Abstract
A newly identified cellulase with a high polysaccharide degrading potential and a processive mode of action, has been evaluated on cellulose fibers. Cellulase Cel9B from Paenibacillus barcinonensis is a modular endoglucanase with the domain structure GH9-CBM3c-Fn3-CBM3b, consisting of a family nine catalytic module GH9, an auxiliary module CBM3c, a fibronectin-like module Fn3, and a functional cellulose binding module CBM3b. The whole cellulase Cel9B (E1) and two truncated forms of the enzyme that consist of the catalytic module linked to the auxiliary module, GH9-CBM3c (E2), and of the cellulose binding module of the enzyme, CBM3b (CBD), were applied to softwood dissolving pulp. The changes in the supramolecular structure and morphology of the fibres after the enzymatic treatment were evaluated by viscosimetry, X-ray diffraction (XRD), thermogravimetric analysis, differential scanning calorimetry and scanning electron microscopy (SEM). XRD studies provided the crystallite size, interplanar distances and crystallinity index of the samples before and after the enzymatic treatment. The treatment with cellulases E1 and E2 decreased the degree of polymerization and increased the crystallinity index of the pulp. Both E1 and E2 had a pronounced capacity for removing fuzz and improved the smoothness and surface appearance of the fibers, as shown by SEM. On the other hand, CBD proved to be less effective under the tested conditions. Moreover, the solubility of dissolving pulp in alkaline solutions has been evaluated as an indirect measure of cellulose accessibility. A notable enhancement in alkaline solubility of the samples treated with the cellulases was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, the most abundant and common biopolymer on our planet, is used in a wide range of applications, from building materials and clothing to biomaterials and pharmaceutical products. Cellulose consists of linear chains of β-1,4-linked glucose residues that in the native state form an extensive hydrogen-bonded network that gives rise to tightly packed crystalline structures (elementary fibrils) that are involved in a hierarchical supramolecular organization that also includes microfibrils and the cellulose fibres (Zhang and Lynd 2004). Despite the large number of hydroxyl groups, the packed structure of cellulose gives it low reactivity and makes it recalcitrant to biodegradation. Only the cellulose molecules on the surfaces of the fibrils or fibril aggregates and those between the crystallites in the cell wall are accessible to most solvents and reagents (Engstrom et al. 2006).
The accessibility of cellulose is of great importance for the production of cellulose derivatives and regenerated cellulose, where a homogeneous substitution of the hydroxyl groups of cellulose chains is desirable in order to obtain quality products (Klemm et al. 2002). Nevertheless, the compact structure of the cellulose complicates the accessibility of functional groups heterogeneous substitution frequently results. The activation of cellulose, which is defined as the opening of the supramolecular structure of cellulose to solvent and chemicals, is a necessary step for its dissolution. This can be done for example by chemical, mechanical, thermal or enzymatic treatments, either separated or in combination, and consists of opening the inner pore structure, disrupting fibrillar aggregation, and reducing crystallinity to increase availability of hydroxyl groups. Among other methods, the one that best suits the current environmental requirements is the treatment with cellulose-degrading enzymes. Evaluation of different types of these enzymes has shown that endoglucanases have potential application to increase the reactivity of cellulose (Rahkamo et al. 1996, 1998; Cao and Tan 2005, 2006; Henriksson et al. 2005).
Cellulases are widely used in industrial applications such as in food, pulp and paper, textile, surfactants and bioethanol production (Bhat 2000). Cellulases are usually multidomain enzymes, comprising, apart from the catalytic domain (GH), one or more ancillary domains such as carbohydrate binding modules (CBMs). These binding modules improve enzyme performance by increasing enzyme–substrate proximity, enhancing accessibility, and modifying the surface of cellulose (Tomme et al. 1998). They can differ widely in their binding kinetics and specificity (Carrard et al. 2000; Boraston et al. 2004).
Paenibacillus barcinonensis is a newly identified species that shows a multiple-enzyme β-glycanase system correlated with its high polysaccharide degrading potential (Pastor et al. 2001; Sánchez et al. 2003, 2005). Cellulase Cel9B from Paenibacillus barcinonensis is a modular family nine endoglucanase with a processive mode of action. It possesses the modular structure GH9-CBM3c-Fn3-CBM3b, where GH9 is a family nine catalytic module, CBM3c is an auxiliary module, Fn3 is a fibronectin-like module, and CBM3b is a functional cellulose binding module (Pastor et al. 2001). In previous work the enzyme was applied to eucalyptus pulps and resulted in a biorefining effect that notably improved mechanical properties of paper (García et al. 2002). Several truncated enzymes derived from Cel9B were recently constructed and evaluated in order to determine the module(s) of the enzyme responsible for the beneficial effect found (Chiriac et al. 2010). One of the cellulases constructed from the structure GH9-CBM3c showed an increased effect when compared to the whole enzyme, while the isolated binding module CBM3b showed only a minor effect on mechanical properties, although SEM analysis clearly showed that both enzymes modified fiber surfaces (Cadena et al. 2010).
To analyze whether the beneficial action on paper grade pulp could be extrapolated to other pulps and in order to extend the study and seek new applications, we have studied the effect of the whole enzyme Cel9B (E1), the truncated cellulase GH9-CBM3c (E2) and the recombinant cellulose binding module CBM3b (CBD) on dissolving pulp from spruce wood. Modifications of supramolecular structures and the morphology of the fibers have been evaluated by viscosimetric measurements, as well as by X-ray diffraction (XRD), thermogravimetry (TG/DTG), differential scanning calorimetry (DSC), and scanning electron microscopy (SEM). The alkali solubility of cellulose has been also evaluated as an indirect measure of the cellulose accessibility.
Materials and methods
Materials
Spruce dissolving pulp (Extranier F) was purchased from Rayonier, USA and was coded as Dpulp. The enzymes applied were cellulase Cel9B from Paenibacillus barcinonensis, which is a modular endoglucanase with the structure GH9-CBM3c-Fn3-CBM3b (E1), a truncated derivative of the cellulase with the structure GH9-CBM3c (E2), and a recombinant cellulose binding module CBM3b (CBD) derived from the enzyme. They were purified from recombinant Escherichia coli clones carrying the respective encoding genes. E1 and E2 show cellulase activity as they contain the catalytic module GH9, while CBD does not show hydrolytic activity on cellulose (Chiriac et al. 2010). The whole enzyme E1 and the truncated form E2 have typical hydrolytic properties of endoglucanases. Their saccharification activities against amorphous celluloses, such as carboxymethyl cellulose (CMC), are much higher (100–200×) than activities against crystalline cellulosic substrates, such as Avicel. However, the ratio between activity on CMC (CMCase) and activity on Avicel (Avicelase) is different in the two cellulases. E1 and E2 show the same activity on CMC, but E1 is twice more active on Avicel than E2 (Chiriac et al. 2010). The activities of E1 and E2 are shown as international units (IU) (Table 1). One unit of enzymatic activity was defined as the amount of enzyme that releases 1 μmol of reducing sugar equivalent per minute under the assay conditions described. All other chemicals and reagents used were of analytical grade.
