Abstract
A novel clean and simple technique for the heterogenization of l-proline organocatalyst has been introduced. This procedure is based on non-covalent immobilization of l-proline on the surface of an anion-exchange resin amberlite IRA900OH (AmbIRA900OH) as an efficient, cheap, and commercially accessible cationic polymer support. The ion-pair immobilization of l-proline on the surface of AmbIRA900OH was achieved by the treatment of a MeOH/H2O solution of l-proline with AmbIRA900OH at 60 °C. l-Proline anions were exchanged with hydroxyl anions and immobilized via ionic interaction between a carboxylate group of l-prolinate and quaternary ammonium cations of the cationic amberlite support. The prepared heterogeneous organocatalyst was well characterized by using FTIR, TGA, DTG, XRD, and elemental analysis techniques. The prepared heterogeneous organocatalyst was used as a catalyst for the one-pot, three-component synthesis of 2-amino-4H-chromene derivatives in EtOH at 80 °C. The reactions were efficiently performed in the presence of the title catalyst with short reaction times and gave the desired products at high yields. This catalyst can be straightforwardly recovered by simple filtration and recycled up to eight consecutive runs. Very low leaching of then organocatalyst into the reaction mixture occurred.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decade, there has been a revolution of organocatalysis in the field of organic chemistry. Organocatalysts are readily available, much easier to handle than other catalysts, and free of metal contamination, which is problematic in pharmaceutical processes. Subsequently, organocatalytic reactions are commonly considered to be environmentally benign and sustainable processes. Despite these attractive features, organocatalysts have been rarely applied in industry [1]. This unpleasant fact can be principally attributed to the unavoidable drawbacks of homogeneous catalytic processes such as unsatisfactory efficiency, low thermal stability, and the difficulties in catalyst separation and recycling [2].
One viable approach to address these challenges would be the heterogenization of organocatalysts. For this reason, the immobilization of organocatalysts has been achieved through covalent attachment onto supports such as polymers [3–6], silica [7–9], ionic liquid (IL) [10, 11], ß-cyclodextrin [12], Merrifield resin [13], and magnetite [14]. Although the covalent bond gives robustness to the system and allows a high number of recycling [15–17], this creativity may induce a partial loss of efficiency due to a lower mobility of the catalyst that reduces the activity and selectivity compared with their small molecular parent catalysts. Consequently, higher catalyst loading (both w/w% and mol%) is required to reach favorable yields [18]. Moreover, laborious synthetic operations are necessary to reach a covalent immobilization, and substantial structural perturbations to the parent catalyst skeletons are generally not avoidable in such circumstances.
Noncovalent immobilization strategies recently appeared as creative alternative solutions with their powers being well-demonstrated in supported organocatalysis. Several noncovalent immobilization strategies have been used to achieve recoverable organocatalysts. For example, immobilization via hydrophobic interaction [12, 19], biphasic immobilization [20, 21], self-supported gel-types, and ion-pair immobilization [22, 23] have been reported. Among these methods, ion-pair immobilization is a conceivable way to immobilize this type of catalysts via ion-exchange with cationic or anionic supports. By this method, anionic organocatalysts can be combined with an organic cation or cationic supports, and cationic organocatalysts can also be hybridized with an organic anion or anionic supports leading to noncovalently supported catalysts.
For instance, Itsuno and co-workers reported a novel type of supported chiral quaternary ammonium comprising an ionic bond between the ammonium moieties and the polymer anchored sulfonate anions [24, 25]. In 2008, Kaneda and co-workers prepared the montmorillonite immobilized Macmillan catalyst via cation-exchange strategy [26]. Layered double hydroxide (LDH) appears as inorganic solid cationic support. LDH consists of stacks of positively charged metal hydroxide layers and interlayer anions. Both inorganic and organic anions could be entrapped into the interlayer. Choudary et al. [27] reported the synthesis of LDH entrapped proline via anion-exchange method. Imidazolium and its supported forms have been used as cationic supports for prolinate anion and other anionic organocatalysts [28–32].
