Abstract
A TCF sulphite pulp, bleached at the laboratory scale with a laccase–violuric acid system and complemented with a pressurized hydrogen peroxide stage, was treated with two endoglucanases, one obtained from Paenibacillus barcinonensis (B) and the other one produced from Cerrena unicolor (F) to improve cellulose reactivity. The treated pulps were evaluated in terms of brightness, viscosity, α-cellulose, water retention value, fibre morphology, Fock solubility, NMR and carbohydrate composition of pulps and liquors. Results revealed that both endoglucanases improved cellulose reactivity, albeit in a different way; thus, B caused no scissions in the cellulose chain and no significant reduction in fibre length, whereas F decreased viscosity and shortened fibre length, leading to lower reactivity value. The liquor composition of soluble carbohydrates released by the enzymatic treatments revealed the B had a processive mode of action since short oligosaccharides, cellobiose and glucose, were obtained. F hydrolysed, from high to low concentration, cellobiose, glucose and cellotriose. Importantly, environmentally friendly dissolving pulp with 90 % Fock solubility was obtained, combining two enzymatic treatments: a laccase–mediator system and then a cellulase from P. barcinonensis (B). In order to improve the quality of final dissolving pulp, a pulp purification step was introduced before the B endoglucanase treatment. The cold caustic extraction lead to reduce the amount of hemicelluloses by 42 % with respect to biobleached pulp, but Fock solubility was also reduced. However, complementing the purification step with F treatment, reduced the amount of hemicelluloses but also improved Fock solubility by 17 %, although some presence of cellulose II was detected by NMR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Finite fossil fuel reserves and their negative impact on the environment require the shift from fossil to bio-renewable carbon feedstock; therefore lignocellulosic biomass is a promising, renewable and globally accessible alternative to petroleum derived compounds. Cellulose, the most abundant polysaccharides in plant biomass, is a linear homopolymer consisting of anhydro-β-d-glucopyranose units (AGU) that are linked together by (1-β-4) glycosidic bonds. Every AGU contains three hydroxyl groups in the positions C2, C3, and C6. All of the three hydroxyl groups are involved in this hydrogen bond network. In addition to the hydrogen bonding, hydrophobic interactions have been suggested to be present in the structures cellulose forms (Lindman et al. 2010; Glasser et al. 2012; Medronho et al. 2012). The molecular structure of cellulose is simple but the most exceptional properties are due to its supramolecular structure. Cellulose chains have an intense tendency to aggregate. During the cell wall biosynthesis, Van der Waals forces and hydrogen bonding between hydroxyl groups and oxygen from adjacent molecules promote parallel stacking of multiple cellulose chains forming elementary fibrils that aggregate into larger structural units named microfibrils (Moon et al. 2011).
Most cellulolytic microorganisms produce a battery of cellulases which act synergistically to solubilize crystalline cellulose (Lynd et al. 2002; Chiriac et al. 2010, 2013). Hydrolysis of cellulose [i.e. cleavage of the (1-β-4) glycosidic bonds] depends on three classes of enzymes: cellobiohydrolases (CBHs; EC 3.2.1.91), endoglucanases (EGs, EC 3.2.1.4) and β-glucosidases (EC 3.2.1.21). Cellobiohydrolases remove cellobiose from either the non-reducing or the reducing ends of chain with higher apparent activity on crystalline cellulose, and as a result, a rapid production of soluble sugars but slow decrease of the degree of polymerization (DP) is observed. Endoglucanases are typical multi-domain enzymes consisting of a catalytic domain that randomly cleaves β-1,4-glycosidic bonds in the middle of the polysaccharide chains and typically act on more irregular physical structures of the substrates, giving a rapid reduction of the DP but slow release of soluble sugars from crystalline cellulose (Ek et al. 2009). The degradation of amorphous cellulose located on the fibre surface and in between microfibrils, resulted in increasing exposed crystalline surfaces, and improving the swelling ability and reactivity of pulp (Henriksson et al. 2005; Gehmayr et al. 2011). Beta-glucosidases (β-d-glucopyranoside glucohydrolases) are enzymes that hydrolyze glycosidic bonds to release nonreducing terminal glucosyl residues from glycosides and oligosaccharides (Cairns and Esen 2010).
The bioconversion process uses enzymes, such as cellulase, to break down cellulose into sugars that can be later fermented into ethanol. Therefore, following the biorefinery concept, endoglucanases are also used for upgrading paper-grade pulps to dissolving-grade pulp, and obtain hemicellulose free dissolving pulp as a primary product, but also lignin, bioethanol and a number of complementary products. The removal of hemicelluloses from paper kraft pulp is the main challenge to convert it into dissolving pulp. Cold caustic extraction (CCE), commonly used as post treatment to PHK pulp for acetate grade dissolving pulp production can dissolve selectively hemicelluloses (Sixta 2006; Arnoul-Jarriault et al. 2014). The dissolution of hemicelluloses during CCE is related to structural change of cellulose pulps when they are subjected to concentrated sodium hydroxide solution (>8 % w/v).
