Abstract
The [Amb]l-prolinate obtained from the immobilization of l-prolinate anion onto amberlite IRA900OH, was used as a new organocatalyst for the efficient synthesis of 4,4′-(arylmethylene)-bis-(1H-pyrazol-5-ol) derivatives. The prepared heterogeneous organocatalyst was well characterized by using of FTIR, TGA, DTG, XRD and elemental analysis techniques. Short reaction times (5–18 min), excellent yields (82–98 %) and simple experimental procedure with an easy work-up are some of the advantages of the procedure.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the last few decades, green chemistry has been recognized as a culture and methodology for achieving sustainable development [1]. Catalysis (including enzyme catalysis, heterogeneous catalysis, and organocatalysis, in particular) is identified to be at the heart of greening of chemistry [2] because this branch of science is found to reduce the environmental impact of chemical processes [3]. The absence of metal in organocatalyst brings an undeniable advantage considering both the principles of “green chemistry” and the economic point of view [4]. At the same time immobilization and recycling of organocatalysts have experienced a very good growth [5–9]. From both industrial and environmental viewpoints, Immobilization is an effective methodology for heterogenizing an organic molecule onto inorganic material. The two principal mechanisms used to prepare the heterogeneous organocatalysts are of both covalent and noncovalent nature which lead to (i) the formation of covalent adduct between catalyst and substrate, and (ii) the processes that rely on noncovalent interactions such as hydrogen bonding or within the catalytic cycle is the formation of ions pairs [10].
The proline catalyzed Robinson annulation was one of the earliest examples of an enantioselective reaction using an organic catalyst [11]. In recent years, prolines, especially l-proline, have been used to catalyze essential transformations in the fine chemical and pharmaceutical industries, such as Multicomponent reactions [12–14]. These amino acids contain both a nucleophilic secondary amino group and a carboxylic acid moiety functioning as a Brønsted acid [4]. Although the l-proline is commercially available at low cost, however the reaction catalysis by using of l-proline requires high catalyst loading. In general, organocatalytic methods often require catalyst loadings as high as 30 mol% for the achievement of high conversions in reasonable reaction times [15]. This is the main reason for the need of an efficient immobilization and recycling procedure. Therefore, attempts to improve or modify the catalytic activity of l-proline, taking advantages of specific properties of the support, would justify the immobilization in many instances [16].
Presently, pyrazolone derivatives are paid much attention for their different biological activities, such as selective COX-2 inhibitory [17] and antitumor [18]. Among them, compounds that contain two pyrazolone rings can be used as gastric secretion stimulatory [19], antidepressant [20] and antibacterial [21, 22]. In addition, the analogous 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol)s are used as insecticides [23], pesticides [24], fungicides [25] and chelating as well as extracting reagents for different metal ions [26]. The most common method for the preparation of this type of compounds involves one-pot pseudo three-component condensation of aldehydes with 3-methyl-1-phenyl-2-pyrazolin-5-one. Therefore, a variety of catalysts and reagents have been used for this reaction, including H3PW12O40 [27], Nano-SiO2/HClO4 [28], silica-bonded N-propyltriethylenetetramine [29], nano n-propylsulfonated γ-Fe2O3 [30], Poly(4-vinylpyridine)-supported dual acidic ionic liquid [31], 1-sulfopyridinium chloride [32], phosphomolybdic acid [33], 3-amminopropylated silica gel [34], poly(ethylene glycol)-bound sulfonic acid [35], xanthan sulfuric acid [36], LiOH·H2O [37], 1,3-disulfonic acid imidazolium tetrachloroaluminate [38], sulfuric acid ([3-(3-silicapropyl) sulfanyl]propyl)ester [39], poly(ethylene glycol)-400 [40] and silica-bonded S-sulfonic acid [41]. Some of these methods suffer from limitations such as long reaction times, unsatisfactory yield, harsh reaction conditions, complex and expensive catalysts and tedious workup procedures.
Considering the above mentioned reasons and in continuation of our previous studies on the applications of ion-exchange resins for click synthesis of 1,4-disubstituted-1H-1,2,3-triazoles [42, 43], we have prepared and characterized ion-exchange resin Amberlite IRA900OH (AmbIRA900OH) as a cationic polymer support for the ion-pair immobilization of l-proline anion via ionic interaction between carboxylate group of l-prolinate and quaternary ammonium cation of the cationic Amb support. The heterogeneous catalyst was utilized as an efficient and reusable organocatalyst for the one-pot pseudo three-component synthesis of 4,4′-(arylmethylene)-bis-(1H-pyrazol-5-ol)s in ethanol.
2 Experimental
2.1 General
All chemicals were purchased from Merck or Fluka Chemical Companies. The chemicals were used in this study with their purity included: 3-methyl-1-phenyl-2-pyrazolin-5-one (≥98 %), benzaldehyde (≥99 %), 4-nitrobenzaldehyde (≥98 %), 4-chlorobenzaldehyde (≥98 %), 2,4-dichlorobenzaldehyde (≥98 %) 3-chlorobenzaldehyde (≥98 %), 4-hydroxybenzaldehyde (≥98 %), 3-hydroxybenzaldehyde (≥99 %), 3,4-isopropylbenzaldehyde (≥99 %), 3,4-dimethoxybenzaldehyde (≥99 %), 4-methylbenzaldehyde (≥99 %), 3-bromobenzaldehyde (≥98 %), 2-bromobenzaldehyde (≥98 %), ethanol (≥99.5 %), 4-methoxybenzaldehyde (≥98 %), 4-fluorobenzaldehyde (≥98 %), phenylglyoxal monohydrate (≥97 %), 4-methylbenzaldehyde (≥97 %), 4-cyanobenzaldehyde. Amberlite IRA 900 (OH-form) was purchased from Sigma-Aldrich Company and was used in its original form. 1H NMR spectra of samples were recorded at a Bruker Advanced DPX 400-MHz spectrometer. X-ray diffraction (XRD) patterns were recorded on a Philips X’PERT-Pro-MPD diffractometer using Cu Ka radiation (k = 1.542 Å). A continuous scan mode was used to collect 2 h from 5 to 40. Fourier transform infrared (FT-IR) spectra were obtained as potassium bromide pellets in the range 400–4000 cm−1 using an AVATAR 370 Thermo Nicolet spectrophotometer. Elemental analyses (C, H, and N) were performed with a Heraeus CHN-O-Rapid analyzer. The thermogravimetric and differential thermogravimetric (TG–DTG) analysis was performed on Netsch STA449c. The sample weight was ca. 10 mg and was heated from room temperature up to 600 °C with 10 °C/min using alumina sample holders.