Enzymatic treatment of pulp
Before the enzymatic treatment, the pulp was disintegrated in a standard disintegrator Frank-PTI (T 205 sp-02 Tappi Test Method 2002) and left for hydration over night in the reaction buffer. Samples of 0.5 g of oven dry pulp (odp) at 1 % consistency were incubated with the enzymes in 50 mM acetate buffer, pH 5.5 at 50 °C in a 250 ml Erlenmeyer flask, under 200 rpm stirring in a water bath shaker. E1 and E2 were applied at 1 Avicelase U/g odp, for 24 h (E1-C1t1, E2-C2t1) or 72 h (E2-C2t2). E1 was also applied at 2 Avicelase units for 24 h (E1-C2t1), while E2 was also applied at 0.5 Avicelase U/g odp for 24 h (E2-C1t1). In addition, one cellulosic sample was pretreated with ultrasound at 35 kHz for 1 h and subsequently treated with E1 at 2 Avicelase U/g odp for 24 h (E1-C2USt1). The enzyme doses of E1 and E2 are shown in Table 1. As CBD does not have hydrolytic activity, its dose was normalized in μmol/g odp. CBD was applied at 0.5 or 1 μmol/g odp for 24 h (CBD-C1 and CBD-C2). The amount of reducing sugar released from pulps was determined using the method of Nelson and Somogyi (Chiriac et al. 2010).
Determination of alkali solubility
The effect of the different enzymes on the supramolecular structure of dissolving pulp was studied by an indirect method, alkaline solubility. The method was applied to the control (Dpulp) and the enzymatic treated samples (E1-C2t1, E2-C2t1 and CBD-C2).
The solubility of cellulose in 8.5 % alkali was determined as described by Isogai and Atalla (1998). Samples of 0.5 g cellulose (odp) were mixed with 1.25 g of NaOH crystals and 13.45 ml of distilled water. The samples were vigorously stirred and then kept at −25 °C for 24 h. Samples were then brought to room temperature and the NaOH concentration was adjusted to 5 % by adding distilled water. The soluble cellulose fraction was separated from the insoluble one by filtration on a glass filter (G3). The insoluble fraction was successively washed with solutions of 5 % NaOH, 1 % NaOH, distilled water, 1 % acetic acid and distilled water and was dried in an oven for 3 days at 30 °C. Samples were heated at 105 °C and weighed to determine the absolute dry weight. The dissolution degree of cellulose was expressed with the following formula:
where S is solubility degree, m0, is the initial amount of cellulose and mf is the amount of cellulose retained on glass filter.
Degree of polymerization
The degree of polymerization (DP) of the studied samples was calculated from the intrinsic viscosity of the corresponding solutions of the samples in 1 M cupriethylendiamine, method according to Tappi Test Method T230 om-89 (Tappi Test Method 1997).
X-ray diffraction analysis
The cellulosic fibers were pressed into discs using a cylindrical steel mold with a diameter of 1.3 cm and a pressure of about 500 MPa, in a laboratory press. The diffractograms of the cellulosic samples were recorded by a Bruker D8 X-ray diffractometer using CuKα radiation (λ = 1.5418 Å) generated at 30 mA and 40 kV for a range of diffraction angle (2θ) between 10 and 50°. The Mecury 3.1 program from the Cambridge Crystallographic Data Centre (CSD System) was used to simulate diffraction patterns for comparisons with the experimental patterns. The experimental diffraction patterns exhibited peaks that were deconvoluted from background scattering by using Lorentzian functions, while the diffraction pattern of the amorphous component was approximated by a Gaussian function curve-fitting analysis. The structural parameters were calculated using TOPAS 4.2 software (Bruker-AXS, Germany).
The crystallinity index (Icr) was calculated based on the following formula (Sun et al. 2009; Park et al. 2010):
where SC is the area under the crystalline peaks and SA is the remaining (amorphous) area.
The lattice spacings (d-spacings) were calculated using Bragg equation:
where dhkl is the lattice spacing of the hkl crystallographic planes, λ is the X-ray wavelength, and 2θ is the corresponding Bragg angle.The crystallite size was calculated based on the width of the diffraction peaks, from the Scherrer equation (Krässig 1993):
where D(hkl) is the size of crystallite (nm), k is the Scherrer constant (0.94), λ is the X-ray wavelength (0.15418 nm) and β is the full-width at half-maximum of the reflection hkl, measured (radians)in 2θ, and θ is half of the 2θ value for the corresponding Bragg peak.
Thermogravimetric analysis
Cellulose samples of 5–10 mg were placed in an aluminum oxide crucible and analyzed in a Mettler Toledo TGA/SDTA 851 device with a heating rate of 10 °C/min, at temperatures ranging from 25 to 700 °C, under a nitrogen atmosphere. Prior to analysis, the samples were placed in a desiccator at a constant relative humidity of 65 %, for 7 days and a temperature of 20 °C. The kinetic interpretation was performed with the STARe software offered by Mettler Toledo. To ensure data reproducibility, several thermograms were recorded for each sample.
Differential scanning calorimetry
Differential scanning calorimetry studies were performed using a Mettler Toledo DSC1. The cellulose samples, measured in closed lid aluminum pans, were heated at a rate of 10 °C/min under a nitrogen atmosphere, from 10 to 300 °C. The cellulosic samples were conditioned in a desiccator for 72 h, at a constant relative humidity of 65 % and temperature of 20 °C, until constant weights.
Scanning electron microscopy
The surface appearance of the fibers was analyzed with a HITACHI S 2300 scanning electron microscope. Prior to analysis the samples were covered with a gold layer using a SPS Polos Spin Coater.