The 4H-chromene derivatives have various pharmacological properties such as anti-coagulant, anti-cancer, anti-HIV, anti-Alzheimer, antitumor, diuretic, anti-malarial activities, anti-leukemic, antibacterial, emetic, anti-anaphylactic activities, and spasmolytic [33–38].
Because of these important properties, there has been extensive focus on the improvement of environmentally friendly methodologies to synthesize 2-amino-4H-chromene scaffold. The main strategy for the synthesis of chromene derivatives include the cyclization of an aromatic/aliphatic aldehyde, malononitrile (or ethyl cyanoacetate), and diverse enolizable C–H activated acidic compounds [39, 40]. Several modified methods have been reported for the synthesis of these derivatives using different homogeneous or heterogeneous catalysts such as Zn(l-proline)2 [41], l-proline [42], K2CO3 [43], Na2CO3 under grinding [44], crown ether complex cation ILs [45], silica-bonded S-sulfonic acid [46] polystyrene-supported basic dicationic IL [47], [Et3NH][HSO4] [48], CAN [49], sucrose [50], TBAB [51], 4-nitro-2,6-diacetylpyridinebis(2,4,6-trimethylaniline)FeCl2 [52], [BMIm]BF4 [53], [2-aemim][PF6] [54], DBU [55], and POPINO [56]. However, many proposed methods for the synthesis of these compounds suffer from disadvantages including relying on multi-step conditions, the use of toxic organic solvents or catalysts containing transition metals, tedious work-up procedure, troublesome waste discarding, high reaction time, and low yields.
In continuation of our research devoted to the applications of ion-exchange resins for click synthesis of 1,4-disubstituted-1H-1,2,3-triazoles [57, 58], herein we wish to report ion-exchange resin amberlite IRA900OH (AmbIRA900OH) as an efficient, cheap, and commercially accessible cationic polymer support for the ion-pair immobilization of l-proline anion via ionic interaction between a carboxylate group of l-prolinate and quaternary ammonium cation of the cationic Amb support. This heterogeneous catalyst was used as an efficient recoverable catalyst for the one-pot, three-component synthesis of 2-amino-4H-chromene derivatives in ethanol.
Experimental
General
1H NMR spectra of samples were recorded at a Bruker Advanced DPX 400-MHz spectrometer. X-ray diffraction (XRD) patterns were recorded on a Philips X’PERT-Pro-MPD diffractometer using Cu Ka radiation (k = 1.542 Å). A continuous scan mode was used to collect 2 h from 5 to 40. Fourier transform infrared (FT-IR) spectra were obtained as potassium bromide pellets in the range 400–4000 cm−1 using an AVATAR 370 Thermo Nicolet spectrophotometer. Elemental analyses (C, H, and N) were performed with a Heraeus CHN-O-Rapid analyzer. The thermogravimetric and differential thermogravimetric (TG–DTG) analysis was performed on Netsch STA449c. The sample weight was ca. 10 mg and was heated from room temperature up to 600 °C at 10 °C/min using alumina sample holders.
Preparation of the [Amb]l-prolinate catalyst
Amberlite IRA-900OH (mesh 16–50, 1 g) was suspended in 10 mL of a 1 M solution of l-proline in MeOH:H2O (1:1). The system was heated at 60 °C for 6 h. The filtration of the reaction mixture followed by washing with MeOH:H2O (1:1) (2 × 10 mL) and H2O (2 × 10 mL) afforded [Amb]l-prolinate catalyst. The prepared catalyst was collected and dried under vacuum.
