscose process (Sixta 2006; Gehmayr and Sixta 2012).
Endoglucanase (EG) treatments have become increasingly popular in recent years to improve pulp reactivity and, as a side effect, precisely adjust pulp viscosity (Henriksson et al. 2005; Engström et al. 2006; Köpcke et al. 2008; Ibarra et al. 2010a). Endoglucanases (EG) are typical multi-domain enzymes consisting of a catalytic domain that randomly cleaves β-1,4-glycosidic bonds in polysaccharide chains. These enzymes preferentially degrade amorphous cellulose located on the fibre surface and in between microfibrils, thereby increasing exposed crystalline surfaces, and the swelling ability and reactivity of pulp (Henriksson et al. 2005; Gehmayr et al. 2011). Additionally, cellulose II is attacked by endoglucanases (Rahkamo et al. 1998), which was speculated to play a role in reactivity increase of the pulp after EG treatment (Henriksson et al. 2005; Engström et al. 2006; Kvarnlöf et al. 2006; Gehmayr and Sixta 2012). However, accessibility is indeed a structural parameter that depends on the nature of the interactions between reactants and the cellulose substrate, and such interactions are influenced by the availability of the inner surface and the fibril architecture. The properties of cellulose depend directly on the moisture regain of the particular fibrous material and on environmental moisture (Ciolacu et al. 2011). One other key concept in fully understanding enzymatic degradation of cellulose is the influence of parameters such as accessibility, crystallinity and supramolecular structure of the substrate.
In this work, a commercial dried TCF bleached dissolving pulp was submitted to endoglucanase treatments in order to bring cellulose activation. Also, the effect of a cold caustic extraction (CCE) before or after the enzymatic hydrolysis treatment was studied. Cellulase action was evaluated in terms of Fock solubility, 13C-CP/MAS NMR, water retention value (WRV), fibre morphology, carbohydrate composition and cellulose degradation.
Experimental
Pulp and enzymes
A commercial dried totally chlorine-free (TCF) bleached sulphite dissolving-grade pulp obtained from a mixture of 60 % Norway spruce (Picea abies) and 40 % Scots pine (Pinus sylvestris) was used as a raw material. The pulp was cooked at Domsjö Fabriker mill (Sweden) and presented the following characteristics: 91.70 ± 0.15 % ISO brightness and 474 ± 0.7 mL/g viscosity with a carbohydrate composition as determined by high-performance liquid chromatography (HPLC) of 95.1 ± 0.3 % glucan, 2.8 ± 0.2 % manna, 0.8 ± 0.0 % xylan, 0.2 ± 0.2 % ramnan, 0.2 ± 0.2 % arabinan, 0.3 ± 0.1 % glucuronic acid and 0.2 ± 0.1 % acetic acid.
Two different endoglucanases were used in the hydrolytic treatments. The B (EC. 3.2.1.4) was produced from the newly identified species Paenibacillus barcinonensis by Universitat de Barcelona (Spain) (Chiriac et al. 2010). This endoglucanase is a modular enzyme with the structure GH9-CBM3c-Fn3-CBM3b (E1), a truncated derivative of the cellulase with the structure GH9-CBM3c (E2), and a recombinant cellulose binding module CBM3b (CBD) derived from the enzyme. E1 and E2 exhibit cellulase activity as they contain the catalytic module GH9, whereas CBD has no hydrolytic activity on cellulose (Chiriac et al. 2010, 2013). B is a family 9 enzyme and shows a processive mode of action. The cellulase activity was 60 CMCase U/mL. The F endoglucanase was supplied by Fungal Bioproducts® (Spain) and was produced from Cerrena unicolor. The activity as U/g dry enzyme powder of the cellulase preparation was: 1700 CMCase U/g and 680 U/g for the cellulase and xylanase activity on the cellulase, respectively. In both cases, the cellulase activity was determined by measuring the amount of reducing sugars released from carboxymethyl cellulose (CMC, Sigma) according to Somogyi–Nelson method (Spiro 1966). An activity unit (U) is the amount of enzyme capable of converting 1 µmol of substrate per second.