Results and discussion
Enzymatic hydrolysis
In the present study the effectiveness of cellulase E1, a family 9 modular endoglucanase from Paenibacillus barcinonensis, and of two truncated forms of the enzyme, cellulase E2 and CBD have been evaluated. E1 is the whole enzyme, which contains a functional carbohydrate binding module. E2 consists of the enzyme devoid of this module, and CBD is the isolated carbohydrate binding module. Cellulases E1 and E2 are endoglucanases: they have high activity on amorphous cellulose (CMC) and low activity on crystalline cellulose (Avicel). However, they have differences in substrate specificity, E1 being twice as active as E2 on Avicel. On the other hand, CBD does not have hydrolytic activity on cellulosic substrates.
Dissolving pulp (Dpulp) was treated with the different enzymes (E1, E2 and CBD) and as a first evaluation of their effect on pulps, the rate of cellulose hydrolysis was determined (Fig. 1).
A rapid release of reducing sugars was observed in the first hours of the treatments, but slowed down as the incubation progressed in time. Cellulase E1 released sugars more rapidly than did cellulase E2. When the two cellulases were applied at the same dose (measured as CMCase units—200 U/g odp), cellulase E1 (E1-C2t1) released a remarkably higher amount of sugars from pulps (154.8 μg/mL) than did cellulase E2 (E2-C2t1) (47.5 μg/mL). This probably reflects the differences in the Avicelase units among the enzyme preparations tested, as Avicelase activity of E1-C2t1 (2 U/g odp) is higher than that of E2-C2t1 (1 U/g odp). However, when the dose of E1 was reduced to equivalence the Avicelase units to 1 U/g odp, although the release of sugars was decreased, it was still higher than E2. In this way the amount of sugars released by E1-C1t1 was 86 μg/mL. This is probably a consequence of a better accessibility for E1 to the crystalline portions of cellulosic fibers than for E2, which lacks the carbohydrate binding module contained in the whole enzyme (Chiriac et al. 2010). To improve the enzymatic hydrolysis process, pulp was pretreated with ultrasound for 1 h. An increase of the amount of reducing sugar from 154.8 μg/mL (E1-C2t1) to 171.9 μg/mL (E1-C2USt1) was recorded. Treatment with the isolated cellulose binding module, CBD, did not release sugars from pulps, as was expected.
Influence of enzymatic hydrolysis on the supramolecular structure
X-ray diffraction
X-ray diffraction was applied to investigate the changes in the crystalline structure of the enzymatically treated cellulose pulps. The diffractograms of the reference (Dpulp) and the enzymatic treated samples are shown in Fig. 2. The diffraction patterns of all samples are characteristic of cellulose I.
To display the intensities, positions and Miller indices of the individual diffraction peaks that contribute to the diffractograms, the Mercury program was used to calculate a pattern with very narrow peaks. By using the crystal structure information file (.cif) from the crystal structure of Nishiyama et al. (2002) and Mercury’s default peak width at half height (pwhm) value of 0.1°, a simulated pattern with peaks mostly without overlapping was obtained (French and Santiago Cintrón 2013; French 2014).
For Dpulp, this “deconvolution” reveals three main contributors of intensity to the three peaks that have Miller indices of (200), found at a Bragg angle of 22.55°, as well as the 2θ reflections at 14.71° and 16.63°, respectively, assigned to the (1–10) and (110) lattice planes (Fig. 2a). A sharpening and an increase of the intensity profiles corresponding to the reflection of the (200) crystallographic plane was observed for all enzyme-treated samples.
The greatest increase of the intensity of (200) reflection was for the samples treated with cellulase E2 (Fig. 2b). This observation indicates the presence of better-defined crystalline domains and is confirmed by the increased values of the crystallinity index (Icr). The increase in crystallinity of the residue obtained after the enzymatic hydrolysis (Table 2) suggests that the hydrolysis of amorphous cellulose could be favored over that of the crystalline cellulose. The crystal structure extent within the cellulose structure has an important effect on the performance of the enzymatic hydrolysis, a fact that can be better explained by determining the crystallite size (D) and the lattice spacing (d-spacing) values. The calculation of the crystallite size normal to the hkl planes was done based on the estimation of the peak width at half-maximum amplitude, while the d-spacings of the (1–10), (110) and (200) peaks were calculated with the Bragg equation (Table 2).
The d-spacing values determined based on XRD method for control pulp (D pulp) are 6.24, 5.26 and 3.92 Å, corresponding to (1–10), (110) and (200) crystallographic planes, respectively. An increase of the lattice spacing for (110) and (200) planes can be observed for enzymatically treated samples, in comparison with the plane (1–10).
Moreover, taking into account the crystallite size data from Table 2, an increase corresponding to the (200) plane was observed, which could be due to predominant hydrolysis of smaller crystallites or to lateral aggregation of the remaining crystallites (Penttilä et al. 2013).
The increase of the crystallite size might be surprising since no cellulose was added during the enzymatic treatment, but it could be explained by co-crystallization (Duchemin et al. 2012). The co-crystallization process was observed after wetting and drying of cellulosic pulp (Newman 2004; Rondeau-Mouro et al. 2011) and it consists of the removal of the non-cellulosic polysaccharide chains between exterior cellulose chains and formation of a bigger crystallite. Mazeau (2011) and Mazeau and Charlier (2012) claim that the co-crystallization process occurs mainly on (110) and (200) planes, in particular on (110) crystallographic plane, when both considering. The same trend was observed also in our study, when control pulp was treated with different enzymes, especially in case of the samples treated with the cellulase E2.
In the case of samples CBD-C1 and CBD-C2 the increase of the crystallite size corresponding to (200) plane, could be explained by the adsorptions of CBD on the surface of cellulose crystals, which is known to be the preferred binding site for the cellulose-binding domains (Liu et al. 2011; Penttilä et al. 2013).
The polymerization degree of pulps was also determined. Cellulase-treated samples showed a consistent decrease in DP when compared to control pulps (Table 2). Also, the cellulose substrate was more degraded when was treated with cellulase E1, showing lower DP than pulps treated with E2. Furthermore, the pretreatment of cellulose with ultrasound (E1-C2USt1) resulted in greater depolymerisation of the samples in comparison with E1-C2t1, accompanied by a slight increase in crystallinity index. Pulp samples treated with CBD, without hydrolytic activity, showed only slight changes in DP.