According to FAO (2012), nowadays, pulps for chemical processing, or dissolving pulps constitute only a small share of the global pulp production (around 3 % or 4.22 × 106 ton) but this share is increasing and prospective consumer markets indicate that will continue in subsequent decades. Interestingly, EG treatments have become more and more popular in recent years to improve pulp reactivity and, as a side effect, precisely adjust pulp viscosity (Henriksson et al. 2005; Engström et al. 2006; Köpcke et al. 2008; Ibarra et al. 2010a). Additionally, cellulose II is attacked by endoglucanases (Rahkamo et al. 1998), which was speculated to play a role in reactivity increase of the pulp after EG treatment (Henriksson et al. 2005; Engström et al. 2006; Kvarnlöf et al. 2006; Gehmayr and Sixta 2012). However, cellulose II is unfavourable for the production of cellulose derivatives or regenerated cellulose such as viscose for textile fibres because it has an antiparallel orientation relative to cellulose I and a more entangled and complex hydrogen-bonding network than the natural form (Ibarra et al. 2010a); as a result, the presence of cellulose II considerably affects the accessibility of cellulose.
On the other side, the introduction of new technologies to bleach dissolving pulps using enzymatic treatments and complemented with treatments based on oxygen-derived compound can be an alternative to traditional bleaching processes, such as elemental chlorine free (ECF) and totally chlorine free (TCF) processes. In a previous work, a laccase–mediator system (LMS) proved to be effective to bleach never-dried softwood sulphite pulp (Quintana et al. 2013). The proposed LVAQPO sequence provided acceptable results in terms of dissolving pulp requirements (Quintana et al. 2015) and had a positive contribution from an environmentally point of view. However, in the direction of improving pulp reactivity and the quality of the final product (i.e. reduce the amount of hemicelluloses) further treatments were required. The remarkable results obtained from commercial dissolving pulp and endoglucanase treatments approved the idea to conduct a similar study but on biobleached dissolving pulp (see Quintana et al. under review). Therefore, in this work, cellulose activation by two endoglucanase enzymes one obtained from Paenibacillus barcinonensis (B) and the other from Cerrena unicolor (F) was examined on biobleached sulphite pulp. Cellulase action was evaluated in terms of Fock solubility, 13C-CP/MAS NMR, water retention value (WRV), fibre morphology, cellulose degradation and carbohydrate composition of pulp. Another important contribution of the present study was to examine the composition of dissolved carbohydrate present in the liquors. Understanding the mode of action of the respective cellulases, in terms of pulp activation and cellulose modification, provided interesting information in view of a potential industrial application. The work was completed with a pulp purification step, with 9 % w/v NaOH, at 25 °C, before the endoglucanase treatments. The combination of a CCE followed by a hydrolytic treatment was evaluated in terms of Fock solubility, hemicellulose content and 13C-CP/MAS NMR.
Experimental
Pulp and enzymes
Unbleached sulphite cellulose obtained from a mixture of 60 % Norway spruce (Picea abies) and 40 % Scots pine (Pinus sylvestris) was used as a raw material. The pulp was cooked at Domsjö Fabriker mill (Sweden) and the characteristics were as follows: 4.2 ± 0.2 kappa number, 61.25 ± 0.6 % ISO brightness and 511 ± 11 mL/g viscosity, and a carbohydrate content as determined by HPLC of 90.2 ± 0.38 % glucan, 4.3 ± 0.1 % mannan, 2.1 ± 0.0 % xylan, 0.8 ± 0.0 % glucuronic acid, 0.2 ± 0.0 % arabinan and 0.04 ± 0.01 % acetyl groups. This pulp was biobleached using a laccase treatment (an L sequence) before the hydrolytic treatment with endoglucanases.
The biobleaching treatment involved laccase from Trametes villosa (TvL), which was supplied by Novozymes® (Denmark) with a laccase activity of 746 U/mL. Its activity was measured as the extent of oxidation of 5 mM 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) to its cation radical (ε436 = 29,300 M−1 cm−1) in 0.1 M sodium acetate buffer (pH 5) at 24 °C. One activity unit (U) was defined as the amount of enzyme converting 1 µmol of ABTS per min. Violuric acid (VA), the mediator used for the bleaching treatment, was purchased from Sigma-Aldrich and used as received.
Two different endoglucanases were used in the hydrolytic treatments. The B (EC. 3.2.1.4) was produced from the newly identified species Paenibacillus barcinonensis by Universitat de Barcelona (Spain) (Chiriac et al. 2010). This endoglucanase is a modular enzyme with the structure GH9-CBM3c-Fn3-CBM3b (E1), a truncated derivative of the cellulase with the structure GH9-CBM3c (E2), and a recombinant cellulose binding module CBM3b (CBD) derived from the enzyme. E1 and E2 exhibit cellulase activity as they contain the catalytic module GH9, whereas CBD has no hydrolytic activity on cellulose (Chiriac et al. 2010, 2013). B is a family 9 enzyme and shows a processive mode of action. A cellulase activity of 60 CMCase U/mL was determined by measuring the amount of reducing sugars released from carboxymethyl cellulose (CMC, Sigma) according to Somogyi–Nelson method (Spiro 1966). An activity unit (U) is the amount of enzyme capable of converting 1 µmol of substrate per second. The F endoglucanase was supplied by Fungal Bioproducts® (Spain) and was produced from Cerrena unicolor. The activity as U/g dry enzyme powder of the cellulase preparation was: 1700 CMCase U/g and 680 U/g for the cellulase and xylanase activity on the cellulase, respectively. The activity was also determined in our laboratory using the Somogyi–Nelson method.