2.2 Preparation of the [Amb]l-Prolinate catalyst
Amberlite IRA-900OH (mesh 16–50, 1 g) was suspended in 10 mL of a 1 M solution of l-proline in MeOH:H2O (1:1). The system was heated at 60 °C for 6 h. The filtration of the reaction mixture followed by washing with MeOH:H2O (1:1) (2 × 10 mL) and H2O (2 × 10 mL) afforded [Amb]l-Prolinate catalyst. The prepared catalyst was collected and dried under vacuum.
2.3 General Procedure for the [Amb]l-Prolinate Catalyzed Synthesis of 3,3′-Diaryloxindol Derivatives
A mixture of 3-methyl-1-phenyl-2-pyrazoline-5-one (2 mmol), aromatic aldehyde (1 mmol), were placed together in a round-bottom flask containing 5 mL of EtOH. [Amb]l-prolinate catalyst (0.08 g, 10 mol%), was added to the mixture. The suspension was magnetically stirred at reflux condition for appropriate time according to Table 2. After completion of the reaction as followed by TLC (n-hexane: ethyl acetate; 3:1), the catalyst was filtered and washed with hot ethanol (2 × 5 mL). The recovered catalyst was washed with acetone, dried and stored for other similar consecutive runs. The filtrate mixture was recrystallized to provide the pure crystals of 4,4′-(arylmethylene)-bis-(1H-pyrazol-5-ol) derivatives. The products are known compounds and are characterized by IR and NMR spectroscopy data for new compounds. Their melting points are compared with reported values [27–41].
2.3.1 Selected Spectroscopic Data
2.3.1.1 4,4′-(Phenylmethylene)-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3a)
White powder, recrystallized from ethanol, yield: 0.36 g (82 %), m.p. 169–171 °C (171–172 °C [27]). IR (KBr): \(\overline{\nu }\) = 3400 (OH), 3050 (CH arom), 2900 (CH aliph), 1598 (C=C), 1496 (C=C), 1403 (C=C), 1270 (C=N), 1020, 730, 690 cm−1. 1H NMR: δ = 2.34 (6H, s, 2 CH3), 4.99 (1H, s, CH), 7.16–7.32 (7H, m, 7 CH), 7.45 (4H, t, J = 7.6 Hz, 4 CH), 7.73 (4H, d, J = 8.0 Hz, 4 CH), 13.98 (2H, br.s, 2 OH) ppm. 13C NMR: δ = 12.12 (CH3), 33.60 (CH), 121.0 (C), 126.04 (CH), 126.38 (CH), 127.67 (CH), 128.61(C), 129.40 (CH), 142.68 (C), 146.81(C) ppm.
2.3.1.2 4,4′-[(4-Methylphenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3b)
White powder, recrystallized from ethanol, yield: 0.38 g (85 %), m.p. 203–205 °C (203 °C [27]). IR (KBr): \(\overline{\nu }\) = 3450 (OH), 3025 (CH arom), 2850 (CH aliph), 1598 (C=C), 1499 (C=C), 1410 (C=C), 1290 (C=N), 1022, 800, 745, 690.1H NMR: δ = 2.26 (3H, s, CH3), 2.33 (6H, s, 2 CH3), 4.93 (1H, s, CH), 7.08 (2H, d, J = 8.0 Hz, 2CH), 7.15 (2H, d, J = 8.0 Hz, 2 CH), 7.25 (2H, t, J = 7.6 Hz, 2 CH), 7.45 (4H, t, J = 7.6 Hz, 4 CH), 7.72 (4H, d, J = 7.6 Hz, 4 CH), 13.93 (2H, br.s, 2 OH). 13C NMR: δ = 12.10 (CH3), 21.01(CH3), 33.23 (CH), 120.95 (C), 126.02 (CH), 127.56 (CH), 129.17 (C), 129.40 (CH), 135.31(CH), 139.55 (C), 146.78 (C) ppm.
2.3.1.3 4,4′-[(4-Chlorophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3c)
White powder, recrystallized from ethanol, yield: 0.43 g (92 %), m.p. 211–213 °C (210 °C [27]). IR (KBr): \(\overline{\nu }\) = 3400 (OH), 3050 (CH arom), 2900 (CH aliph), 1600 (C=C), 1590 (C=C), 1578 (C=C), 1498 (C=C), 1415 (C=C), 1280 (C=N), 808, 742, 685 cm−1. 1H NMR: δ = 2.34 (6H, s, 2 CH3), 4.99 (1H, s, CH), 7.24-7.29 (4H, m, 4 CH), 7.35 (2H, d, J = 8.4 Hz, 2 CH), 7.46 (4H, t, J = 7.6 Hz, 4 CH), 7.72 (4H, d, J = 8 Hz, 4 CH), 13.89 (2H, br.s, 2 OH) ppm. 13C NMR: δ = 12.07 (CH3), 33.02 (CH), 121.01(C), 126.11 (CH), 128.50 (C), 129.41(CH), 129.63 (CH), 131.06 (CH), 141.58 (C), 149.76 (C) ppm.