General procedure for the [Amb]l-prolinate catalyzed multicomponent synthesis of 2-amino-4H-chromene derivatives
ß-naphthol or enolizable compound (1 mmol), aldehyde (1 mmol), and malononitrile (1 mmol), were placed together in a round-bottom flask containing 5 mL of EtOH. [Amb]l-prolinate catalyst (0.08 g, 10 mol%) was added to the mixture. The suspension was magnetically stirred at reflux for an appropriate time according to (Table 2). After completion of the reaction, as followed by TLC (n-hexane:ethyl acetate; 3:1), the catalyst was filtered and washed with hot ethanol (2 × 5 mL). The recovered catalyst was washed with acetone, dried, and stored for other similar consecutive runs. The filtrate mixture was recrystallized to provide the pure crystals of 2-amino-4H-chromene derivatives. The products are known compounds and are characterized by IR and NMR spectroscopy data for new compounds. Their melting points are compared with reported values.
Results and discussions
Catalyst preparation
The procedure followed to obtain the ion-pair immobilization of l-prolinate anion on the cationic polymer resin is outlined in Scheme 1. The strategy consists of building up suitable heterogeneous macroporous polymer-supported l-prolinate catalyst on the surface of commercially available amberlite IRA-900OH (mesh 16–50). Preparation of heterogeneous polymer-supported l-prolinate catalyst by this procedure is facile and straightforward. In a typical, procedure AmbIRA900OH was treated with a solution of 0.01 M l-proline at 60 °C to achieve [Amb]l-prolinate hybrid.
Characterization of the catalyst
IR spectra
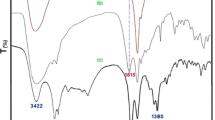
The ion-pair immobilization of l-prolinate anion on the polymer resin can be confirmed by characterizing the pure AmbIRA900OH, pristine l-proline, and [Amb]l-prolinate hybrid using FT-IR spectroscopy, as shown in Fig. 1. The FT-IR spectrum of pristine l-proline shows characteristic stretching frequencies including: N–H asymmetric stretching at 3056 cm−1 and carboxylate (COO−) asymmetric and symmetric stretching at 1622 and 1380 cm−1, respectively (Fig. 1a). These bands are observed as new peaks in the FT-IR spectrum of [Amb]l-prolinate hybrid when compared with the FT-IR spectrum of pure AmbIRA900OH (Fig. 1b vs. a). The carboxylate (COO−) asymmetric and symmetric stretching are presented in [Amb]l-prolinate and found to shift to lower positions at 1615 and 1375 cm−1, respectively (Fig. 1b). In addition, the band at 3056 cm−1 corresponding to the asymmetric stretching vibration of the N–H group in l-proline is also found at 3056 cm−1 in FT-IR spectrum of [Amb]l-prolinate. All the results from the comparison of FT-IR spectra encourage us to anticipate that the l-prolinate anion successfully loaded onto the polymer surface through ionic interaction using ion-pair binding between the carboxylate group of l-prolinate and quaternary ammonium cation of the cationic Amb support.