Enzymatic hydrolytic treatments and cold caustic extraction (CCE)
Prior to the hydrolytic treatments, the pulp was disintegrated according to ISO 5263-1:2004 and stored at 4 °C until use. Commercial dried TCF-bleached dissolving pulp was subjected to an enzymatic hydrolysis treatment followed by a cold caustic extraction (CCE) treatment or vice versa. Two different endoglucanases, cellulase B and cellulase F, were used at three different doses each, namely: 120, 12 and 60 U/g (odp), corresponding to samples B120, F12 and F60, respectively. The enzyme treatments were conducted in an Easydye Ahiba oscillating individual reactor from Datacolor, at 10 % (w/w) consistency in 0.05 M sodium acetate buffer (pH 5.5) at 55 °C for 1 h. The treated pulps were extensively washed with de-ionized water. A control treatment (K) under the same conditions as the enzymatic treatment but in the absence of enzyme was also developed. A cold caustic extraction (CCE) treatment was conducted in an Easydye Ahiba oscillating individual reactor from Datacolor. The treatment was performed at 10 % (w/w) consistency adjusted with 9 % (w/v) NaOH at 25 °C for 1 h. Treated pulps were washed with de-ionized water until the filtrate pH was neutral.
The treatments were named as follows: CCE, K, K_CCE, B120, CCE_B120, B120_CCE, F12, F12_CCE and F60. After each enzymatic or chemical treatment, an amount of pulp sample was withdrawn for subsequent characterization (see Fig. 1).
Analysis of pulp properties
The starting and treated pulp samples were characterized in terms of kappa number, brightness, viscosity and water retention value (WRV) according to ISO 302:2004, ISO 2470:2009, ISO 5351:2004 and ISO 23714, respectively. The degree of polymerization (DP) was calculated from intrinsic viscosity values, using the equation of Evans and Wallis (1987) (SCAN-CM 15:88):
Pulp degradation can also be assessed via the number of scissions in the cellulose chain (CS), which is defined mathematically as (Bouchard et al. 2000):
where DPo is the degree of polymerization of the initial pulp or first treatment and DP that at the end of the completed treatment.
The cellulose reactivity of the pulp samples was determined according to slightly modified version of Fock’s method (Fock 1959; Ibarra et al. 2010b). This is a micro-scale method simulating the industrial viscose process for manufacturing regenerated cellulose. Prior to analysis, the samples were dried and conditioned in a climate room at 23 °C and 50 % RH overnight. In the first step, cellulose was soaked in an alkali medium and then converted into cellulose xanthate with CS2, which makes the cellulose polymer soluble. Then, –CS2 groups were removed in dilute sulphuric acid to obtain re-precipitated cellulose fibres. Finally, regenerated cellulose was oxidized with K2Cr2O7, refluxed and titrated with Na2S2O3. Fock reactivity was expressed as regenerated cellulose yield,
where R denotes reacted cellulose (%), Y sample weight (g), M the molecular mass of glucopyranosyl residues (C6H10O5, 162 g/mol), V 1 the volume of titrant (Na2S2O3, L), C 1 the concentration of K2Cr2O7 (mol/L), C 2 that of Na2S2O3 (mol/L), a the first dilution to 100 g and withdrawal of 10 mL (10.4 g = 100/10.4 = 9.62), and b the second dilution of the sample to 100 mL and withdrawal of 40 mL = 100/40. The respective reactivity results were calculated from 10 replications per sample.
Carbohydrate composition of TCF-bleached commercial dissolving pulp and the resulted treated pulps were determined using high performance liquid chromatography (HPLC). Samples were studied on a duplicate basis using a modified version of TAPPI 249 cm-09 test method. Hydrolysis was carried out in two steps: (i) A strong hydrolysis step with concentrated sulfuric acid. Approximately 50 mg of sample with known moisture content were treated with 5 mL of H2SO4 72 % and kept at 30 °C for 1 h with gentle stirring. (ii) A mild acid step at high temperature. Tube contents were putted into 250 mL-flasks and diluted to 4 % H2SO4. Flasks were putted into an autoclave for 1 h at 103 kPa. Solution was then cooled and passed through a glass filter to remove insoluble lignin. Prior to HPLC analysis samples were filtered using a 0.45 µm pore size Whatman membrane. Chromatographic analysis was performed using a 1200 Agilent HPLC instrument furnished with a Biorad Aminex HPX-87H ion-exchange column. Concentrations were calculated by interpolation into calibration curves ran from standards of glucose, xylose, rhamnose and arabinose. In order to resolve xylose, mannose and galactose peaks, the hydrolyzed effluents were neutralized with barium carbonate (BaCO3), then were filtered through a membrane of 0.45 µm pore size and then were analyzed with a Biorad Aminex HPX-87P column. The chromatographic determination was performed under the following conditions: mobile phase 6 mmol/L (acid samples) or ultrapure water (neutralized samples); flow rate, 0.7 mL/min; column temperature, 60 °C (acid sample) or 80 °C (neutralized sample).