2.3.1.4 4,4′-[(2-Chlorophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3d)
White powder, recrystallized from ethanol, yield: 0.38 g (80 %), m.p. 233–235 °C (236–237 °C [27]). IR (KBr): \(\overline{\nu }\) = 3450 (OH), 3060 (CH arom), 2905 (CH aliph), 1615 (C=C), 1595 (C=C), 1555 (C=C), 1496 (C=C), 1400 (C=C), 1350, 1295 (C=N), 836, 750, 690 cm−1. 1H NMR: δ = 2.30 (6H, s, 2 CH3), 5.17 (1H, s, CH), 7.22–7.34 (4H, m, 4 CH), 7.40 (1H, d, J = 7.82 Hz, CH), 7.44 (4H, t, J = 8.0 Hz, 4 CH), 7.71 (4H, d, J = 8.0 Hz, 4 CH), 7.81 (1H, d, J = 7.2 Hz, CH), 13.92 (2H, br.s, 2 OH) ppm. 13C NMR: δ = 12.33 (CH3), 32.14 (CH), 121.07 (C), 126.13 (CH), 127.4 (CH), 128.55 (C), 129.41(CH), 129.95(CH), 130.71(CH), 132.4 (CH), 137.63 (CH), 139.89 (C), 146.54(C) ppm.
2.3.1.5 4,4′-[(2,4-Dichlorophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3e)
White powder, recrystallized from ethanol, yield: 0.42 g (84 %), m.p. 229–231 °C (228–230 °C [27]). IR (KBr): \(\overline{\nu }\) = 3480 (OH), 3050 (CH arom), 2900 (CH aliph), 1620 (C=C), 1590 (C=C), 1550 (C=C), 1499 (C=C), 1420 (C=C), 1380, 1290 (C=N), 850, 760, 680 cm−1. 1H NMR: δ = 2.33 (6H, s, 2 CH3), 5.22 (1H, s, CH), 7.11 (1H, d, J = 7.8 Hz, CH), 7.25 (1H, d, J = 7.83 Hz, CH), 7.45 (2H, t, J = 7.72 Hz, 2 CH), 7.58 (4H, t, J = 7.76 Hz, 4 CH), 7.62 (4H, d, J = 7.75 Hz, 4 CH),7.68 (1H, s, CH), 13.88 (2H, br.s, 2 OH) ppm. 13C NMR: δ = 12.46 (CH3), 33.54 (CH), 122.3 (C), 122.8 (CH), 126.54 (CH), 126.8 (CH), 128.76 (C), 129.85 (CH), 130.3 (CH), 131.8 (CH), 132.7 (C), 135.7 (C), 136.2 (C), 137.5 (C), 145.2 (C) ppm.
2.3.1.6 4,4′-[(4-Nitrophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3f)
Yellow powder, recrystallized from ethanol, yield: 0.46 g (96 %), m.p. 225–227 °C (224–226 °C [27]). IR (KBr): \(\overline{\nu }\) = 3420 (OH), 3060 (CH arom), 2800 (CH aliph), 1598 (C=C), 1510 (C=C), 1490 (C=C), 1419 (C=C), 1400 (C=C), 1290 (C=N), 1342, 750, 690 cm−1. 1H NMR: δ = 2.37 (6H, s, 2 CH3), 5.15 (1H, s, CH), 7.26 (2H, t, J = 7.6 Hz, 2 CH), 7.46 (4H, t, J = 8.0 Hz, 4 CH), 7.53 (2H, d, J = 8.4 Hz, 2 CH), 7.72 (4H, d, J = 8.0 Hz, 4 CH), 8.18 (2H, d, J = 8.8 Hz, 2 CH), 13.88 (2H, br.s, 2 OH) ppm. 13C NMR: δ = 12.06 (CH3), 33.65 (CH), 121.07 (C), 123.82 (CH), 126.2 (CH), 129.1 (C), 129.42 (CH), 137.64 (C), 146.4 (C), 146.79 (C), 151.79 (C) ppm.
2.3.1.7 4,4′-[(3-Nitrophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3g)
Pale yellow powder, recrystallized from ethanol, yield: 0.41 g (86 %), m.p. 152–154 °C (149–150 °C [27]). IR (KBr): \(\overline{\nu }\) = 3400 (OH), 3050 (CH arom), 2915 (CH aliph), 1610 (C=C), 1590 (C=C), 1510 (C=C), 1498 (C=C), 1420 (C=C), 1345 (C=N), 760, 725, 690, 598 cm−1. 1H NMR: δ = 2.37 (6H, s, 2 CH3), 5.17 (1H, s, CH), 7.27 (2H, t, J = 7.2 Hz, 2 CH), 7.46 (4H, t, J = 8.0 Hz, 4 CH), 7.62 (1H, t, J = 8.4 Hz, CH), 7.71-7.76 (5H, m, 5 CH), 8.09 (2H, d, J = 6.8 Hz, 2 CH), 13.89 (2H, br.s, 2 OH) ppm. 13C NMR: δ = 12.09 (CH3), 33.31(CH), 121.10 (C), 121.69 (CH), 122.23 (CH), 126.23(CH), 129.45 (C), 130.18 (CH), 134.83 (CH), 145.06 (C), 146.82 (C), 148.24 (C) ppm.
2.3.1.8 4,4′-[(4-Hydroxyphenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3h)
Yellow powder, recrystallized from ethanol, yield: 0.38 g (84 %), m.p. 153–155 °C (152–153 °C [27]). IR (KBr): \(\overline{\nu }\) = 3480 (OH), 3050 (CH arom), 2900 (CH aliph), 1595 (C=C), 1585 (C=C), 1450 (C=C), 1360, 1280 (C=N), 750, 690, 600, 595 cm−1. 1H NMR: δ = 2.31 (6H, s, 2 CH3), 4.86 (1H, s, CH), 6.67 (2H, d, J = 8.7 Hz, 2 CH), 7.06 (2H, d, J = 8.5 Hz, 2 CH), 7.25 (2H, t, J = 7.33 Hz, 2 CH), 7.45 (4H, t, J = 7.83 Hz, 4 CH), 7.72 (4H, d, J = 7.98 Hz, 4 CH), 9.16 (1H, s, OH), 12.2 (1H, br.s, OH), 13.96 (1H, br.s, OH) ppm. 13C NMR: δ = 12.50 (CH3), 33.25 (CH), 115.73 (CH), 121.37 (C), 126.38 (CH), 128.96 (C), 129.77 (CH), 133.18 (CH), 147.04 (C), 156.37 (C) ppm.