TGA and DTG analysis
Thermogravimetric analysis (TGA) and differential thermal analysis (DTG) associated with the decomposition profiles of the AmbIRA900OH, pristine l-proline, and [Amb]l-prolinate hybrid under a nitrogen atmosphere provide further evidence for the immobilization of l-prolinate anion onto the polymer surface (Figs. 2, 3). The 100 % weight loss of pristine l-proline appears at 215–250 °C on the base of its TGA and DTG curves and is assigned to the successive cleavage of the l-proline at this interval (Figs. 2c, 3c). The TGA curve of pure AmbIRA900OH shows three weight loss step intervals at 65–100, 150–250, and 370–470 °C. The first weight loss interval at 65–100 °C is most probably due to a loss of adsorbed water (weight loss = ca. 11 wt%). The second weight loss interval at 150–250 °C is presumably assigned to the loss of some functional groups (weight loss = ca. 19 wt%), and finally the third weight loss interval at 370–470 °C (weight loss = ca. 34 wt%) is presumably assigned to partial polymer decomposition (Fig. 2b). Figure 3b displays the DTG curve of AmbIRA900OH and is in accordance with the weight loss steps from its TGA curve. The TGA curve of [Amb]l-prolinate hybrid displays four weight loss steps including: 60–100, 150–250, 250–370, and 370–470 °C intervals (Fig. 2a). These four weight loss peaks are well distinguished in the corresponding DTG curve (Fig. 3a). Obviously, in comparison with AmbIRA900OH, a new decomposition interval is observed in TGA and DTG curves of [Amb]l-prolinate hybrid (weight loss = ca. 15 wt%). This weight loss is assigned to the successive cleavage of l-prolinate anion loaded on the surface of the polymer and also refers to the content of l-prolinate moiety on the Amb cationic support. The calculation from the TG curve indicated that 1.3 mmol of the l-prolinate organocatalyst is loaded per 1 g of the [Amb]l-prolinate hybrid. It is noticeable that the decomposition temperature of l-prolinate anion in [Amb]l-prolinate hybrid has been increased to 250–370 °C in comparison with the decomposition temperature of pristine l-proline (215–250 °C). These observations mean that the thermal stability of the l-prolinate has been increased in comparison with the pristine l-proline and also explains the carboxylate asymmetric and symmetric stretching shifts to lower positions in the FT-IR spectrum of [Amb]l-prolinate hybrid (Fig. 1b). The increased decomposition temperature of the l-prolinate suggests that the guest/host interaction was done through the ion-pair exchanges between hydroxyl and l-prolinate anions on the surface of ion-exchange resin and is an indirect proof for the presence of ion-pair interaction between l-prolinate anions and quaternary ammonium cations on the surface of cationic support (Fig. 3a vs. c). The high loading of l-prolinate on the surface of Amb (15 wt%), together with the unique ion-pair binding behaviors between l-prolinate and Amb cation, makes the [Amb]l-prolinate hybrid efficient and stable in the reaction system.
XRD
The crystalline nature of [Amb]l-prolinate hybrid confirms that l-prolinate is non-covalently supported on the cationic polymer support via ion-pair immobilization. The main intense diffraction peaks of pristine l-proline based on the standard spectrum (Fig. 4a) are observed in the XRD pattern of [Amb]l-prolinate hybrid due to the presence of the l-prolinate on the Amb support thanks to a favorable ion-pair binding with quaternary ammonium cations of the ion-exchange resin (Fig. 4b vs. a). This technique gives robustness to the catalytic system and, on the other hand, allows the l-prolinate organocatalyst to be flexible, mobile, and free on the surface of the polymer at the same time. Moreover, the thermal stability of the organocatalyst has been improved by this way. These mentioned advantages are characteristic properties of homogeneous and heterogeneous catalysts that have been included in [Amb]l-prolinate hybrid.
The one-pot multicomponent synthesis of 2-amino-4H-chromene using [Amb]l-prolinate catalyst
After preparation and characterization of [Amb]l-prolinate catalyst, its catalytic activity was investigated in a multicomponent reaction for the synthesis of 2-amino-4H-chromene derivatives. To optimize the reaction conditions, the reaction of 4-hydroxychomarine, benzaldehyde, and malononitrile was selected as a model to examine the effect of [Amb]l-prolinate catalyst (2–15 mol%) under a variety of conditions (Table 1).
The present optimization studies revealed that the best result was achieved by carrying out the reaction in the presence of 10 mol% of [Amb]l-prolinate under reflux in ethanol (Table 1, entry 9). The yield smoothly increased with the catalyst load up to 10 mol% and use of larger amounts of the catalyst (15 mol%) did not improve the yield while decreasing the amount of the catalyst led to decreased yield. Using these optimized conditions, the reaction of enole or enolizable ketones, malononitrile, and various aromatic aldehydes was explored (Scheme 2).