Even though endoglucanases preferentially degrade amorphous cellulose and an increase of the total percentage of crystalline cellulose would be expected as indicated by some studies (Cao and Tan 2005, 2006; Ciolacu et al. 2008), other authors show that the crystallinity index (Icr) remains unchanged after enzymatic hydrolysis (Lenze et al. 1990; Puls and Wood 1991; Zhang and Lynd 2004). In the present study the treatment with the endoglucanases tested, cellulases E1 and E2, led to an increase of the Icr. The lower values recorded for the crystallinity index of samples treated with E1, in comparison with E2, may be due to the fact that these endoglucanases have a minor activity on crystalline cellulose, that is higher in E1 than in E2. Alternatively the difference among the cellulases can be related to the hydrolysis mechanism of cellulase E1, which is a processive endoglucanase and not a typical endoglucanase (Chiriac et al. 2010).
Thermal analysis
Thermal analysis may be defined as a set of techniques (DTA, DSC and TG) used to describe the physical or chemical changes of substances, as a function of temperature (Ciolacu and Popa 2006). Changes in latent heat in a chemical substance are detectable by DSC, while the progressive mass loss is detectable by thermogravimetry (TG) or differential thermogravimetry (DTG).
Thermogravimetric analysis
The behavior during the thermooxidative destruction of the cellulose samples treated with the different enzymes was studied by thermogravimetric analysis (Fig. 3). The thermal decomposition of cellulose involves at least two processes in addition to simple desorption of physically bound water (Ciolacu and Popa 2006). The first is the increase of cellulose–cellulose hydrogen bonding of cellulose chains, with the evaporation of absorbed water (until 115 °C, dehydration), the second is the unzipping of the cellulose chain and levoglucosan formation from the monomer units (until 380 °C, the main decomposition stage) and the third reaction is the decomposition of the dehydrated products to yield char and volatile products.
Several parameters can be identified within the thermograms, such as Tonset which is the initial temperature of each thermogravimetric step. Tpeak is the temperature corresponding to the maximum rate of mass loss, and Tend is the temperature of the end of step. Also, the mass loss for each stage can be determined, i.e., WI which is the amount of desorbed water (10–115 °C), and WII, the mass loss for the stage corresponding to the thermal degradation (115–380 °C).
Figure 3a–c shows that untreated cellulose begins to decompose at a lower temperature (Tonset = 314 °C) when compared to enzymatically treated samples (Table 3). This behavior is explained by the fact that the non-crystalline region decomposes more actively than the crystalline region. Also, the intermolecular hydrogen bonds between chains are stronger in the crystalline region than those from amorphous area and require more energy to break before the decomposition process can proceed (Morgado and Frollini 2011). This means that more crystalline samples, such as the enzymatically treated pulps, have higher thermal stability than the control pulp (Dpulp). These results are in good agreement with the data obtained from X-ray diffraction method, regarding the crystallinity index.
From DTG curves (Fig. 3d–f) can be seen that the temperatures corresponding to the maximum rate of decomposition (Tpeak) were shifted towards higher temperatures for the enzyme-treated samples, as from 355 °C corresponding to Dpulp to 359 °C for the samples treated with E1, 362 °C for the samples treated with E2 and 360 °C for those treated with CBD (Table 3). This fact can be correlated with the data of the crystallinity index (Icr) and the crystallite size (D). Thus, an increase in the crystallinity index and in the crystallite size corresponding to (200) reflection, determined an increase of the decomposition temperature of cellulose residues (Calahorra et al. 1989; Ciolacu and Popa 2006; Kim et al. 2010; Poletto et al. 2012).
The char fraction remaining after thermal decomposition of celluloses is an expression of the crystallinity index of the samples. Thus, the increase of the crystallinity index of enzyme treated samples leads to an increase of the obtained char (R600). Similar results were also observed by Yue et al. (2012). Moreover, an increase of the char with the decrease of the polymerization degree was recorded (Gurgel et al. 2012).
To evaluate the influence of the supramolecular organization of cellulose over the course of thermal degradation reaction, the kinetic parameters were determined. The application of the Freemann-Caroll method led to a kinetic processing of the thermogravimetric data, based on the equation (Grigoriu et al. 2009):
where dα/dT is the conversion degree; α is the conversion defined as α = (w0 − w)/(w0 − w∞), w0 and w∞ being the initial and final weights, and w the weight at any time; T is the absolute temperature recorded on the thermogram and R is the gas constant.
The weight loss kinetic parameters, as the activation energy (Ea), pre-exponential factor (A) and the reaction order (n), for the control pulp and the enzymatic treated samples were determined (Table 3).
The activation energy corresponding to the II stage of cellulose decomposition increased sharply for the samples treated with enzymes, showing a maximum value at enzyme concentration of C1 and at a time t1 (from 215.24 kJ/mol of Dpulp, to 347.47 kJ/mol of E1-C1t1, 340.71 kJ/mol of E2-C1t1 and 313.63 kJ/mol of CBD-C1, respectively). For each enzyme a progressive decrease of Ea was observed with increasing enzyme concentration or time of treatment. This behavior was confirmed also by the data reported by Gurgel et al. (2012). The differences that appear between the Ea values (determined according to the Freeman-Carroll method) correspond to different enzyme actions and could be correlated with the degree of polymerization. Thus, the decrease of Ea for the samples of each enzyme corresponds to a decrease in the DP of cellulose (Calahorra et al. 1989; Gurgel et al. 2012).
The reaction order (n) for dissolving pulp was 0.56 and increased during the enzymatic treatments to 1.18 (for the samples treated with E2) and until 1.31 (for the samples treated with E1). Thus, it can be considered that the thermal decomposition of enzyme treated celluloses follows the first order kinetics. Also, similarly to what was found for Ea, when we compare the samples treated with the same enzyme, it was found that the reaction order decreased as the degree of polymerization decreased, as also observed by Calahorra et al. (1989).
The results obtained from the thermogravimetric analysis indicate changes in the supramolecular structure of samples, as a consequence of the enzymatic degradation process, and thus, this method could be used as a complementary characterization technique for these types of materials.
Differential scanning calorimetry
The modifications induced by the enzymes on the supramolecular structure of pulps and implicitly on the accessibility of the samples, were evaluated through DSC. The samples were first conditioned at a constant relative humidity of 65 %, at a temperature of 20 °C, for 72 h and after that the thermograms were recorded until 300 °C. The action of the enzymes on cellulosic samples was investigated by measuring the changes in the amount of water that was bound to fibers, expressed as the enthalpy of evaporation.