2.3.1.9 4,4′-[(3-Hydroxyphenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3i)
Yellow powder, recrystallized from ethanol, yield: 0.40 g (88 %), m.p. 169–170 °C (165–168 °C [27]). IR (KBr): \(\overline{\nu }\) = 3460 (OH), 3050 (CH arom), 2950 (CH aliph), 1599 (C=C), 1580 (C=C), 1455 (C=C), 1369, 1250 (C=N), 790, 680, 600, 590 cm−1. 1H NMR: δ = 2.36 (6H, s, 2 CH3), 4.91 (1H, s, CH), 6.76 (1H, d, J = 8.21 Hz, CH), 6.91 (1H, d, J = 8.26 Hz, CH), 6.97 (1H, s, CH), 7.16 (1H, t, J = 8.0 Hz, CH), 7.48 (2H, t, J = 7.46 Hz, 2 CH), 7.56 (4H, t, J = 7.67 Hz, 4 CH), 7.62 (2H, d, J = 7.31 Hz, 2 CH), 9.43 (1H, s, OH), 12.5 (1H, br.s, OH), 13.88 (1H, br.s, OH) ppm. 13C NMR: δ = 13.3 (CH3), 32.9 (CH), 112.9 (CH), 114.7 (CH), 121.3 (C), 121.6 (CH), 122.5 (CH), 126.2 (CH), 128.8 (C), 129.3 (CH), 130.0 (CH), 137.5 (C), 139.2 (C), 147.1(C), 156.7 (C) ppm.
2.3.1.10 4,4′-[(3,4-Dimethoxyphenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3j)
White cream powder, recrystallized from ethanol, yield: 0.40 g (81 %), m.p. 194–196 °C (19–197 °C [27]). IR (KBr): \(\overline{\nu }\) = 3420 (OH), 3150 (CH arom), 3090 (CH arom), 2920 (CH aliph), 1590 (C=C), 1500 (C=C), 1420 (C=C), 1270 (C=N), 1120 (C–O), 745, 690 cm−1. 1H NMR: δ = 2.29 (6H, s, 2 CH3), 3.63 (3H, s, OCH3), 3.67 (3H, s, OCH3), 4.86 (1H, s, CH), 6.78–6.83 (2H, m, 2 CH), 6.87 (1H, s, CH), 7.20 (2H, t, J = 6.8 Hz, 2 CH), 7.40 (4H, t, J = 7.4 Hz, 4 CH), 7.68 (4H, d, J = 7.8 Hz, 4 CH), 13.99 (1H, s, OH) ppm. 13C NMR: δ = 12.53 (CH3), 33.82 (CH), 56.39 (OCH3), 56.43 (OCH3), 112.56 (CH), 112.68 (CH), 120.20 (C), 121.46 (CH), 126.44 (CH), 129.78 (C), 135.85 (CH), 138.19 (C), 147.07(C), 148.15 (C), 149.32 (C) ppm.
2.3.1.11 4,4′-[(4-Methylthiophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3k)
Yellow powder, recrystallized from ethanol, yield: 0.41 g (86 %), m.p. 205–207 °C (201–203 °C [27]). IR (KBr): \(\overline{\nu }\) = 3440 (OH), 3055 (CH arom), 2900 (CH aliph), 1590 (C=C), 1500 (C=C), 1450(C=C), 1300 (C=N), 1040, 790, 750, 680 cm−1. 1H NMR: δ = 2.52 (6H, s, 2 CH3), 2.53 (3H, s, CH3), 4.81 (1H, s, CH), 7.22 (2H, d, J = 7.92 Hz, 2 CH), 7.31 (2H, d, J = 7.81 Hz, 2 CH), 7.38 (2H, t, J = 7.1 Hz, 2 CH), 7.48 (4H, t, J = 7.5 Hz, 4 CH), 7.79 (4H, d, J = 7.72 Hz, 4 CH), 13.91 (2H, br.s, OH) ppm. 13C NMR: δ = 12.62 (CH3), 21.54 (CH3), 34.24 (CH), 121.30 (C), 122.84 (CH), 122.09 (CH), 127.68 (CH), 128.98 (C), 129.33 (CH), 129.89 (CH), 134.39 (C), 134.85 (C), 137.54 (C), 138.65 (C), 145.84 (C) ppm.
2.3.1.12 4,4′-[(4-Cyanophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3l)
Yellow powder, recrystallized from ethanol, yield: 0.43 g (93 %), m.p. 206–208 °C (210–212 °C [27]). IR (KBr): \(\overline{\nu }\) = 3400 (OH), 3050 (CH arom), 2900 (CH aliph), 2210 (CN), 1595 (C=C), 1500 (C=C), 1458 (C=C), 1410, 1280 (C=N), 808, 748, 682 cm−1. 1H NMR: δ = 2.36 (6H, s, 2 CH3), 5.09 (1H, s, CH), 7.26 (2H, t, J = 7.2 Hz, 2 CH), 7.43–7.49 (6H, m, 6 CH), 7.73–7.79 (6H, m, 6 CH), 14.01 (1H, br.s, OH) ppm. 13C NMR: δ = 12.10 (CH3), 33.81(CH), 109.28 (C), 119.51 (C), 120.69 (C), 121.06 (CH), 125.35 (CH), 126.12 (CH), 128.88 (C), 129.4 (CH), 132.58 (CH), 137.74 (C), 146.79 (C), 148.71(C) ppm.