13C-CP/MAS NMR spectra were recorded in a Bruker AMX-300 instrument operating at 7.05 T and at 75.5 MHz for 13C. Samples were immersed in deionized water for at least 2 h. All measurements were performed at 290 ± 1 K. The magic angle spinning (MAS) rate was 4 kHz. The cross-polarization contact time was 1 ms and the recycle delay time 2.5 s. Acquisition time was 98.3 ms and sweep-width was 31.2 kHz. The number of scans was 5100. The quantification method was based on the evolution of two independent and isolated signal arising from cellulose I and II, respectively: the decrease of the height of the peak near 65 ppm, assigned to the C6 in the crystalline part of cellulose I and the increase of the peak near 107 ppm, assigned to the C1 in the crystalline part of cellulose II (Janzon et al. 2008b; Arnoul-Jarriault et al. 2014).
The morphological properties of the fibres (viz., length, width and curl), and the content in fines of the pulp samples were determined in accordance with TAPPI T 271 on a Metso kajaaniFS300 fibre analyser.
Results and discussion
During the manufacturing of cellulose derivatives and regenerated cellulose, the accessibility of cellulose is a key parameter to control, since a no homogeneous substitution of the hydroxyl groups of the cellulose chain might lead to the production of low-quality derivatives (Köpcke 2010). In the production of regenerated cellulose, the ideal achievement is to obtain a complete dissolution of the cellulose structure. However, due to its supramolecular structure is difficult to achieve. In addition, the non-uniform and compact structure of cellulose limits its dissolution to some special solvents. On the other hand, the solvents used to dissolve cellulose are usually expensive, highly toxic and have harmful effects on the environment. Hence, the great interest to improve cellulose accessibility and as a result reduce the consumption of solvents.
With the previous observations in mind, the present work deals with the intention to improve pulp reactivity. Therefore, cellulase treatments in combination with strong alkaline treatments were performed. Fock solubility, carbohydrate composition, fibre morphology and WRVof treated pulps were analysed.
Fock solubility
The reactivity of pulp was assessed using Fock’s method, which measures the amount of pulp that is dissolved in a viscose-like solution (Fock 1959; Ibarra et al. 2010a; Gehmayr and Sixta 2012). As can be seen from Table 1, both enzymes increased Fock solubility: from 67.3 % (starting pulp, Com) to 76.1 % with the F12 treatment and 95.8 % with the F60 treatment. The B120 treatment also increased Fock solubility up to 78.7 %. The high reactivity obtained with F60 is comparable to previously reported values of (Henriksson et al. 2005; Ibarra et al. 2010a) for similar softwood TCF bleached dissolving pulp. According to Henriksson et al. (2005), endoglucanase attacks the less ordered cellulose regions between and on the surface of the fibrils, leading to fibre wall swelling and therefore an increase in the accessibility to solvents.
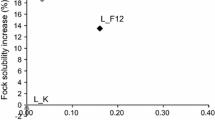
The Fock solubility increase was calculated with respect to the value obtained from the previous stage of each treatment. As can be seen from Fig. 2, a cold caustic extraction by itself or the combination of a cold caustic extraction after an enzymatic treatment diminished the Fock solubility of the final pulp due to the hornification phenomena, which is an aggregation of microfibrils. Gehmayr et al. (2011) previously found drying or strong dewatering of pulp after a treatment at high alkalinity induce the formation of molecular aggregates via inter- and intra fibrillar hydrogen bonding of the cellulose microfibrils. In other words, these high values of Fock solubility obtained by F60 and F12 treatments can be expressed as a 42 and 13 % of reactivity increase with respect to the starting pulp. Fock solubility was also considerably improved by alkaline extraction and then followed by enzymatic treatment. Although the increase in reactivity was similar with CCE_F12 and F60, the final Fock solubility level obtained was 69 % with the former and 96 % with the latter. In the case of B120, the hydrolytic treatment caused a 17 % reactivity increase with respect to commercial pulp, and led to a reactivity value of 79 %. Using a subsequent alkaline stage had an adverse effect, but inserting it prior to the hydrolytic treatment increased Fock solubility by a further 39 % reaching a 69 % value. These results can be explained by the fact that the cold caustic treatment (concentration: 8 wt%) transforms cellulose I into cellulose II and endoglucanases have greater affinity for the latter form (Engström et al. 2006; Köpcke et al. 2008; Gehmayr et al. 2011).