Because the area of the endothermic peak is directly correlated to the variation in the enthalpy of the cellulosic samples, the DSC method can be applied to measure the enthalpy of evaporation with the following equation (Grigoriu et al. 2009):
where ΔH is the enthalpy of evaporation (J/g), E is the sensitivity of the calorimeter (4.02 μV/mW), S is the peak area (mm2), k is the correction factor (μV) and mx is the mass of the dried sample (mg).
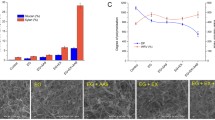
During the enzymatic hydrolysis of the dissolving pulp, with the different enzymes (E1, E2 and CBD) at different times (24 and 72 h) and concentrations (C1 and C2), an increase of the endothermic peak was recorded, explained by the sorption of a higher amount of water (Fig. 4). This indicated an increase of the accessibility of the cellulosic samples.
It is well known that the capacity of the cellulose fiber for water absorption depends largely on the availability of the free hydroxyl groups and it is generally considered that water absorption occurs almost entirely in the amorphous regions of cellulose, neglecting the free hydroxyl groups that may be present on the surfaces of crystallites. Thus, the modifications that appear in the dimensions of the amorphous area within cellulose fibers can be identified through the DSC method, by the changes in the amount of water bound to cellulose.
Taking into account the data regarding the enthalpy of evaporation of cellulosic samples (Table 4), differences in actions of enzyme E2 in comparison with E1 can be observed as a function of the specific activity of each enzyme.
The endo-cleavages from the amorphous regions increased at the higher enzyme concentration and the fragments were released from the cellulose molecules with an increase in the amount of reducing sugars and a decrease in the absorbed water and of the enthalpy of evaporation, respectively (Table 4). Also, a good correlation was observed between the enthalpy of evaporation and the degree of polymerization data. Thus, the effect of enzymatic hydrolysis is reflected in a decrease of the DP accompanied by a decrease of ΔH values. Hoshino et al. (1999) studied the effect of enzymatic treatment on the hygroscopic properties of cotton cellulose, using a highly purified endo-type cellulase from Streptomyces sp. KSM-26, and also observed that the rapid decrease in the size of the amorphous regions decreases the number of sites for adsorption of moisture to a low level. These observations are in agreement with the increase in XRD crystallinity of the Dpulp after enzymatic hydrolysis and suggest that the hydrolysis of amorphous cellulose could be favored over that of the crystalline cellulose.
Comparing the group of samples treated with the same enzyme, we can observe that for cellulase E1, which possesses the cellulose binding module, a higher concentration leads to an increase of the evaporation enthalpy, from 134.9 J/g (E1-C1t1) to 147.64 J/g (E1-C2t1), a fact explained by a pronounced action of cellulase on crystalline region, and thus an increase of the amorphous area. These data are correlated with the values obtained for the amount of the reducing sugar which increases from 92 μg/mL (E1-C1t1) to 154 μg/mL (E1-C2t1). At the same time, the action of cellulase E2, which is more active on amorphous regions, leads to a decrease of the evaporation enthalpy from 161.32 J/g (E2-C1t1) to 126.04 J/g (E2-C2t1) due to a decrease of the less ordered regions and increased crystalline fraction, which is more resistant to the cellulose action.
The treatment with the isolated CBD affects the supramolecular structure of cellulose in a small percentage, as was expected. The decrease of the ΔH values for the CBD treated samples could be explained by the binding of enzyme at the surface of cellulose.
Analysis of fiber morphology
Scanning electron microscopy analysis of fibers morphology showed that cellulases E1 and E2 change the surface appearance of the fibers (Fig. 5).
The control pulp (Dpulp) and the sample treated with buffer (Blank pulp, Bk) presented a fibrillated or hairy aspect. Similar behavior was shown by fibers treated with the cellulose binding module, CBD. On the other hand, fibers treated with cellulases E1 or E2 had much smoother and cleaner surfaces. In this way, E2-treated fibers had a smoother appearance and only a few outbound fibrils compared to the control fibers. E1 cellulase showed a more pronounced peeling effect than E2. This effect increased with the enzyme dose, yielding fibers with a completely clean surface when treated with the higher E1 dose. Both cellulases seem to weaken the fibrils and mechanical stirring lead to detachment of the salient fibrils from the fiber surface. The wall delamination increased with the enzyme dose and the time of the treatment, and was enough in the case of E1 to get totally clean fibers. This cleaning effect is probably due to the ability of the cellulases tested to weaken the wall and suggests an improvement in the accessibility and chemical reactivity of this type of pulp by cellulases E1 and E2. In fact, the pilling effect lead to an increased surface area and so far increased accessibility for the reagents as observed by Ibarra et al. (2010). Other researchers have also described a cleaning effect for cellulases (Mansfield et al. 1997; Buschle-Diller et al. 1994; Liu et al. 2009).
These observations are in agreement with the ability of these cellulases to enhance the mechanical refining of the pulp as described in a previous work, which can be equally explained by a softening of the external wall of cellulose fibers (Cadena et al. 2010). Nevertheless, for cellulases E1 and E2, the modifying ability of fibers is of interest since cellulases are used for this purpose in laundry and textile industry, namely to restore cloth softness and cotton finishing, respectively, as already discussed above. They have a pronounced capability to remove pilling without attacking the structural integrity of the fibers, since a weakening of the fibers is undesired.
Alkali solubility
One of the most important activation methods, which could be used also at industrial level, is the swelling of cellulose in NaOH solutions. Cellulose is partially soluble in aqueous solutions of NaOH, the degree of dissolution depending on the nature and characteristics of cellulose (Ciolacu and Popa 2005). Alkali solubility is a related parameter to accessibility of cellulose to reactants, an important factor for the production of cellulose derivatives.
In order to analyze the influence of enzyme action on the accessibility of dissolving pulp, the alkali solubility of cellulose (in 8.5 % NaOH solution at low temperature) of pulps treated with E1-C2t1, E2-C2t1 and CBD-C2t1 was determined.
Pulps treated with enzymes showed a notable increase of solubility in alkali. In this way while untreated dissolving pulp showed a degree of dissolution of 19 %, samples treated with E1 or E2 showed a solubility of 25 and 22 %, respectively. Treatment with CBD did not produce a significant effect on cellulose solubility (Fig. 6). Taking into account the results it can be concluded that an enzymatic pre-treatment of cellulose leads to an increased solubility by increasing the accessibility of samples.