2.3.1.13 4,4′-[(2-Hydroxyphenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3m)
Yellow powder, recrystallized from ethanol, yield: 0.36 g (80 %), m.p. 228–230 °C (230–231 °C [28]). IR (KBr): \(\overline{\nu }\) = 3400 (OH), 3050 (CH arom), 2900 (CH aliph), 1596 (C=C), 1570 (C=C), 1498 (C=C), 1450 (C=C), 1366, 1280 (C=N), 750, 687 cm−1. 1H NMR: δ = 2.32 (6H, s, 2 CH3), 5.21 (1H, s, CH), 6.72–6.79 (2H, m, 2 CH), 6.99 (1H, t, J = 7.6 Hz, CH), 7.25 (2H, t, J = 7.2 Hz, 2 CH), 7.45 (4H, t, J = 7.6 Hz, 4 CH), 7.59 (1H, d, J = 7.6 Hz, CH), 7.72 (4H, d, J = 8.0 Hz, 4 CH), 9.58 (1H, br.s, OH), 12.33 (1H, br.s, OH), 14.39 (1H, br.s, OH) ppm. 13C NMR: δ = 12.24 (CH3), 27.77 (CH), 115.29 (CH), 119.08 (C), 121.08 (CH), 125.97 (C), 127.39 (CH), 129.27 (C), 129.38 (CH), 129.69 (CH), 146.81(C), 154.35 (C) ppm.
2.3.1.14 4,4′-[(4-Fluorophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3n)
White powder, recrystallized from ethanol, yield: 0.43 g (94 %), m.p. 180–182 °C (182 °C [28]). IR (KBr): \(\overline{\nu }\) = 3480 (OH), 3050 (CH arom), 2800 (CH aliph), 1600 (C=C), 1585 (C=C), 1498 (C=C), 1400 (C=C), 1370, 1318, 1219 (C=N), 1150 (C–F), 1020, 800, 790, 745, 680 cm−1. 1H NMR: δ = 2.34 (6H, s, 2 CH3), 4.99(1H, s, CH), 7.12 (2H, t, J = 8.8 Hz, 2 CH), 7.24-7.32 (4H, m, 4 CH), 7.45 (4H, t, J = 8.0 Hz, 4 CH), 7.73 (4H, d, J = 8.0 Hz, 4 CH), 13.95 (1H, br.s, OH) ppm. 13C NMR: δ = 12.09 (CH3), 32.92 (CH), 115.11(CH), 115.32 (CH), 121.02 (CH), 126.08 (CH), 129.4 (C), 129.47 (CH), 129.55 (C), 138.65 (C), 146.71 (C), 159.95–162.36 (C–F, 1 J CF = 241 Hz) ppm.
2.3.1.15 4,4′-[(4-Isopropylphenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3o)
White cream powder, recrystallized from ethanol, yield: 0.39 g (82 %), m.p. 132–134 °C (132–134 °C [28]). IR (KBr): \(\overline{\nu }\) = 3400 (OH), 3090 (CH arom), 2950–2850 (CH aliph), 1610 (C=C), 1598 (C=C), 1578 (C=C), 1495 (C=C), 1419, 1410, 1370, 1358, 1280 (C=N), 1020, 780, 745, 682 cm−1. 1H NMR: δ = 1.17 (6H, d, J = 6.8 Hz, 2 CH3), 2.36 (6H, s, 2 CH3), 2.83 (1H, sep, J = 6.8 Hz, CH), 4.93 (1H, s, CH), 7.15 (2H, d, J = 8.0 Hz, 2 CH), 7.19-7.27 (4H, m, 4 CH), 7.45 (4H, t, J = 7.6 Hz, 4 CH), 7.74 (4H, d, J = 8.0 Hz, 4 CH), 14.11 (1H, br.s, OH) ppm. 13C NMR: δ = 12.14 (CH3), 24.40 (CH3), 33.32 (CH), 33.47 (CH), 118.81 (C), 120.96 (CH), 125.98 (CH), 126.54 (CH), 127.55 (C), 129.38 (CH), 137.92 (C), 140.21 (C), 146.33 (C), 146.77 (C) ppm.
2.3.1.16 4,4′-[(4-Methoxyphenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3p)
White cream powder, recrystallized from ethanol, yield: 0.41 g (89 %), m.p. 174–176 °C. IR (KBr): \(\overline{\nu }\) = 3450 (OH), 3050 (CH arom), 2800 (CH aliph), 1605 (C=C), 1598 (C=C), 1577 (C=C), 1505 (C=C), 1490 (C=C), 1385, 1245 (C=N), 1180, 1110, 1038, 870, 812, 785, 750, 690 cm−1. 1H NMR: δ = 2.33 (6H, s, 2 CH3), 3.71 (3H, s, OCH3), 4.92 (1H, s, CH), 6.85 (2H, d, J = 8.8 Hz, 2 CH), 7.19 (2H, d, J = 8.4 Hz, 2 CH), 7.25 (2H, t, J = 7.2 Hz, 2 CH), 7.45 (4H, t, J = 7.6 Hz, 4 CH), 7.73 (4H, d, J = 8.0 Hz, 4 CH), 14.03 (1H, br.s, OH) ppm. 13C NMR: δ = 12.13 (CH3), 32.87 (CH), 55.46 (OCH3), 113.98 (CH), 120.94 (C), 125.97 (C), 128.65 (C), 129.38 (CH), 134.61(CH), 137.91(CH), 146.68 (C), 157.99 (C) ppm. Anal. Calcd for C27H26N4O3: C, 72.09; H, 5.62; N, 12.01 (%). Found: C, 72.03; H, 5.45; N, 12.14(%).