In Fig. 3 is shown the relation of Fock solubility increase and viscosity results. The cold caustic extraction, the control treatment and the control treatment followed by a cold caustic extraction had the same range of viscosities but slightly different reduction of Fock solubility. Comparing K and K_CCE samples, the latter suffered a higher reduction of Fock solubility, but with respect to the CCE treatment a similar solubility loss was found. This result indicates that the conditions of the control treatment had less influence on Fock solubility than the cold caustic treatment. The action of F endoglucanase can be evaluated comparing F60 and F12 samples. As can be seen, both treatments exhibited comparable viscosities, but at higher enzyme dose (F60) the Fock solubility improvement was 26 % higher with respect to the lower enzyme dose treatment (F12). This result confirms that the effect on Fock solubility was due to the enzyme action itself. Nevertheless, introducing a cold caustic extraction treatment before the F12 treatment enabled to reach practically the same Fock solubility improvement as F60 treatment, with no differences on viscosity. Therefore, the endoglucanase treatment was responsible for the Fock solubility improvement since a strong alkaline treatment provided a negative effect on that property. It is known that endoglucanases have greater affinity to cellulose II. From B endoglucanase treatment interesting conclusions can be drawn. Firstly, the B treatment and the B_CCE treatment showed the same viscosity values but a big difference on Fock solubility increase was found. This variation was caused by the strong alkaline concentration which is responsible for the transformation of cellulose I to cellulose II. The cellulose II polymorph has a closer and denser structure and as a result present lower reactivity (Sixta 2006). On the other hand, when the caustic extraction was introduced before the hydrolytic treatment (CCE_B120) a Fock solubility increase was observed with respect to the enzymatic treatment alone. However, since respective treatments did not exhibit identical viscosity values, the Fock solubility improvement cannot only be related to the enzyme action.
Carbohydrate composition of treated pulps
Carbohydrate composition was determined by HPLC, with special attention on the hemicelluloses content (Fig. 4). The control treatment presented the following carbohydrate composition: 0.5 % xylan, 0.2 % rahmnan, 0.6 % galactan, 2.8 % mannan and 0.2 % arabinan. As can be seen from Fig. 4a, with respect to control treatment, all treatments reduced the amount of xylans with exception of B120 treatment. A comparison of control treatment (K) and control treatment followed by strong alkaline treatment (K_CCE) let to distinguish the action resulted from the own alkali treatment. With K_CCE treatment the amount of xylan and mannan was reduced by 57 and 25 %, respectively. The B endoglucanase treatment did not diminish the global amount of hemicelluloses, however, with the introduction of a cold caustic extraction a variation was found. Interestingly, no differences were detected with the combination of an alkaline extraction before (CCE_B120) or after the enzymatic treatment (B120_CCE) and the final amount of hemicelluloses was 3.1 % in both cases. The action of F endoglucanase on mannan fraction was higher than the one produced by B. The CCE_F12 treatment reduced the amount of hemicelluloses to a final content of 3.4 %, affecting principally the xylan fraction. Working at higher enzyme dose (F60 treatment) did not result in higher reduction of hemicelluloses; in fact, the final amount of hemicelluloses was the same as the control treatment.
Cellulase effects
Once already detected that Fock solubility was significantly increased by the endoglucanases treatments, and a diminution of the hemicellulose content was observed by CCE treatment; the ISO brightness and viscosity properties were also measured after each enzymatic and chemical treatment (Table 2).
The results showed that both enzymes lead to substantially reduced brightness at the end of the process. Thus, the B120 and F60 treatments decreased brightness by 9.21 and 3.32 %, respectively, from the initial pulp (Com); by contrast, the control treatment (K) decreased it by only 1.1 %. Interestingly, the increase of the enzyme dose from F12 to F60 resulted in no significantly greater brightness reduction. The brightness loss observed is thought to be caused by the enzyme solution (i.e., the mixture where the enzymes were prepared) since inserting a treatment at high sodium hydroxide concentration after the enzymatic treatment (B120_CCE) raised brightness to virtually the same level as in the starting pulp. Also, B and F cellulases caused a marked increase in colour saturation (C*); however, removal of adsorbed enzyme products by subsequent cold caustic treatment decreased chroma, which suggests that cellulose chains were not damaged or modified. In order to clarify these results, the enzymes were denatured at a high temperature (>90 °C) before use and then the enzymatic treatments were performed following the same conditions. The results revealed a significant brightness loss and hence that the enzymes itself did not contribute to the adverse effect on brightness.
In general, F cellulases degraded cellulose chains and decreased viscosity, but using a high enzyme dose of F (F60 sample) resulted in no further viscosity loss with respect to F12. According to Gurnagul et al. (1992), a heterogeneous attack of cellulase on fibre wall can weaken fibres without significantly diminishing cellulose degree of polymerization or viscosity, which is consistent with our results at high enzyme doses. The B120 treatment did not cause an adverse effect on viscosity (Cadena et al. 2010; Garcia-Ubasart et al. 2013), even with the combination of a cold caustic extraction and a posterior enzymatic treatment. The treatment with buffer solution alone (K), and the cold caustic extraction applied after the buffer treatment (K_CCE), resulted in no cellulose degradation with respect to starting pulp. The gain of viscosity observed after a CCE with respect to initial pulp can be explained by the alkaline pH used, which is responsible for dissolving degraded cellulose and low molecular weight hemicelluloses.