Biobleaching sequence (L) for unbleached sulphite pulp
Prior to the bleaching treatment, unbleached sulphite fibres were conditioned at pH 4 adjusted with H2SO4, stirred at 2 % pulp consistency for 30 min and washed with de-ionized water in a glass filter funnel. This step was needed to remove contaminants and metals, and also to bring the pulp to the pH required for the enzymatic treatment.
The bleaching process was conducted at the laboratory scale. A TCF biobleaching sequence including a laccase–mediator treatment was applied to unbleached sulphite cellulose. The sequence was designated LVAPO, where L denotes the enzymatic treatment and PO a hydrogen peroxide stage reinforced with oxygen. The enzymatic stage (L) was carried out with the laccase–violuric acid system in an oxygen pressurized reactor (0.6 MPa) at stirring rate of 30 rpm, using 50 mM sodium tartrate buffer (pH 4) to adjust 5 % (w/w) pulp consistency, at 50 °C for 4 h. The enzyme dose was 20 U/g odp (oven dry weight of pulp) of laccase and 1.5 % odp of violuric acid (Quintana et al. 2013). The enzymatic treatment was followed by a chemical bleaching stage involving alkaline hydrogen peroxide reinforced with oxygen. PO was carried out at 5 % (w/w) consistency in an oxygen pressurized (0.6 MPa) reactor at a stirring rate of 30 rpm under the following conditions: 10 % odp H2O2, 1.5 % odp NaOH, 0.3 % odp DTPA and 0.2 % odp MgSO4, at 90 °C for 1 h. Subsequently, the treated pulps were extensively washed with de-ionized water and stored until use.
Enzymatic hydrolytic treatments and cold caustic extraction
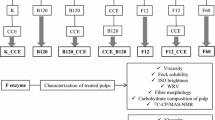
The resulting biobleached pulp (LVAPO) was subjected to enzymatic hydrolysis with the two endoglucanases, B and F. The former enzyme was used at 120 U/g odp (L_B120 treatment) and the latter at 12 U/g odp (L_F12 treatment). An amount of 20 g of dry pulp at 10 % consistency in 0.05 M sodium acetate buffer at pH 5.5 at 55 °C was treated with each enzyme for 1 h in each run (Cadena et al. 2010). The enzymatic treatments were performed in polyethylene bags that were placed in a laboratory water bath. The samples were periodically kneaded. The reaction was stopped by washing the pulp with de-ionized water in a porous glass filter funnel of porosity grade 2. A control treatment (L_K) was also performed under the same conditions but in the absence of enzyme. In parallel, the biobleached pulp (LVAPO) was also treated with a CCE in an Easydye Ahiba oscillating individual reactor from Datacolor. The treatment was performed at 10 % (w/w) consistency adjusted with 9 % (w/v) NaOH at 25 °C for 1 h. Then the hydrolytic treatments with B or F enzymes were conducted, following the same conditions described earlier but using an Easydye Ahiba oscillating individual reactor (see Schema 1).
Analysis of pulp properties
The starting and treated pulp samples were characterized in terms of kappa number, brightness, viscosity, α-cellulose and WRV according to ISO 302:2004, ISO 2470:2009, ISO 5351:2004, TAPPI method T-203 cm-99 and ISO 23714, respectively. The bleaching process was also characterized in terms of chroma (C *), which is the perpendicular distance of a point from the lightness axis [C * = (a *2 + b *2)1/2] and represents the amount of colour of a sample. The DP was calculated from intrinsic viscosity values, using the equation of Evans and Wallis (1987) (SCAN-CM 15:88):
Pulp degradation can also be assessed via the number of scissions in the cellulose chain (CS), which is defined mathematically as (Bouchard et al. 2000):
where DPo is the degree of polymerization of the initial pulp or previous stage and DP that at the end of any chemical or enzymatic treatment.
The cellulose reactivity of the pulp samples was determined according to slightly modified version of Fock’s method (Fock 1959; Ibarra et al. 2010b). This is a micro-scale method simulating the industrial viscose process for manufacturing regenerated cellulose. Prior to analysis, the samples were dried and conditioned in a climate room at 23 °C and 50 % RH overnight.