2.3.1.17 4,4′-[(2-Bromophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3q)
White cream powder, recrystallized from ethanol, yield: 0.44 g (86 %), m.p. 201–204 °C (198–200 °C [28]). IR (KBr): \(\overline{\nu }\) = 3450 (OH), 3050 (CH arom), 2900 (CH aliph), 1618 (C=C), 1596 (C=C), 1565 (C=C), 1550 (C=C), 1495 (C=C), 1395, 1365, 1310 (C=N), 1020, 900, 838, 790, 750, 690 cm−1. 1H NMR: δ = 2.31 (6H, s, 2 CH3), 5.12 (1H, s, CH), 7.15 (1H, t, J = 7.2 Hz, CH), 7.6 (2H, t, J = 7.2 Hz, 2 CH), 7.35 (1H, t, J = 7.6 Hz, CH), 7.45 (4H, t, J = 7.6 Hz, 4 CH), 7.55 (1H, d, J = 8.0 Hz, CH), 7.71 (4H, d, J = 8.0 Hz, 4 CH), 7.83 (1H, m, CH), 12.58 (1H, br.s, OH), 13.76 (1H, s, OH) ppm. 13C NMR: δ = 12.55 (CH3), 34.82 (CH), 121.03 (CH), 123.30 (CH), 126.06 (C), 127.92 (CH), 128.84 (C), 129.41 (CH), 131.01(CH), 133.30 (CH), 141.48 (C), 146.53 (C) ppm.
2.3.1.18 4,4′-[(3-Bromophenyl)Methylene)]-Bis-(3-Methyl-1-Phenyl-1H-Pyrazol-5-ol) (3r)
White cream powder, recrystallized from ethanol, yield: 0.45 g (88 %), m.p. 164–166 °C. IR (KBr): \(\overline{\nu }\) = 3400 (OH), 3050 (CH arom), 2950–2800 (CH aliph), 1610 (C=C), 1595 (C=C), 1578 (C=C), 1563 (C=C), 1490, 1450,1398, 1362, 1318, 1299 (C=N),1000, 862, 790,768, 755, 684 cm−1; 1H NMR: δ = 2.35 (6H, s, 2 CH3), 5.02 (1H, s, CH), 7.24–7.32 (4H, m, 4 CH), 7.39–7.48 (6H, m, 6 CH), 7.73 (4H, d, J = 8.4 Hz, 4CH), 14.01 (1H, br.s, OH) ppm. 13C NMR: δ = 12.10 (CH3), 33.37 (CH), 121.06 (C), 122.08 (CH), 126.13 (C), 126.96 (CH), 129.42 (C), 130.37 (CH), 130.81(CH), 137.75 (CH), 145.67 (CH), 146.76 (C), 157.66 (C) ppm. Anal. Calcd for C27H23BrN4O2: C, 62.92; H, 4.50; N, 10.87 (%). Found: C, 62.64; H, 4.59; N, 10.78 (%)0.3
3 Results and Discussion
3.1 Catalyst Preparation
The procedure followed to obtain the ion-pair immobilization of l-prolinate anion on the cationic polymer resin is outlined in Scheme 1. The strategy consists of building up suitable heterogeneous macroporous polymer-supported l-prolinate catalyst on the surface of commercially available amberlite IRA-900OH (mesh 16–50). Preparation of the heterogeneous polymer-supported l-prolinate catalyst by this procedure is facile and straightforward. In a typically procedure AmbIRA900OH was treated with a solution of 0.01 M l-proline at 60 °C to achieve [Amb]l-prolinate hybrid.
3.2 Characterization of the Catalyst
3.2.1 IR Spectra
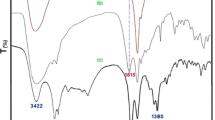
The ion-pair immobilization of l-prolinate anion on the polymer resin can be confirmed by characterizing the pure AmbIRA900OH, pristine l-proline and [Amb]l-prolinate hybrid using FT-IR spectroscopy, as shown in Fig. 1. The FT-IR spectrum of pristine l-proline shows characteristic stretching frequencies include: N–H asymmetric stretching at 3056 cm−1 and carboxylate (COO−) asymmetric and symmetric stretching at 1622 and 1380 cm−1, respectively (Fig. 1a). These bands are observed as new peaks in the FT-IR spectrum of [Amb]l-prolinate hybrid when compared with the FT-IR spectrum of pure AmbIRA900OH (Fig. 1b vs. a). The carboxylate (COO−) asymmetric and symmetric stretching are presented in [Amb]l-prolinate and found to shift to lower positions at 1615 and 1375 cm−1 respectively (Fig. 1b). In addition, the band at 3056 cm−1 corresponding to the asymmetric stretching vibration of the N–H group in l-proline is also found at 3056 cm−1in FT-IR spectrum of [Amb]l-prolinate. All the results from the comparison of FT-IR spectra encourage us to anticipate that the l-prolinate anion successfully loaded onto the polymer surface through ionic interaction using ion-pair binding between carboxylate group of l-prolinate and quaternary ammonium cation of the cationic Amb support.
3.2.2 TGA and DTG Analysis
Thermogravimetric analysis (TGA) and differential thermal analysis (DTG) associated with the decomposition profiles of the AmbIRA900OH, pristine l-proline and [Amb]l-prolinate hybrid under a nitrogen atmosphere provide further evidence for the immobilization of l-prolinate anion onto the polymer surface(Figs. 2, 3). The 100 % weight loss of pristine l-proline appears at 215–250 °C on the base of its TGA and DTG curves and assigned to the successive cleavage of the l-proline at this interval (Figs. 2c, 3c).The TGA curve of pure AmbIRA900OH shows three weight loss step intervals at 65–100, 150–250 and 370–470 °C. The first weight loss interval at 65–100 °C is most probably due to a loss of adsorbed water (weight loss = ca. 11 wt%). The second weight loss interval at 150–250 °C presumably assigned to the loss of some functional groups(weight loss = ca. 19 wt%) and finally the third weight loss interval at 370–470 °C (weight loss = ca. 34 wt%) could presumably assigned to partial polymer decomposition (Fig. 2b). Figure 3b displays the DTG curve of AmbIRA900OH and is in accordance with the weight loss steps from its TGA curve. The TGA curve of [Amb]l-prolinate hybrid displays four weight loss steps include: 60–100, 150–250, 250–370, and 370–470 °C intervals (Fig. 2a). These four weight loss peaks are well distinguished in the corresponding DTG curve (Fig. 3a).