Intrinsic viscosity data allowed us to calculate the number of cellulose chain scissions (CS) caused by the enzymatic treatments and cold caustic extraction. CS represents the number of chain cleavage steps per initial cellulose chain. As can be seen from Fig. 5a, B cellulase-treated pulp exhibited none chain scission value while F cellulase had a chain scission value around 0.2. These results indicated that B and F endoglucanases must have acted differently on cellulose chains. Cold caustic extraction (CCE) gave a negative CS value that was assumed to be zero; however, a subsequent enzymatic treatment (CCE_B120) increased CS by 35 %. This behaviour was not observed when the caustic treatment was applied after the enzymatic treatment, B120_CCE, which failed to raise the number of scissions of CCE_B120. Also, in the case of CCE_F12 a higher CS was obtained compared to the simple F12 enzymatic treatment. From these results can be concluded, that the presence of cellulose II promoted during the cold caustic extraction facilitated the action of the enzymes. On the other hand, the combined treatment CCE_F12 led to more scissions than F60 at a high enzyme dose, which further underlines the favourable effect of cold caustic extraction. Janzon et al. (2008a) reported that the conversion of cellulose I into cellulose II starts at a NaOH concentration about 6–7 wt% in wood pulps and above 10 wt% in cotton linters. Also, cellulose II has proved especially susceptible to hydrolysis by endoglucanases (Atalla 1979; Rahkamo et al. 1998). Similarly to us, Gehmayr and Sixta (2012) stated that pulps with an increasing proportion of cellulose II experienced an increasing extent of maximal chain scission and this effect may be attributed to enhanced accessibility of the cellulose II modification.
Plotting the proportion of fines as a function of the number of chain scissions with F treatments (Fig. 5b) afforded some interesting conclusions. F cellulase reduced fibre size and as a result the amount of fines (viz., fibres with a particle size between 0.0 and 0.2 mm) relative to the starting pulp increased significantly, whereas the control treatment (K) did not (results not shown). The higher the cellulase dose used was, the higher was the content in fines, which suggests increased degradation of cellulose chains. The F60 treatment increased the amount of fines by 42 and 18 % relative to initial and F12 treatment. Again, introducing a cold caustic extraction before the enzymatic treatment increased the content in fines by up to 9 % and the number of chain scissions by 46 % in CCE_F12 treated pulps relative to F12. However, a cold caustic extraction (CCE) by itself did not modify the number of chain scissions. In addition, the application of a strong alkaline treatment after the buffer treatment neither caused greater scissions than control treatment alone. In terms of fibre morphology, the fibre length and the proportion of fines of K_CCE were identical to the values for K. However, the content in fines of control treatment was increased by 13.5 % with respect to the starting pulp although no degradation of cellulose fibres was apparent from viscosity results. This result can be explained by the oscillating agitation used during the treatment that helped to release small particles. On the other hand, the combination of an enzymatic treatment and a cold caustic extraction had a significant effect: the chain scission increased and the content in fines became slightly greater. These results suggest that the high sodium hydroxide concentration contributed to fibre swollen and opened-up the cellulose structure, thereby facilitating the action of the posterior enzyme.
13C-CP/MAS NMR
The solid state 13C-NMR spectra of commercial dissolving pulp, the enzymatic treated pulps and the combination of 9 % NaOH extracted pulps before or after endoglucanase treatments are shown in Fig. 6. The C-6 signal of commercial dissolving pulp consisted of two peaks nearly identical heights at 64 and 66 ppm. This spectrum suggests a small presence of cellulose II and this cellulose polymorph is more susceptible to enzymatic hydrolysis by endoglucanases (Krässig 1993; Teleman 2001). Therefore, B endoglucanase treatment was dominated by cellulose I signal at 66 ppm while the F12 treatment increased the signal intensity but in less extent. The 9 % NaOH extraction presented different polymorphic form from commercial dissolving pulp, but no signals of cellulose II. The most pronounced structural changes were detected with CCE_F12 sample. A high presence of cellulose II was apparent by a perceptible shoulder at 108 ppm of C-1 signal and an increased C-6 signal intensity at 64 ppm.