Carbohydrate composition of initial (TCF-bleached dissolving pulp and unbleached sulphite pulp) and treated pulps was determined using high performance liquid chromatography (HPLC). Samples were studied on a duplicate basis using a modified version of TAPPI 249 cm-09 test method. Hydrolysis was carried out in two steps: (1) A strong hydrolysis step with concentrated sulfuric acid. Approximately 50 mg of sample with known moisture content were treated with 5 mL of H2SO4 72 % and kept at 30 °C for 1 h with gentle stirring. (2) A mild acid step at high temperature. Tube contents were putted into 250 mL-flasks and diluted to 4 % H2SO4. Flasks were putted into an autoclave for 1 h at 103 kPa. Solution was then cooled and passed through a glass filter to remove insoluble lignin. Prior to HPLC analysis samples were filtered using a 0.45 µm pore size Whatman membrane. Chromatographic analysis was performed using a 1200 Agilent HPLC instrument furnished with a Biorad Aminex HPX-87H ion-exchange column. Concentrations were calculated by interpolation into calibration curves ran from standards of glucose, xylose, rhamnose and arabinose. In order to resolve xylose, mannose and galactose peaks, the hydrolyzed effluents were neutralized with barium carbonate (BaCO3), then were filtered through a membrane of 0.45 µm pore size and then were analyzed with a Biorad Aminex HPX-87P column. The chromatographic determination was performed under the following conditions: mobile phase 6 mmol/L (acid samples) or ultrapure water (neutralized samples); flow rate, 0.7 mL/min; column temperature, 60 (acid samples) or 85 °C (neutralized samples).
13C-CP/MAS NMR spectra were recorded in a Bruker AMX-300 instrument operating at 7.05 T and at 75.5 MHz for 13C. Samples were immersed in deionized water for at least 2 h. All measurements were performed at 290 ± 1 K. The magic angle spinning (MAS) rate was 4 kHz. The cross-polarization contact time was 1 ms and the recycle delay time 2.5 s. Acquisition time was 98.3 ms and sweep-width was 31.2 kHz. The number of scans was 5100. The quantification method was based on the evolution of two independent and isolated signal arising from cellulose I and II, respectively: the decrease of the height of the peak near 65 ppm, assigned to the C6 in the crystalline part of cellulose I and the increase of the peak near 107 ppm, assigned to the C1 in the crystalline part of cellulose II (Janzon et al. 2008b; Arnoul-Jarriault et al. 2014).
The morphological properties of the fibres (viz., length, width and curl), and the content in fines of the pulp samples were determined in accordance with TAPPI T 271 on a Metso kajaaniFS300 fibre analyser. All samples were analysed in duplicate. Surface SEM images of the handsheets were taken on a JEOL JSM-6400 microscope. Samples were placed on the SEM sample holding stub with the aid of conductive double side sticky carbon film and coated with Au/Pd alloy prior to analysis.
Effluent properties
Dissolved carbohydrates present in the liquors released from the cellulase treatments of biobleached dissolving pulps were quantified by HPLC (Agilent 1200 HPLC instrument), using a Biorad Aminex HPX-87H ion-exchange resin column that affords the separation of glucose, xylose and arabinose. Prior to analysis, the liquors were subjected to acid hydrolysis with 4 % H2SO4 and the flasks placed in an autoclave at 103 ± 7 kPa for 20 min in order to convert oligomers into monomers for easier on-column separation. Then, the hydrolyzed solutions were filtered through Whatman membranes of 0.45 µm pore size and the chromatographic determination was performed under the following conditions: mobile phase, 6 mmol/L sulphuric acid; flow rate, 0.7 mL/min; column temperature, 60 °C.
The concentration of oligosaccharides present in the treatment liquors was also determined by HPLC, using a Biorad Aminex HPX-42A ion-exchange column (Garcia-Ubasart et al. 2013). Effluents were filtered using a 0.45 µm pore size Whatman membrane and their pH was neutralized (to pH 7) using HCl or NaOH. The determination was performed under the following conditions: mobile phase, ultrapure water; flow rate, 0.35 mL/min; column temperature, 65 °C. Identification and quantification of compounds was done by interpolation into calibration curves run from standards.
Results and discussion
In previous works (Quintana et al. 2013, 2015), sulphite pulp was satisfactorily biobleached by a combination of a laccase–violuric acid treatment, chelant stage and a pressurized hydrogen peroxide treatment. These biobleached pulps (LVAQPO) were characterized in terms of Fock solubility, viscosity, ISO brightness, alkali resistance, α-cellulose, metal ion content and thermal degradation by TGA. The pulps exhibited acceptable market dissolving pulp characteristics but in the direction of improving pulp reactivity and the quality of the final product (i.e. lower amount of hemicelluloses) further treatments were required. Therefore, this part of the work want to elucidate the effect of two endoglucanases, one from Paenibacillus barcinonensis (B) and the other one from Cerrena unicolor (F), on biobleached sulphite pulps (LVAPO). Furthermore, the outstanding results obtained from a bleached commercial dissolving pulp, CCE and the respective endoglucanase treatments (Quintana et al. under review), supported the idea to conduct the same investigation with LVAPO pulp. Therefore, the cellulase treatments were compared with those where a CCE with 9 % (w/v) of NaOH was introduced before the hydrolytic treatment. Based on the previous results, the K_CCE treatment was eliminated since no differences were observed with respect to control treatment (K). As a novelty, the carbohydrate composition of liquors was analyzed and leads to understand the mechanism of action of each enzyme.