Obviously, in comparison with AmbIRA900OH, a new decomposition interval is observed in TGA and DTGcurves of [Amb]l-prolinate hybrid (weight loss = ca. 15 wt%). This weight loss is assigned to the successive cleavage of l-prolinate anion loaded on the surface of the polymer and also referred to the content of l-prolinate moiety on the Amb-cationic support. The calculation from TG curve was indicated that 1.3 mmol of l-prolinate organocatalyst is loaded per 1 g of the [Amb]l-prolinate hybrid. It is noticeable that the decomposition temperature of l-prolinate anion in [Amb]l-prolinate hybrid has been increased to 250–370 °C in comparison with the decomposition temperature of pristine l-proline (215–250 °C). These observations mean that the thermal stability of the l-prolinate has been increased in comparison with the pristine l-proline and also explain the carboxylate asymmetric and symmetric stretching shifts to lower positions in FT-IR spectrum of [Amb]l-prolinate hybrid (Fig. 1b).
The increased decomposition temperature of the l-prolinate suggests that the guest/host interaction was done through the ion-pair exchanges between hydroxyl and l-prolinate anions on the surface of ion-exchange resin and is an indirect proof for the presence of ion-pair interaction between l-prolinate anions and quaternary ammonium cations on the surface of cationic support (Fig. 3a vs. c).The high loading of l-prolinate on the surface of Amb (15 wt%), together with the unique ion-pair binding behaviors between l-prolinate and Amb-cation, makes the [Amb]l-prolinate hybrid efficient and stable in the reaction system.
3.2.3 XRD
The crystalline nature of [Amb]l-prolinate hybrid confirms that l-prolinate is non-conveniently supported on cationic polymer support via ion-pair immobilization. The main intense diffraction peaks of pristine l-proline based on the standard spectrum (Fig. 4a) are observed in the XRD pattern of [Amb]l-prolinate hybrid due to the presence of the l-prolinate on the Amb support thanks to a favourable ion-pair binding with quaternary ammonium cations of the ion-exchange resin (Fig. 4b vs. a). This technique gives robustness to the catalytic system and on the other hand lets the l-prolinate organocatalyst to be flexible, mobile and free on the surface of the polymer at the same time. Moreover, the thermal stability of organocatalyst has been improved by this way. These mentioned advantages are characteristic properties of homogeneous and heterogeneous catalysts which have been included in [Amb]l-prolinate hybrid.
3.3 Optimization of the Reaction Conditions
After preparation and characterization of [Amb]l-prolinate catalyst, its catalytic activity was investigated in a one-pot pseudo three-component reaction for the synthesis of 4,4′-(arylmethylene)-bis-(1H-pyrazol-5-ol)s. To search for the optimal conditions, the reaction of benzaldehyde and two equivalents of 3-methyl-1-phenyl-2-pyrazolin-5-one was selected as the model reaction to examine the effect of [Amb]l-prolinate catalyst (2–15 mol%) under a variety of conditions (Table 1).

The present optimization studies revealed that the best result was achieved by caring out the reaction in the presence of 10 mol% of [Amb]l-prolinate under reflux condition in ethanol (Table 1, entry 9). The yield smoothly increased with the catalyst load up to 10 mol% and use of larger amounts of the catalyst (15 mol%) did not improve the yield while decreasing the amount of the catalyst led to decreased yield.
Using these optimized reaction conditions, the efficiency of this approach was explored for the synthesis of a wide variety of 4,4′-(arylmethylene)-bis-(1H-pyrazol-5-ol)s (Scheme 2; Table 2). All the reactions (Table 2) delivered excellent product yields and accommodated a wide range of aromatic aldehydes bearing both electron-donating and electron-withdrawing substituents (3a–r).
We proposed a plausible mechanism for the formation of 3a–r from aromatic aldehydes (1) and 3-methyl-l-phenyl-2-pyrazolin-5-one (2) using [Amb]l-prolinate as catalyst. Initially, 1 reacts with l-prolinate anion of catalyst to form an iminium carboxylate (I). Then l-prolinate anion abstracts a proton from the 3-methyl-l-phenyl-2-pyrazolin-5-one (2) to form the enolate intermediate (II). In the next step, Michael addition of enolate (II) to the iminium carboxylate (I) furnishes intermediate III. Finally the second molecule of 2 is added to intermediate III by the Michael addition fashion to give final product 3 (Scheme 3).
The recovery of a catalyst is highly preferable for a greener process. For this purpose, the reusability of [Amb]l-prolinate was examined for eight consecutive cycles (fresh + seven cycles) for the synthesis of 4,4′-(phenylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ol) (3a). From Fig. 5, It can be seen that [Amb]l-prolinate can be reused up to 8 runs without need to reload and the yield difference between the first and 8th runs is only 5 % which indicated that the catalyst efficiency is almost completely maintained during 8 consecutive runs. The nitrogen content of the fresh and reused catalyst was measured by using of elemental analysis and the comparison of the nitrogen contents indicated that the catalyst lost only 3 % of its nitrogen content after 8 runs. This is a good proof for very low leaching account of l-proline organocatalyst from [Amb]l-prolinate catalyst into the reaction mixture during 8 runs and also confirms that the catalytic ability of [Amb]l-prolinate almost completely has been remained stable after 8 runs in agreement with the recyclability study.
In addition, to show the efficiency of this method in comparison with other reported procedures, we selected the reaction of benzaldehyde and two equivalents of 3-methyl-1-phenyl-2-pyrazolin-5-one for the synthesis of 4,4′-(phenylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ol) (3a) as a representative model. This comparison is shown in Table 3. It is clear from the data that our method has short reaction times and provides higher yields of the products.