Fiber morphology
The outcomes of the F cellulase treatments were examined in greater detail (Table 3). The water retention value (WRV) provides information about the integral pore volume of a pulp and the content of hydrophilic groups (Gehmayr et al. 2011). Fines are thought to increase WRV improvement since these particles have more accessible –OH groups and plays a major role in dewatering. Treating commercial dissolving pulp with the lowest dose of F cellulase (F12 treatment), reduced fibre length and substantially increased the proportion of fines, and as a result of that the WRV rose to 1.1 %. By contrast, a high enzyme dose (F60 treatment) had virtually no effect on WRV even though fibre length was shortened by 57 %, and the amount of fines increased to 41 %, with respect to commercial dissolving pulp. The conditions used in the control treatment, i.e. oscillating agitation and the buffer solution had slight effect on fibre morphology. Introducing a cold caustic extraction (K_CCE) stage resulted in no change in fibre morphology or WRV relative to the control treatment (K). The greatest change in WRV was produced by CCE_F12, which suggests increased hydrogen bonding; fibres were shortened by 43 % and the content in fines grew by up to 34 %, with respect to starting pulp. In terms of fibre width, a slight increase was observed with F12 treatment, but a substantial greater size was found with F60 treatment, with respect to commercial pulp. Interestingly, K_CCE, CCE_F12 and F60 presented the same value of fibre width. A comparison of K with K_CCE on the one hand, and F12 with CCE_F12 on the other hand, revealed that the incorporation of an alkaline step into the process enhanced the fibre width.
Combining fibre length results with WRV results (Fig. 7) some conclusions can be drawn. In general, the lower the fibre length resulted, the higher the WRV became, with the exception of F60 treatment. Focusing the attention on the control treatment followed by a caustic extraction (K_CCE), it failed to improve WRV and to alter cellulose fibre size relative to control treatment (K). The CCE_F12 suffered a slight improvement in WRV but a 10 % reduction in fibre length, with respect to F12 treatment; although these differences were more significant with respect to starting pulp.
Figure 8 represents the population fractions determined according to TAPPI standard. As can be seen, the proportion of fibres in the length range of 0.5–3.2 mm was considerably diminished when the F60 treatment was performed, and thereby the content of fines increased by up to 41.3 % (length range of 0.0–0.2 mm) with respect to starting pulp. However, the effect caused by the enzyme was not detected in the length range of 0.2–0.5 mm since the same population as starting pulp was obtained. After F60 treatment, the increase of fines was followed by CCE_F12 and then F12. The CCE_F12 treatment increased the amount of fines by up to 35 % and the F12 treatment by up to 28 %. F12 and CCE_F12 treatments presented similar proportion of fibres in the length range of 0.2–1.2 mm, but the differences in the long length fraction became more accentuated. As observed earlier, the control treatment had similar population fractions as starting pulp, except for slight difference in the content of fines and therefore different proportion of fibres in the range of 0.2–1.2 mm. These results can be explained due to the oscillating agitation used in the treatments which facilitated the release of small particles.
The microscopy images (Fig. 9) provided visual confirmation of the previous results. Thus, the F60 sample exhibited a high content in fines but also in long fibers. In addition, slight difference between F12 and CCE_F12 were detected.
Conclusions
Two different cellulases, named as B and F, were used to increase the reactivity according to Fock method of dried bleached commercial dissolving pulp with the intention to bring a satisfactory advantage to the viscose process. Working at high enzyme dose, F60 treatment, provided a reactivity increase of 42 %, which belongs to a 96 % Fock solubility. In terms of fibre morphology, the amount of fines increased significantly after F60 treatment, which is in connection with viscosity loss. The F12 treatment provided a 13 % Fock solubility improvement. Interestingly, applying a cold caustic extraction (CCE) treatment before the hydrolytic treatment (CCE_F12) resulted in further increased Fock solubility than an enzymatic hydrolysis alone (F12), although the viscosity loss was more pronounced. In terms of WRV, this property was also improved with the combination of a cold caustic extraction and hydrolytic treatment (CCE_F12). The B enzyme did not cause viscosity loss, although the Fock solubility was improved by 17 % with respect to commercial pulp, which corresponds to a 79 % of Fock solubility. The NMR results revealed that the starting pulp had already an important proportion of cellulose II, but after the CCE_F12 treatment this amount of cellulose II became higher.