Fock solubility
Table 1 shows the Fock solubility results. The initial pulp had a Fock solubility value of 76.6 %, while L_F12 treatment had an 88 % and the L_B120 treatment reached a Fock solubility value of 91 %.
In Fig. 1 is shown the relation of Fock solubility increase and chain scission values (CS), calculated from the viscosity results. As expected, the control cellulase treatment did not undergo Fock solubility improvement, although the viscosity value was slightly different from biobleached pulp. With respect to biobleached pulp, the L_B120 improved Fock solubility by 18.7 % with no variation on viscosity. Therefore, this change was due to the action of the own endoglucanase treatment. The L_F12 treatment also suffered a Fock solubility increase but a viscosity loss with respect to initial pulp was found. Therefore, the improvement on Fock solubility cannot only be related to the enzyme action.
The relationship between pulp solubility according to Fock method and chain scission values provided useful information to understand the action that each enzyme caused on biobleached cellulose fibres. Special attention had the B endoglucanase which provided the highest increase in Fock solubility (18.7 % respect to LVAPO) even though CS was virtually zero, suggesting that this enzyme produced a fibrillated effect rather than cutting the fibres, as previously demonstrated by some authors (Cadena et al. 2010; Garcia-Ubasart et al. 2013). With F endoglucanase, the L_F12 improved Fock solubility by 13.5 % and exhibited slightly greater CS number of 0.16 with respect to starting pulp, which is in agreement with its slightly greater viscosity loss. As can be seen from Table 1 and Fig. 1, the buffer solution used in the control treatment (L_K) did not contribute to improve Fock solubility or shorten fibres and CS value remained 0.
Carbohydrate composition was determined by HPLC, with special interest on the hemicelluloses content (Fig. 2a). The hemicellulose composition of biobleached pulp (LVAPO) was as followed: 2.4 % of xylan, 0.3 % of mannan, 3.0 % galactan and 6.0 % mannan. As can be seen, B and F endoglucanases exhibited the same pattern of action for rhamnan and galactan but acted differently on mannan and xylan fraction. With B endoglucanase treatment, the hemicellulose content was diminished by 37 % and the final amount of hemicelluloses was 7.4 %. As can be seen, the highest reduction was observed with xylan fraction (77 %), then galactan (47 %) and finally mannan (19 %), with respect to initial pulp. The amount of rhamnan was not modified with enzymatic treatment. F endoglucanase decreased the amount of hemicelluloses by 39 % which corresponds a 7.1 % of final hemicelluloses. The xylan fraction suffered a reduction of 53 %, the galactan a 47 % and the mannan a 30 %. In both treatments, the xylan removal was superior to mannan. As the results of commercial pulp and endoglucanases treatments revealed (see Quintana et al. under review), the action caused on mannan fraction was higher with F than with B endoglucanase.
In terms of pulp purification step, the strong alkaline treatment before the hydrolytic treatment contributed to reduce the total amount of hemicelluloses by 14.7 % with respect to initial pulp (Fig. 2b). However, hemicelluloses removal was further extended with the respective cellulase treatments. The final amount of hemicelluloses for L_CCE_B120 and L_CCE_F12 was 6.2 and 5.8 %, respectively. As previously observed, the B endoglucanase had slightly higher action on xylan fraction than F. In particular, B reduced the amount of xylans by 79 % while the F a 70 %. With the hydrolytic treatments alone some changes in the mannan fraction were observed. However, incorporating a CCE and then the hydrolytic treatments, the mannan fraction was not affected, although the respective amounts were in the range of L_B120 and L_F12 samples. These results may indicate that the remaining fraction of mannan is not susceptible to alkali treatment. Gehmayr and Sixta (2012) described that during a CCE80 (80g/L of NaOH) at 30 °C, mannan was resistant to physical dissolution due to its highly ordered structure but xylan was removed because of its good solubility under these conditions.
Brightness, chromatic coordinate, viscosity, α-cellulose and fibre morphology results
The B and F treatments led to decrease brightness in the biobleached sulphite pulp, mainly through adsorption of products present in the enzyme solution (Quintana et al. under review). Also, the viscosity results manifested that the enzymatic treatments had no effect on cellulose integrity, although L_F12 seemed to present a slightly lower viscosity. On the other hand, α-cellulose was adversely affected by the enzymatic treatments, particularly with B. It should be noted that viscosity represents the average molecular weight of all cellulose chains, whereas α-cellulose indicates the content of undegraded, higher molecular weight cellulose in pulp. Therefore, although no damage of cellulose chains was perceived, the content in high-molecular weight cellulose (α-cellulose) was considerably altered by B enzyme. Regarding the proportion of fines, enzymatic treatments increased the amount of fines by up to 19 % with L_B120 and 34 % with L_F12, relative to LVAPO sample. Likewise, fibre length was reduced by 19 and 41 %, respectively. The latter results suggest that both endoglucanases act on cellulose chains but have a different effect on fibre length, which is also suggestive of a different mechanism of action.