All the products were cleanly isolated with simple filtration and recrystallization from hot ethanol. As Table 2 shows, in all the cases, aromatic aldehydes substituted with either electron-donating or electron-withdrawing groups smoothly underwent the reaction and gave the target products in good to excellent yields.
For a true heterogeneous catalyst, supported catalyst should not leach into the reaction mixture and the recyclability of the supported catalyst is also important. To investigate these properties for the introduced catalyst, the reaction of 4-hydroxychomarine, benzaldehyde, and malononitrile in ethanol was selected again as model (Table 3).
After completion of the reaction, the mixture was filtered and the recovered catalyst was washed with acetone and dried before using for next consecutive runs (seven runs). Almost consistent activity was observed over eight consecutive runs. From Table 3, It can be seen that [Amb]l-prolinate can be reused up to eight runs without need to reload and the yield difference between the first and eighth runs is only 5 %, which indicates that the catalyst efficiency is almost completely maintained during eight consecutive runs.
The nitrogen content of the fresh and reused catalyst was measured by using elemental analysis, and the comparison of the nitrogen contents indicated that the catalyst lost only 3 % of its nitrogen content after eight runs. This is a good proof for very low leaching account of l-proline organocatalyst from [Amb]l-prolinate catalyst into the reaction mixture during eight runs and also confirms that the catalytic ability of [Amb]l-prolinate remained almost completely stable after eight runs, in agreement with the recyclability study.
Next, in order to show the superiority of the [Amb]l-prolinate to the homogeneous l-proline and sodium prolinate, the reaction of 4-hydroxychomarine, benzaldehyde, and malononitrile in ethanol was selected again as model reaction. The best results were achieved by using 20 mol% of l-proline and 15 mol% of sodium prolinate while at the same condition, with 10 mol% of [Amb]l-prolinate the reaction time was shorter. Moreover, the [Amb]l-prolinate catalyst can be simply recovered and reused up to eight runs (Table 3) while we failed to recover and reuse the l-proline and sodium prolinate catalysts.
A proposed mechanism to demonstrate the role of catalyst is shown in Scheme 3. A Knoevenagel reaction, Michael addition, and intra-molecular cyclization are involved simultaneously in the synthesis of 2-amino-4H-chromene derivatives. In the first step, the [Amb]l-prolinate catalyst abstracts a proton from the active methylene group of malononitrile leading to formation of carbanion. The formed carbanion of malononitrile makes a nucleophilic attack on the carbonyl carbon atom of aromatic aldehydes, followed by loss of a water molecule to form a-cyanocinnamonitrile derivative. In the second step, [Amb]l-prolinate catalyst reacts with dimedone and makes an enamine intermediate. Michael addition of enamine intermediate to Knoevenagel products, followed by ring closing and deprotonation, furnish 2-aminochromene derivative.
Conclusion
In conclusion, a new heterogeneous organocatalyst based on ion-pair immobilization of l-proline on the surface of amberlite hydroxide has been developed. The non-covalent immobilization strategy was used for the heterogenization of l-proline via ion-pair immobilization on the surface of amberlite hydroxide. This strategy made the organocatalyst to be mobile and flexible which not only helped the supported catalyst to be as powerful as its non-supported form, but also made it easily recoverable with simple filtration. The novel prepared heterogeneous organocatalyst efficiently catalyzed the one-pot synthesis of chromene derivatives using variety of aromatic aldehydes, malononitrile, and enols or enolizable carbonyl compounds to furnish the corresponding chromenes in good to excellent yields under green conditions.