Abbreviations
- B120:
-
B endoglucanase at 120 U/g
- B120_CCE:
-
B endoglucanase at 120 U/g + cold caustic extraction
- CCE:
-
Cold caustic extraction
- CCE_B120:
-
Cold caustic extraction + B endoglucanase at 120 U/g
- CCE_F12:
-
Cold caustic extraction + F endoglucanase at 12 U/g
- CS:
-
Chain scission
- DP:
-
Degree of polymerization
- EG:
-
Endoglucanase
- F60:
-
F endoglucanase at 60 U/g
- F12:
-
F endoglucanase at 12 U/g
- K:
-
Control treatment
- K_CCE:
-
Control treatment + cold caustic extraction
- PO:
-
Hydrogen peroxide reinforced with pressurized oxygen
- TCF:
-
Totally chlorine free
- n.d.:
-
Not determined
- odp:
-
Oven dried pulp
- WRV:
-
Water retention value
- 13C-CP/MAS NMR:
-
Solid state cross polarization/magic angle spinning carbon 13 nucelar magnetic resonance
References
Arnoul-Jarriault B, Lachenal D, Chirat C, Heux L (2014) Upgrading softwood bleached kraft pulp to dissolving pulp by cold caustic treatment and acid-hot caustic treatment. Ind Crops Prod. doi:10.1016/j.indcrop.2014.09.051
Atalla RH (1979) Conformational effects of the hydrolysis of cellulose. Adv Chem Ser 181:55–69
Bouchard J, Morelli E, Berry RM (2000) Gas phase addition of solvent to ozone bleaching of kraft pulp. J Pulp Pap Sci 26:30–35
Cadena EM, Chriac AI, Javier Pastor FI et al (2010) Use of cellulases and recombinant cellulose binding domains for refining TCF kraft pulp. Biotechnol Prog 26:960–967. doi:10.1002/btpr.411
Chiriac AI, Cadena EM, Vidal T et al (2010) Engineering a family 9 processive endoglucanase from Paenibacillus barcinonensis displaying a novel architecture. Appl Microbiol Biotechnol 86:1125–1134. doi:10.1007/s00253-009-2350-8
Chiriac AI, Pastor FIJ, Popa VI et al (2013) Changes of supramolecular cellulose structure and accessibility induced by the processive endoglucanase Cel9B from Paenibacillus barcinonensis. Cellulose 21:203–219. doi:10.1007/s10570-013-0118-x
Ciolacu D, Pitol-Filho L, Ciolacu F (2011) Studies concerning the accessibility of different allomorphic forms of cellulose. Cellulose 19:55–68. doi:10.1007/s10570-011-9620-1
Engström A-C, Ek M, Henriksson G (2006) Improved accessibility and reactivity of dissolving pulp for the viscose process: pretreatment with monocomponent endoglucanase. Biomacromolecules 7:2027–2031
Evans R, Wallis AFA (1987) Comparison of cellulose molecular weights determined by high performance size exclusion chromatography and viscometry. In: 4th International symposium wood pulping chemistry, pp 201–205
Fock W (1959) A modified method for determining the reactivity of viscose-grade dissolving pulps. Papier 13:92–95
Garcia-Ubasart J, Torres AL, Vila C et al (2013) Biomodification of cellulose flax fibers by a new cellulase. Ind Crops Prod 44:71–76. doi:10.1016/j.indcrop.2012.10.019
Gehmayr V, Sixta H (2012) Pulp properties and their influence on enzymatic degradability. Biomacromolecules 13:645–651
Gehmayr V, Schild G, Sixta H (2011) A precise study on the feasibility of enzyme treatments of a kraft pulp for viscose application. Cellulose 18:479–491. doi:10.1007/s10570-010-9483-x
Gurnagul N, Page D, Paice M (1992) The effect of cellulose degradation on the strength of wood pulp fibres. Nord Pulp Pap Res J 7:152–154
Henriksson G, Christiernin M, Agnemo R (2005) Monocomponent endoglucanase treatment increases the reactivity of softwood sulphite dissolving pulp. J Ind Microbiol Biotechnol 32:211–214. doi:10.1007/s10295-005-0220-7
Ibarra D, Köpcke V, Ek M (2010a) Behavior of different monocomponent endoglucanases on the accessibility and reactivity of dissolving-grade pulps for viscose process. Enzyme Microb Technol 47:355–362. doi:10.1016/j.enzmictec.2010.07.016
Ibarra D, Köpcke V, Larsson PT et al (2010b) Combination of alkaline and enzymatic treatments as a process for upgrading sisal paper-grade pulp to dissolving-grade pulp. Bioresour Technol 101:7416–7423. doi:10.1016/j.biortech.2010.04.050
Janzon R, Puls J, Bohn A et al (2008a) Upgrading of paper grade pulps to dissolving pulps by nitren extraction: yields, molecular and supramolecular structures of nitren extracted pulps. Cellulose 15:739–750. doi:10.1007/s10570-008-9224-6