It can also be appreciated in Table 2 that the viscosity, the α-cellulose and the contents in fines, of the treated control pulp was practically identical with those of the starting pulp.
The biobleached pulp treated with B increased the WRV by 17 % but fibre length was only reduced by 19 % and the content of fines increased by 19 %, with respect to LVAPO pulp (Fig. 3). By contrast, L_F12 treatment did not modify the WRV (4 % increase) but raised the content in fines by 53 % and reduced fibre length by 42 %, regarding biobleached pulp. The respective results suggest that B enzyme might alter fibre surface via external fibrillation (Cadena et al. 2010; Garcia-Ubasart et al. 2013) while F enzyme showed a preferable action to cut the fibres.
The differences found between fibre length, fine content, WRV and CS pointed out that B and F endoglucanases had different performing mechanism.
13C-CP/MAS NMR
The solid state 13C-NMR spectra of biobleached pulp, the enzymatic treated pulps and 9 % (w/v) NaOH extracted pulps followed by endoglucanase treatments are illustrated in Fig. 4. The spectra of L_CCE_B120 and L_CCE_F12 samples exhibited different polymorphic form than samples submitted to an endoglucanase treatment alone. The introduction of a 9 % (w/v) NaOH extraction converted cellulose I to cellulose II, since the C-6 signal at 64 ppm increased, obtaining two peaks with nearly identical heights at 66 and 64 ppm. Nevertheless, the C-1 signal did not exhibit a shoulder at 108 ppm, which is characteristic of cellulose II (Janzon et al. 2008a). The greater proportion of cellulose II of L_CCE_B120 and L_CCE_F12 samples can be related to the low Fock solubility values, in comparison to L_B120 and L_F12 (Krässig 1993; Janzon et al. 2008a).
Figure 5 shows SEM images of handsheets made from LVAPO, L_B120, L_F12 and L_K treated pulps. Interestingly, the action of F enzyme was apparently observed in increased amount of free particles and disruption of the cellulose wall structure. With the L_B120 treatment, differences in appearance with respect to control and starting pulp were inappreciable; however, the treated pulp was 19 % more reactive, but similar in viscosity and fibre length to the starting pulp.
Mode of action of B and F endoglucanases
In order to further explain the results, the concentration and composition of dissolved sugars present in the liquors released from the enzymatic treatments were determined by HPLC. Comparing the results for the different treatments required expressing the composition of dissolved monosaccharides relative to the effluent volume resulted from each treatment. As can be seen from Table 3, the LVA treatment produced a greater release of glucose and xylose than did LVAPO. After the biobleaching treatment, pulps were subjected to an endoglucanase treatment with B or F. As also observed with Fock solubility and fibre morphology, the results for the control treatment (L_K) revealed that the buffer solution by itself had no effect on carbohydrate composition, while L_F12 treatment caused the greatest release of glucose, followed by L_B120. These results are consistent with the number of chain scissions caused by each enzyme and with the extent cellulose degradation, expressed as viscosity. As expected, the amount of xylose in the liquors was smaller than that of glucose in all treatments with an exception of control treatment (L_K), which led to very similar amounts of the two monomers. Although F had a xylanase activity of 680 U/g, the liquors contained little xylose monomer—probably due to the low xylan content in the starting pulp, as determined by HPLC.
Table 4 shows the concentration and composition of oligosaccharides in the effluents as determined by HPLC. Glucose standard oligomers were used as references. The pattern of products released by the L_F12 treatment decreased in the following sequence: cellobiose > glucose > cellotriose. The fact that oligosaccharides of different size and glucose monomer are found is consistent with the mode of action of F, which randomly cleaves glycosidic bonds. With L_B120 treatment, cellobiose in major proportion and glucose in lower were identified in the liquors but no presence of cellotriose or larger oligosaccharides. The contents in hydrolysis products suggest that B is an endoglucanase with an exo type mode of action (Chiriac et al. 2010). According to Chiriac et al. (2010), the absence of long oligosaccharides among the hydrolysis products from crystalline cellulose would indicate a processive mode of action of B, as previously reported for some endoglucanases of family 9 (Irwin et al. 1998; Zverlov 2003). In addition, the low amount of oligosaccharides detected in L_B120 treatment is consistent with the low decrease of pulp viscosity.
Conclusions
Two different cellulases, B and F, were studied in never-dried biobleached sulphite pulp (LVAPO) with the intention to increase Fock solubility and bring a satisfactory advantage to the viscose process. The enzymes, however, exhibited a different mode of action. Thus, while B increased Fock solubility strongly but had little effect on fibre length, F increased Fock solubility to a lesser extent but produced a strong reduction in fibre length and increased notably the amount of fines. In terms of the number of chain scission, the F endoglucanase produced higher values than B endoglucanase. The soluble carbohydrate composition present in the liquors as determined by HPLC confirmed that B endoglucanase had an exo type mode of action since cellobiose and glucose in different proportion were detected. On the other hand, the F liquors contained cellobiose, glucose and traces of cellotriose soluble oligomers.