Similar results have been obtained by Cao and Tan (2006) who described an increase of solubility and accessibility of samples treated with crude cellulases from Humicola insolens, and also with an endoglucanase from Aspergillus sp. The authors found that the action of these cellulases increases both alkaline solubility and crystallinity of samples by the ability of these enzymes to decrease the polymerization degree and to break down some of the hydrogen bonds of cellulose. Rahkamo et al. (1996) described a 26 % increased alkali solubility using Tricoderma reesei endoglucanase II (EGII). Henriksson et al. (2005) reported considerable increased reactivity of the dissolving pulp even for very low dose treatment with a commercial cellulase.
The small difference between the effects of the two cellulases is undoubtedly due to the presence of the cellulose binding domain CBM3b in the E1 molecule (whole enzyme). The molecular binding mechanism of CBDs might destabilize the intermolecular hydrogen bonding within crystalline cellulose fibrils (Tormo et al. 1996) that could explain the ability of non-hydrolytic disruption of fibers proposed for CBDs (Din et al. 1991; Kataeva et al. 2002). Relying on this, the relaxation of the crystal structure should induce an improvement in the accessibility of cellulose. However, we didn’t observe an improvement in the alkali solubility of cellulose after treatment with CBD (CBM3b) in the tested conditions. An extended study of the application parameters would be necessary to determine the effect of the CBD on the accessibility and reactivity of cellulose. This would be interesting since its presence in E1 influenced the effectiveness of this cellulase as a biorefining aid (Cadena et al. 2010) even though treatment with CBD did not substantially affect mechanical properties of eucalyptus pulps,. As reported in this work the catalytic domain of E1 and E2 (GH9) was responsible for the endoglucanase effect on pulp. Meanwhile the CBD had an intramolecular synergistic effect. The importance of the catalytic domain for the endoglucanase action on pulps has been demonstrated also by Ibarra et al. (2010). They showed that the retaining mechanism of endoglucanases is an ineffective way to improve the cellulose reactivity, while endoglucanases with inverting mechanism, E1 and E2 falling into this category, can be efficiently applied. Therefore, they propose inverting cellulases, preferably with a CBD, as agents in the viscose process to improve the cellulose accessibility and reactivity and thus lower demand for carbon disulfide and its environmental impact.
Cellulose fibers that remained insoluble after the alkali treatment were analyzed by XRD and SEM.
From X-ray diffractograms, the first aspect that can be observed is the presence of crystalline structure of cellulose II, obtained after the treatment with NaOH solution (Fig. 7). The three main peaks have Miller indices of (1–10), (110) and (020), which correspond to Bragg angles of 12.18°, 20.17° and 21.62°, respectively (French 2014). The peak positions of cellulose II (Fig. 7a) will not agree exactly with those corresponding to the alkali-treated samples (Fig. 7b, c), possibly because of crystallite size variations resulting in different long-range compressive forces on the crystals and unit cells (French and Santiago Cintrón 2013; Nishiyama et al. 2012) as well as incomplete conversion to cellulose II.
X-ray diffraction patterns of a cellulose II (thick line—pattern obtained by using Mercury’s pwhm of 1.8°; thin line—pattern obtained by using Mercury’s default pwhm of 0.1°); b cellulose II (Dpulp-NaOH) with deconvoluted peaks; c alkali treated dissolving pulp and alkali treated pulps obtained after enzymatic treatment
Calculation of the crystallite sizes normal to the hkl planes were based on the estimation of the peak width at half-the-maximum amplitude (Table 5). The crystallite dimensions of three main equatorial reflections were determined, (1–10), (110) and (020), which measured the crystallite width (Ciolacu et al. 2011).
Table 5 shows that the (1–10) and (020) d-spacings of the alkali treated samples increase in all cases, in comparison with the samples not treated with alkali (Table 2), while the crystallite size normal to the (110) plane recorded a decrease. The same behavior was observed by Wang et al. (2008) who studied the dissolution behavior of enzyme pre-treated cotton fibers in NaOH/urea solution. The alkali treatment reduced the crystallinity index of all samples and the Icr of Dpulp, was reduced from 56.43 to 45.64 % (Dpulp-NaOH). Regarding the Icr of enzyme treated samples, the highest value was recorded by the pulp treated with E1 (48.88 %), while pulp treated with E2 showed an Icr of 46.28 %. These results are in good correlation with the solubility data, because a greater solubility reflects a higher release of the low-molecular weight fractions from the amorphous regions of celluloses which are more accessible as a result of the first step of enzymatic degradation.
The results obtained from X-ray diffraction suggest that as a first step, cellulose was attacked by cellulase in the direction of the (020) plane, cutting cellulose molecules. Then, the NaOH solution interacts with the cellulose crystal to break the inter- and intramolecular hydrogen bonds, reducing the length of cellulose crystal. Thus, enzymatic attack enhances the dissolution of cellulose, without changing the crystal type but slightly increases the crystallinity index. Subsequent treatment with NaOH solution at low temperature modified the crystal form from cellulose I to cellulose II, accompanied by a decreased crystallinity index and an increase of the accessibility of the cellulosic, results that are important factors in controlling the fibers’ reactivity.
The SEM micrographs reveal the effect on the NaOH solution on the surface morphology of the enzyme treated pulps (Fig. 8).
Treatment with NaOH caused a pronounced effect on the morphology of cellulosic fibers. The surfaces of the alkali-treated samples look smoother than those of fibers before the alkaline treatment (Fig. 5), as a result of the elimination of the salient fibrils and of the fragments stuck to the fibers. Moreover, the alkali treatment seems to permit a separation of the fiber bundles into individual fibers that show a more swollen and straightened state in comparison with the highly twisted untreated fibers. The NaOH solution influenced the fine structure of cellulosic samples by changing the fibers from the crystalline form of cellulose I–cellulose II, as was demonstrated by XRD. In addition, alkali solution has been reported to fill the central cavity (the lumen) almost entirely, so the fibers become more cylindrical and lose their convolutions, inducing a smoother and shinier texture (Navard et al. 2012). In case of the E1-NaOH sample it is obvious that the surfaces of the fibers became a bit rougher as a result of the pronounced action of cellulase E1, which permitted a rapid release of sugars and thus left the surface more accessible to reagent.