Janzon R, Saake B, Puls J (2008b) Upgrading of paper-grade pulps to dissolving pulps by nitren extraction: properties of nitren extracted xylans in comparison to NaOH and KOH extracted xylans. Cellulose 15:161–175. doi:10.1007/s10570-007-9154-8
Klemm D, Philipp B, Heinze T et al (1998) General considerations on structure and reactivity of cellulose. Compr Cellul Chem 1:9–29
Köpcke V (2010) Conversion of wood and non-wood paper-grade pulps to dissolving-grade pulps. Doctoral Thesis, KTH Chemical Science and Engineering
Köpcke V, Ibarra D, Ek M (2008) Increasing accessibility and reactivity of paper grade pulp by enzymatic treatment for use as dissolving pulp. Nord Pulp Pap Res J 23:363–368
Krässig HA (1993) Cellulose-structure, accessibility and reactivity, vol 11. Gordon and Breach Science, New York
Kvarnlöf N, Germgård U, Jönsson LJ, Söderlund C-A (2006) Enzymatic treatment to increase the reactivity of a dissolving pulp for viscose preparation. Appita J 59:242–246
Langan P, Nishiyama Y, Chanzy H (2001) X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2:410–416. doi:10.1021/bm005612q
Langan P, Sukumar N, Nishiyama Y, Chanzy H (2005) Synchrotron X-ray structures of cellulose Iβ and regenerated cellulose II at ambient temperature and 100 K. Cellulose 12:551–562. doi:10.1007/s10570-005-9006-3
Mozdyniewicz DJ, Nieminen K, Sixta H (2013) Alkaline steeping of dissolving pulp. Part I: cellulose degradation kinetics. Cellulose 20:1437–1451. doi:10.1007/s10570-013-9926-2
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082. doi:10.1021/ja0257319
Nishiyama Y, Kim U-J, Kim D-Y et al (2003) Periodic disorder along ramie cellulose microfibrils. Biomacromolecules 4:1013–1017. doi:10.1021/bm025772x
Patrick K (2011) Dissolving pulp gold rush in high gear. Paper360 8–12. http://www.tappi.org/Hide/The-Dissolving-Pulp-Gold-Rush.aspx
Pönni R, Pääkkönen T, Nuopponen M et al (2014) Alkali treatment of birch kraft pulp to enhance its TEMPO catalyzed oxidation with hypochlorite. Cellulose 21:2859–2869. doi:10.1007/s10570-014-0278-3
Rahkamo L, Viikari L, Buchert J et al (1998) Enzymatic and alkaline treatments of hardwood dissolving pulp. Cellulose 5:79–88. doi:10.1023/A:1009268713757
Sixta H (2006) Handbook of pulp. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim
Spiro R (1966) Analysis of sugars found in glycoproteins. Methods Enzymol 566:7–9
Teleman LPT (2001) On the accessibility and structure of xylan in birch kraft pulp. Cellulose 8:209–215. doi:10.1023/A:1013195030404
Testova L, Borrega M, Tolonen LK et al (2014) Dissolving-grade birch pulps produced under various prehydrolysis intensities: quality, structure and applications. Cellulose 21:2007–2021. doi:10.1007/s10570-014-0182-x
Van de Weyenberg I, Chi Truong T, Vangrimde B, Verpoest I (2006) Improving the properties of UD flax fibre reinforced composites by applying an alkaline fibre treatment. Compos Part A Appl Sci Manuf 37:1368–1376. doi:10.1016/j.compositesa.2005.08.016
Wada M, Chanzy H, Nishiyama Y, Langan P (2004) Cellulose III I crystal structure and hydrogen bonding by synchrotron X-ray and neutron fiber diffraction. Macromolecules 37:8548–8555. doi:10.1021/ma0485585
Acknowledgments
The authors thank the "Ministerio de Economía y Competitividad" of Spain for their support in this work under the projects BIOSURFACEL CTQ2012-34109 (funding also from the "Fondo Europeo de Desarrollo Regional FEDER”) and BIOPAPμFLUID CTQ2013-48995-C2-1-R. The authors are grateful to the consolidated group with the Universitat de Barcelona (UB) AGAUR 2014 SGR 534. The authors would like to thank DÖMSJO (Sweden) for providing the starting pulp, Fungal Bioproducts (Spain) for kindly supplying the F cellulase and the Department of Microbiology (University of Barcelona, Spain) for warmly providing the B cellulase.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Quintana, E., Valls, C., Vidal, T. et al. Comparative evaluation of the action of two different endoglucanases. Part I: On a fully bleached, commercial acid sulfite dissolving pulp. Cellulose 22, 2067–2079 (2015). https://doi.org/10.1007/s10570-015-0623-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0623-1