The most salient contribution of this study is that the introduction of a biobleaching sequence involving a laccase–mediator system (LMS) provides an environmentally friendly process without detracting the final characteristics of a dissolving pulp, and the subsequent endoglucanase treatment (L_B120) reached a 91 % of Fock solubility. Moreover, the CCE introduced before the hydrolytic treatment could reduce the percentage of hemicellulose although the final Fock solubility was lower due to the formation of cellulose II as observed by 13C-CP/MAS NMR. However, complementing the purification step with F12 treatment, lead to improve Fock solubility and reach a final value of 82.4 %, and obtain the lowest amount of hemicelluloses (5.3 %).
Abbreviations
- CCE:
-
Cold caustic extraction
- CS:
-
Chain scission
- DP:
-
Degree of polymerization
- EG:
-
Endoglucanase
- K_CCE:
-
Control treatment with buffer acetate + cold caustic extraction
- L:
-
Laccase–mediator treatment
- L_B120:
-
Biobleached pulp + B endoglucanase at 120 U/g
- L_CCE:
-
Biobleached pulp + cold caustic extraction
- L_CCE_B120:
-
Biobleached pulp + cold caustic extraction + B endoglucanase at 120 U/g
- L_CCE_F12:
-
Biobleached pulp + cold caustic extraction + F endoglucanase at 12 U/g
- L_F12:
-
Biobleached pulp + F endoglucanase at 12 U/g
- L_K:
-
Biobleached pulp + control treatment with buffer acetate
- PO:
-
Hydrogen peroxide reinforced with pressurized oxygen
- TCF:
-
Totally chlorine free
- n.d.:
-
Not determined
- odp:
-
Oven dried pulp
- VA:
-
Violuric acid
- WRV:
-
Water retention value
- 13C-CP/MAS NMR:
-
Solid state cross polarization/magic angle spinning carbon 13 nuclear magnetic resonance
References
Arnoul-Jarriault B, Lachenal D, Chirat C, Heux L (2014) Upgrading softwood bleached kraft pulp to dissolving pulp by cold caustic treatment and acid-hot caustic treatment. Ind Crops Prod. doi:10.1016/j.indcrop.2014.09.051
Bouchard J, Morelli E, Berry RM (2000) Gas phase addition of solvent to ozone bleaching of kraft pulp. J Pulp Pap Sci 26:30–35
Cadena EM, Chriac AI, Javier Pastor FI et al (2010) Use of cellulases and recombinant cellulose binding domains for refining TCF kraft pulp. Biotechnol Prog 26:960–967. doi:10.1002/btpr.411
Cairns JRK, Esen A (2010) Beta-glucosidases. Cell Mol Life Sci 67:3389–3405. doi:10.1007/s00018-010-0399-2
Chiriac AI, Cadena EM, Vidal T et al (2010) Engineering a family 9 processive endoglucanase from Paenibacillus barcinonensis displaying a novel architecture. Appl Microbiol Biotechnol 86:1125–1134. doi:10.1007/s00253-009-2350-8
Chiriac AI, Pastor FIJ, Popa VI et al (2013) Changes of supramolecular cellulose structure and accessibility induced by the processive endoglucanase Cel9B from Paenibacillus barcinonensis. Cellulose 21:203–219. doi:10.1007/s10570-013-0118-x
Ek M, Gellerstedt G, Henriksson G (2009) Pulp and paper chemistry and technology. In: Wood chemistry and wood biotechnology, vol 1. pp 247–249
Engström A-C, Ek M, Henriksson G (2006) Improved accessibility and reactivity of dissolving pulp for the viscose process: pretreatment with monocomponent endoglucanase. Biomacromolecules 7:2027–2031
Evans R, Wallis AFA (1987) Comparison of cellulose molecular weights determined by high performance size exclusion chromatography and viscometry. In: 4th International Symposium on Wood and Pulping Chemistry, pp 201–205
FAO (2012) FAO. Food and Agriculture Organitzation of the United Nations
Fock W (1959) A modified method for determining the reactivity of viscose-grade dissolving pulps. Papier 13:92–95
Garcia-Ubasart J, Torres AL, Vila C et al (2013) Biomodification of cellulose flax fibers by a new cellulase. Ind Crops Prod 44:71–76. doi:10.1016/j.indcrop.2012.10.019
Gehmayr V, Sixta H (2012) Pulp properties and their influence on enzymatic degradability. Biomacromolecules 13:645–651
Gehmayr V, Schild G, Sixta H (2011) A precise study on the feasibility of enzyme treatments of a kraft pulp for viscose application. Cellulose 18:479–491. doi:10.1007/s10570-010-9483-x
Glasser WG, Atalla RH, Blackwell J et al (2012) About the structure of cellulose: debating the Lindman hypothesis. Cellulose 19:589–598. doi:10.1007/s10570-012-9691-7
Henriksson G, Christiernin M, Agnemo R (2005) Monocomponent endoglucanase treatment increases the reactivity of softwood sulphite dissolving pulp. J Ind Microbiol Biotechnol 32:211–214. doi:10.1007/s10295-005-0220-7