Conclusions
Current investigation demonstrated the influence of different treatments with a family GH9 modular endoglucanase from Paenibacillus barcinonensis (E1) and two derivatives of this enzyme (E2 and CBD), on the supramolecular structure of dissolving pulp. E1 and E2 have endoglucanase activity while CBD is an isolated cellulose binding domain without hydrolytic activity. Application of E1 and E2 produced a rapid release of sugars in the first hours of the treatments, followed by a slowed rate as the incubation progressed in time. A decrease of the degree of polymerization was observed, with the lowest DP values found for pulps obtained after hydrolysis with cellulase E1. XRD measurements of the cellulosic fibers reveal an increase of the crystallinity index during the enzymatic hydrolysis, most evident for cellulase E2. Moreover, there was an increase in the crystallite size perpendicular to the (200) plane. Ultrasonication caused a more accentuated depolymerization of the cellulosic samples and increased the crystallinity of the sample. The thermal decomposition revealed a decrease in the activation energy correlated with the decreased degree of polymerization and it was also observed that the enzyme-treated samples have higher thermal stability than the untreated cellulose. SEM shown a pronounced capacity of the enzymes to remove fuzz and improved the smoothness and surface appearance of the fibers, a fact more pronounced for cellulase E1. In addition, the results from this work showed that enzymatic hydrolysis enhanced the dissolution degree of cellulose in NaOH solution at low temperature, which indicates the potential application of the cellulases E1 and E2 for increasing the reactivity of cellulose.
References
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Boraston AB, Bolam DN, Gilbert HJ, Davies GJ (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382:769–781
Buschle-Diller G, Zeronian S, Pan N, Yoon M (1994) Enzymatic hydrolysis of cotton, linen, ramie, and viscose rayon fabrics. Text Res J 64:270–279
Cadena EM, Chriac AI, Pastor FIJ, Diaz P, Vidal T, Torres AL (2010) Use of cellulases and recombinant cellulose binding domains for refining TCF kraft pulp. Biotechnol Progress 26:960–967
Calahorra ME, Cortázar M, Eguiazábal JI, Guzmán GM (1989) Thermogravimetric analysis of cellulose—effect of the molecular-weight on thermal-decomposition. J Appl Polym Sci 37:3305–3314
Cao Y, Tan H (2005) Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzyme Microb Tech 36:314–317
Cao Y, Tan H (2006) Improvement of alkali solubility of cellulose with enzymatic treatment. Appl Microbiol Biot 70:176–182
Carrard G, Koivula A, Söderlund H, Béguin P (2000) Cellulose-binding domains promote hydrolysis of different sites on crystalline cellulose. Proc Natl Acad Sci USA 97:10342–10347
Chiriac AI, Cadena EM, Vidal T, Torres AL, Diaz P, Pastor FIJ (2010) Engineering a family 9 processive endoglucanase from Paenibacillus barcinonensis displaying a novel architecture. Appl Microbiol Biotechnol 86:1125–1134
Ciolacu D, Popa VI (2005) Structural changes of cellulose determined by dissolution in aqueous alkali solution. Cell Chem Technol 39:179–188
Ciolacu D, Popa VI (2006) Study on the thermal degradation of cellulose allomorphs. Cell Chem Technol 40:445–449
Ciolacu D, Ciolacu F, Popa VI (2008) Supramolecular structure—a key parameter for cellulose biodegradation. Macromol Symp 272:136–142
Ciolacu D, Gorgieva S, Tampu D, Kokol V (2011) Enzymatic hydrolysis of different allomorphic forms of microcrystalline cellulose. Cellulose 18:1527–1541
Din N, Gilkes NR, Tekant B, Miller RC, Warren RA, Kilburn DG (1991) Non-hydrolytic disruption of cellulose fibres by the binding domain of a bacterial cellulase. Nat Biotechnol 9:1096–1099
Duchemin B, Thuault A, Vicente A, Rigaud B, Fernandez C, Eve S (2012) Ultrastructure of cellulose crystallites in flax textile fibres. Cellulose 19:1837–1854
Engstrom A, Ek M, Henriksson G (2006) Improved accessibility and reactivity of dissolving pulp for the viscose process: pretreatment with monocomponent endoglucanase. Biomacromolecules 7:2027–2031
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose. doi:10.1007/s10570-013-0030-4
French AD, Santiago Cintrón M (2013) Cellulose polymorphy, crystallite size, and the Segal crystallinity index. Cellulose 20:583–588
García O, Torres AL, Colom JF, Pastor FIJ, Díaz P, Vidal T (2002) Effect of cellulase-assisted refining on the properties of dried and never-dried Eucalyptus pulp. Cellulose 9:115–125
Grigoriu AM, Luca C, Lisa G, Grigoriu A (2009) On the thermal stability of flax fabrics grafted with monochlorotriazinyl-β-cyclodextrin and treated with cinnamic derivatives. Cell Chem Technol 43:153–161
Gurgel LVA, Marabezi K, Ramos LA, Curvelo AAS (2012) Characterization of depolymerized residues from extremely low acid hydrolysis (ELA) of sugarcane bagasse cellulose: effects of degree of polymerization, crystallinity and crystallite size on thermal decomposition. Ind Crop Prod 36:560–571
Henriksson G, Christiernin M, Agnemo R (2005) Monocomponent endoglucanase treatment increases the reactivity of softwood sulphite dissolving pulp. J Ind Microbiol Biot 32:211–214
Hoshino E, Wada Y, Nishizawa K (1999) Improvements in the hygroscopic properties of cotton cellulose by treatment with an endo-type cellulase from Streptomyces sp. KSM-26. J Biosci Bioeng 88:519–525
Ibarra D, Köpcke V, Ek M (2010) Behavior of different monocomponent endoglucanases on the accessibility and reactivity of dissolving-grade pulps for viscose process. Enzyme Microb Tech 47:355–362
Isogai A, Atalla RH (1998) Dissolution of cellulose in aqueous NaOH solutions. Cellulose 5:309–319
Kataeva IA, Seidel RD, Shah A, West LT, Li X, Ljungdahl LG (2002) The fibronectin type 3-like repeat from the Clostridium thermocellum cellobiohydrolase CbhA promotes hydrolysis of cellulose by modifying its surface. Appl Environ Microbiol 68:4292–4300