4 Conclusions
In summary, we have reported the one-pot pseudo three-component synthesis of 4,4′-(arylmethylene)-bis-(1H-pyrazol-5-ol) derivatives using ion-pair immobilization of l-proline on the surface of amberlite hydroxide as a new, heterogeneous and reusable organocatalyst. This methodology made the organocatalyst to be mobile and flexible which not only helped the supported catalyst to be as powerful as its non-supported form, but also made it to be easily recoverable with simple filtration. Some attractive features of this protocol are simple procedure, excellent yields, short reaction times, easy work-up, high catalytic activity and recyclability, and reusability of the catalyst. The catalyst can be used at least eight times without substantial reduction in its catalytic activity.
References
Lancaster M (2002) Green chemistry: an introductory text. The Royal Society of Chemistry, Cambridge
Clark JH (2001) Pure Appl Chem 73:103–111
Clark JH, Rhodes CN (2000) Clean synthesis using porous inorganic solid catalysts. RSC Clean Technology Monographs, Cambridge
Rajjak-Shaikh I (2014) J Catal 2014:1–35
Gruttadauria M, Giacalone F, Noto R (2008) Chem Soc Rev 37:1666
Cozzi F (2006) Adv Synth Catal 348:1367
Ding K, Uozumi Y (eds) (2008) Handbook of asymmetric heterogeneous catalysis. Wiley-VCH, Weinhein
Bart ok M (2010) Chem Rev 110:1663-1705 Castillo´n Castillo´n
Tsubogo T, Ishiwata T, Kobayashi S (2013) Angew Chem Int Ed 52:6590–6604
Zhang L, Luo S, Cheng JP (2011) Catal Sci Technol 1:507–516
Yamada SI, Otani G (1969) Tetrahedron Lett 10:4237–4240
Shi F, Tan W, Zhu RY, Xing GJ, Tu SJ (2013) Adv Synth Catal 355:1605–1622
Yang JW, Hechavarria Fonseca MT, List B (2005) J Am Chem Soc 127:15036–15037
Bartok M (2015) Catal Rev Sci Eng 57:192–255
Martín-Rapún R, Fan X, Sayalero S, Bahramnejad M, Cuevas F, Pericàs MA (2011) Chem Eur J 17:8780–8783
Jimeno C, Sayalero S, Pericàs MA (2010) In: Barbaro P, Liguori F (eds) Heterogenized homogeneous catalysts for fine chemicals, vol 4. Springer, New York
El-Sayed MA, Abdel-Aziz NI, Abdel-Aziz AA, El-Azab AS, El-Tahir KE (2012) Bioorg Med Chem 20:3306–3316
Balbi A, Anzaldi M, Macciò C, Aiello C, Mazzei M, Gangemi R, Castagnola P, Miele M, Rosano C, Viale M (2011) Eur J Med Chem 46:5293–5309
Rosiere CE, Grossman MI (1951) Science 113:651
Bailey DM, Hansen PE, Hlavac AG, Baizman ER, Pearl J, Defelice AF, Feigenson ME (1985) J Med Chem 28:256–260
Mahajan RN, Havaldar FH, Fernandes PS (1991) J Indian Chem Soc 68:245–246
Chauhan PMS, Singh S, Chatterjee RK (1993) Indian J Chem Sect B 32:858–861
Lubs HA (1970) The chemistry of synthetic dyes and pigments. American Chemical Society, Washington, DC
Londershausen M (1996) Pestic Sci 48:269
Singh D, Singh DJ (1991) Indian Chem Soc 68:165
Garnovskii AD, Uraev AI, Minkin VI (2004) Arkivoc 3:29
Vafaee A, Davoodnia A, Pordel M (2015) Res Chem Intermed, Published online, 09 January
Sadeghi B, Ghorbani M (2014) Iran J catal 4:67–70
Niknam K, Habibabad MS, Deris A, Aeinjamshid N (2014) Monatsh Chem 144:987–992
Sobhani S, Pakdin-Parizi Z, Nasseri R (2013) J Chem Sci 125:975–979
Parvanak-Boroujeni K, Shojaei P (2013) Turk J Chem 37:756–764
Moosavi-Zare AR, Zolfigol MA, Zarei M, Zare A, Khakyzadeh V, Hasaninejad A (2013) Appl Catal A Gen 467:61–68
Phatangare KR, Padalkar VS, Gupta VD, Patil VS, Umape PG, Sekar N (2012) Synth Commun 42:1349–1358
Sobhani S, Hasaninejad AR, Faal-Maleki M, Pakdin-Parizi Z (2012) Synth Commun 42:2245–2255
Hasaninejad A, Shekouhy M, Zare A, Hoseini-Ghattali SMS, Golzar N (2011) J Iran Chem Soc 8:411–423
Suresh-Kuarm B, Rajitha B (2012) Synth Commun 42:2382–2387
Gouda MA, Abu-Hashem AA (2012) Green Chem Lett Rev 5:203–209
Khazaei A, Zolfigol MA, Moosavi-Zare AR, Asgari Z, Shekouhy M, Zare A, Hasaninejad A (2012) RSC Adv 2:8010–8013
Tayebi S, Baghernejad M, Saberi D, Niknam K (2011) Chin J Catal 32:1477–1483
Hasaninejad A, Zare A, Shekouhy M, Golzar N (2011) Org Prep Proced Int 43:131–137
Niknam K, Saberi D, Sadegheyan M, Deris A (2010) Tetrahedron Lett 51:692–694
Keshavarz M, Iravani N, Ghaedi A, ZareiAhmady A, Vafaei-Nezhad M, Karimi S (2013) Springerplus 2:64
Albadi J, Keshavarz M (2013) Synth Commun 43:2019
Acknowledgments
The authors gratefully acknowledge the Research Council of Yasouj University for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keshavarz, M., Vafaei-Nezhad, M. Design and Characterization of l-Prolinate-Amberlite as a Novel Heterogeneous Organocatalyst and Its Catalytic Application in the Synthesis of Pyrazol-Derivates. Catal Lett 146, 353–363 (2016). https://doi.org/10.1007/s10562-015-1668-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1668-3