Ibarra D, Köpcke V, Ek M (2010a) Behavior of different monocomponent endoglucanases on the accessibility and reactivity of dissolving-grade pulps for viscose process. Enzyme Microb Technol 47:355–362. doi:10.1016/j.enzmictec.2010.07.016
Ibarra D, Köpcke V, Larsson PT et al (2010b) Combination of alkaline and enzymatic treatments as a process for upgrading sisal paper-grade pulp to dissolving-grade pulp. Bioresour Technol 101:7416–7423. doi:10.1016/j.biortech.2010.04.050
Irwin D, Shin DH, Zhang S et al (1998) Roles of the catalytic domain and two cellulose binding domains of Thermomonospora fusca E4 in cellulose hydrolysis. J Bacteriol 180:1709–1714
Janzon R, Puls J, Bohn A et al (2008a) Upgrading of paper grade pulps to dissolving pulps by nitren extraction: yields, molecular and supramolecular structures of nitren extracted pulps. Cellulose 15:739–750. doi:10.1007/s10570-008-9224-6
Janzon R, Saake B, Puls J (2008b) Upgrading of paper-grade pulps to dissolving pulps by nitren extraction: properties of nitren extracted xylans in comparison to NaOH and KOH extracted xylans. Cellulose 15:161–175. doi:10.1007/s10570-007-9154-8
Köpcke V, Ibarra D, Ek M (2008) Increasing accessibility and reactivity of paper grade pulp by enzymatic treatment for use as dissolving pulp. Nord Pulp Pap Res J 23:363–368
Krässig HA (1993) Cellulose-Structure, Accessibility and Reactivity, vol 11. Gordon and Breach Science Publisher, Yverdon, p 376
Kvarnlöf N, Germgård U, Jönsson LJ, Söderlund C-A (2006) Enzymatic treatment to increase the reactivity of a dissolving pulp for viscose preparation. Appita J 59:242–246
Lindman B, Karlström G, Stigsson L (2010) On the mechanism of dissolution of cellulose. J Mol Liq 156:76–81. doi:10.1016/j.molliq.2010.04.016
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. doi:10.1128/MMBR.66.3.506-577.2002
Medronho B, Romano A, Miguel MG et al (2012) Rationalizing cellulose (in)solubility: reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 19:581–587. doi:10.1007/s10570-011-9644-6
Moon RJ, Martini A, Nairn J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994. doi:10.1039/c0cs00108b
Quintana E, Valls C, Vidal T, Roncero MB (2013) An enzyme-catalysed bleaching treatment to meet dissolving pulp characteristics for cellulose derivatives applications. Bioresour Technol 148:1–8. doi:10.1016/j.biortech.2013.08.104
Quintana E, Valls C, Barneto AG et al (2015) Studying the effects of laccase treatment in a softwood dissolving pulp: cellulose reactivity and crystallinity. Carbohydr Polym 119:53–61. doi:10.1016/j.carbpol.2014.11.019
Quintana E, Valls C, Vidal T, Roncero MB Comparative evaluation of the action of two different endoglucanases. Part I: On a fully bleached, commercial acid sulfite dissolving pulp. Cellulose (under review)
Rahkamo L, Viikari L, Buchert J et al (1998) Enzymatic and alkaline treatments of hardwood dissolving pulp. Cellulose 5:79–88. doi:10.1023/A:1009268713757
Sixta H (2006) Handbook of pulp. Wiley, Weinheim
Spiro R (1966) Analysis of sugars found in glycoproteins. Methods Enzymol. 566:7–9
Zverlov VV (2003) Two new cellulosome components encoded downstream of celI in the genome of Clostridium thermocellum: the non-processive endoglucanase CelN and the possibly structural protein CseP. Microbiology 149:515–524. doi:10.1099/mic.0.25959-0
Acknowledgments
The authors thank the "Ministerio de Economía y Competitividad" of Spain for their support in this work under the projects BIOSURFACEL CTQ2012-34109 (funding also from the "Fondo Europeo de Desarrollo Regional FEDER") and BIOPAPμFLUID CTQ2013-48995-C2-1-R. The authors are grateful to the consolidated group with the Universitat de Barcelona (UB) AGAUR 2014 SGR 534. The authors would like to thank DÖMSJO (Sweden) for providing the starting pulp, Fungal Bioproducts (Spain) for kindly supplying the F cellulase and the Department of Microbiology (University of Barcelona, Spain) for warmly providing the B cellulase.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Quintana, E., Valls, C., Vidal, T. et al. Comparative evaluation of the action of two different endoglucanases. Part II: On a biobleached acid sulphite pulp. Cellulose 22, 2081–2093 (2015). https://doi.org/10.1007/s10570-015-0631-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0631-1