Kim UJ, Eom SH, Wada M (2010) Thermal decomposition of native cellulose: influence on crystallite size. Polym Degradr Stabil 95:778–781
Klemm D, Schmauder HP, Heinze T (2002) Cellulose. In: Vandamme EJ, De Baets S, Steinbüchel A (eds) Biopolymers, vol 6. Wiley-VCH, Weinheim, pp 275–319
Krässig HA (1993) Methods of fiber structure characterization. In: Huglin MB (ed) Cellulose: structure, accessibility and reactivity, polymer monographs, chap 3, vol 11. Gordon and Breach Science Publishers, Philadelphia, pp 43–149
Lenze J, Esterbauer H, Sattler W, Schurz J, Wrentschur E (1990) Changes of structure and morphology of regenerated cellulose caused by acid and enzymatic hydrolysis. J Appl Poly Sci 41:1315–1326
Liu H, Fu S, Zhu JY, Li H, Zhan H (2009) Visualization of enzymatic hydrolysis of cellulose using AFM phase imaging. Enzyme Microb Tech 45:274–279
Liu YS, Baker JO, Zeng Y, Himmel ME, Haas T, Ding SY (2011) Cellobiohydrolase hydrolyzes crystalline cellulose on hydrophobic faces. J Biol Chem 286:11195–11201
Mansfield SD, Jong ED, Stephens RS, Saddler JN (1997) Physical characterization of enzymatically modified kraft pulp fibers. J Biotechnol 57:205–216
Mazeau K (2011) On the external morphology of native cellulose microfibrils. Carbohydr Polym 84:524–532
Mazeau K, Charlier L (2012) The molecular basis of the adsorption of xylans on cellulose surface. Cellulose 19:337–349
Morgado DL, Frollini E (2011) Thermal decomposition of mercerized linter cellulose and its acetates obtained from a homogeneous reaction. Polímeros 21:111–117
Navard P, Wendler F, Meister F, Bercea M, Budtova T (2012) Preparation and properties of cellulose solutions. In: Navard P (ed) The European Polysaccharide network of excellence (EPNOE), chap 5. Springer, Wien, pp 91–152
Newman RH (2004) Carbon-13NMR evidence for cocrystallization of cellulose as a mechanism for hornification of bleached kraft pulp. Cellulose 11:45–52
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124(31):9074–9082
Nishiyama Y, Johnson GP, French AD (2012) Diffraction from nonperiodic models of cellulose crystals. Cellulose 19:319–336
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10
Pastor FIJ, Pujol X, Blanco A, Vidal T, Torres AL, Díaz P (2001) Molecular cloning and characterization of a multidomain endoglucanase from Paenibacillus sp BP-23: evaluation of its performance in pulp refining. Appl Microbiol Biot 55:61–68
Penttilä PA, Várnai A, Pere J, Tammelin T, Salmén L, Siika-Aho M, Viikari L, Serimaa R (2013) Xylan as limiting factor in enzymatic hydrolysis of nanocellulose. Biores Technol 129:135–141
Poletto M, Zattera AJ, Forte MMC, Santana RMC (2012) Thermal decomposition of wood: influence of wood components and cellulose crystallite size. Biores Technol 109:148–153
Puls J, Wood TM (1991) The degradation pattern of cellulose by extracellular cellulases of aerobic and anaerobic microorganisms. Biores Technol 36:15–19
Rahkamo L, Siika-Aho M, Vehviläinen M, Dolk M, Viikari L, Nousiainen P, Buchert J (1996) Modification of hardwood dissolving pulp with purified Trichoderma reesei cellulases. Cellulose 3:153–163
Rahkamo L, Viikari L, Buchert J, Paakkari T, Suortti T (1998) Enzymatic and alkaline treatments of hardwood dissolving pulp. Cellulose 5:79–88
Rondeau-Mouro C, Bizot H, Bertrand D (2011) Chemometric analyses of the 1H–13C cross-polarization build-up of celluloses NMR spectra: a novel approach for characterizing the cellulose crystallites. Carbohydr Polym 84:539–549
Sánchez MM, Pastor FIJ, Diaz P (2003) Exo-mode of action of cellobiohydrolase Cel48C from Paenibacillus sp. BP-23, a unique type of cellulase among Bacillales. Eur J Biochem 270:2913–2919
Sánchez MM, Fritze D, Blanco A, Spröer C, Tindall BJ, Schumann P, Kroppenstedt RM, Diaz P, Pastor FIJ (2005) Paenibacillus barcinonensis sp. nov., a xylanase-producing bacterium isolated from a rice field in the Ebro River delta. Int J Syst Evol Microbiol 55:935–939
Sun Y, Zhuang J, Lin L, Ouyang P (2009) Clean conversion of cellulose into fermentable glucose. Biotechnol Adv 27:625–632
Tappi Test Method (1997) Viscosity of pulp. T230 om-94
Tappi Test Method (2002) Forming handsheets for physical tests of pulp. T205 sp-02
Tomme P, Boraston A, McLean B, Kormos J, Creagh AL, Sturch K, Gilkes NR, Haynes CA, Warren RA, Kilburn DG (1998) Characterization and affinity applications of cellulose-binding domains. J Chromatogr B Biomed Sci Appl 7(15):283–296
Tormo J, Lamed R, Chirino AJ, Morag E, Bayer EA, Shoham Y, Steitz TA (1996) Crystal structure of a bacterial family-III cellulose-binding domain: a general mechanism for attachment to cellulose. EMBO J 15:5739–5751
Wang Y, Zhao Y, Deng Y (2008) Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohyd Polym 72:178–184
Yue Y, Zhou C, French AD, Xia G, Han G, Wang Q, Wu Q (2012) Comparative properties of cellulose nano-crystals from native and mercerized cotton fibers. Cellulose 19:1173–1187
Zhang YP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng 88:797–824
Acknowledgments
This work was partially supported by the Spanish Ministry of Education and Science, grant No CTQ2010-20238-C03-02. Iulia Chiriac held a FI grant from Generalitat de Catalunya. Dr. Diana Ciolacu acknowledges the financial support of European Social Fund—“Cristofor I. Simionescu” Postdoctoral Fellowship Programme (ID POSDRU/89/1.5/S/55216), Sectoral Operational Programme Human Resources Development 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiriac, A.I., Pastor, F.I.J., Popa, V.I. et al. Changes of supramolecular cellulose structure and accessibility induced by the processive endoglucanase Cel9B from Paenibacillus barcinonensis . Cellulose 21, 203–219 (2014). https://doi.org/10.1007/s10570-013-0118-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0118-x