References
F. Xu, M. Zacuto, N. Yoshikawa, R. Desmond, S. Hoerrner, T. Itoh, M. Journet, G.R. Humphrey, C. Cowden, N. Strotman, P. Devine, J. Org. Chem. 75, 7829 (2010)
H.U. Blaser, B. Pugin, F. Spindler, J. Mol. Catal. A Chem. 231, 1 (2005)
M. Gruttadauria, F. Giacalone, A.M. Marculescu, R. Notoa, Adv. Synth. Catal. 350, 1397 (2008)
M. Gruttadauria, A.M.P. Salvo, F. Giacalone, P. Agrigento, R. Noto, Eur. J. Org. Chem. 2009, 5437 (2009)
S. Calogero, D. Lanari, M. Orrù, O. Piermatti, F. Pizzo, L. Vaccaro, J. Catal. 282, 112 (2011)
J. Zou, W. Zhao, R. Li, H. Zhang, Y. Cui, J. Appl. Polym. Sci. 118, 1020 (2010)
A. Lu, T.P. Smart, T.H. Epps, D.A. Longbottom, R.K. ÓReilly, Macromolecules 44, 7233 (2011)
A. Zamboulis, N.J. Rahier, M. Gehringer, X. Cattoën, G. Niel, C. Bied, J.J.E. Moreau, M.W.C. Man, Tetrahedron Asymmetry 20, 2880 (2009)
A. Khalafi-Nezhad, E. Shaikhi Shahidzadeh, S. Sarikhani, F. Panahi, J. Mol. Catal. A Chem. 379, 1 (2013)
W. Miao, T.H. Chan, Adv. Synth. Catal. 348, 1711 (2006)
S. Luo, X. Mi, L. Zhang, S. Liu, H. Xu, J. Cheng, Angew. Chem. Int. Ed. 45, 3093 (2006)
J. Huang, X. Zhang, D.W. Armstrong, Angew. Chem. Int. Ed. 46, 9073 (2007)
J. Li, G. Yang, Y. Qin, X. Yang, Y. Cui, Tetrahedron Asymmetry 22, 613 (2011)
H. Yang, S. Li, X. Wang, F. Zhang, X. Zhong, Z. Dong, J. Ma, J. Mol. Catal. A Chem. 404, 363 (2012)
X. Fan, S. Sayalero, M.A. Pericas, Adv. Synth. Catal. 354, 2971 (2012)
P. Kasaplar, P. Riente, C. Hartmann, M.A. Pericas, Adv. Synth. Catal. 354, 2905 (2012)
E. Ozkal, S. Ozcubukcu, C. Jimeno, M.A. Pericas, Catal. Sci. Technol. 2, 195 (2012)
J. Horn, F. Michalek, C.C. Tzschucke, W. Bannwarth, Top. Curr. Chem. 242, 43 (2004)
K. Liu, D. Haeussinger, W.D. Woggon, Synlett 14, 2298 (2007)
K. Huang, Z.Z. Huang, X.J. Li, J. Org. Chem. 71, 8320 (2006)
H.M. Guo, L.F. Cun, L.Z. Gong, A.Q. Mi, Y.Z. Jiang, Chem. Commun. 11, 1450 (2005)
B. Escuder, F. Rodríguez-Llansola, J.F. Miravet, New. J. Chem. 34, 1044 (2010)
F. Rodríguez-Llansola, B. Escuder, J.F. Miravet, J. Am. Chem. Soc. 131, 11478 (2009)
Y. Arakawa, A. Chiba, N. Haraguchi, S. Itsuno, Adv. Synth. Catal. 350, 2295 (2008)
Y. Arakawa, N. Haraguchi, S. Itsuno, Angew. Chem. Int. Ed. 47, 8232 (2008)
T. Mitsudome, K. Nose, T. Mizugaki, K. Jitsukawa, K. Kaneda, Tetrahedron Lett. 49, 5464 (2008)
B.M. Choudary, B. Kavita, N.S. Chowdari, B. Sreedhar, M.L. Kantam, Catal. Lett. 78, 373 (2002)
K. Fukumoto, M. Yoshizawa, H. Ohno, J. Am. Chem. Soc. 127, 2398 (2005)
X. Zheng, Y. Qian, Y. Wang, Eur. J. Org. Chem. 2010, 515 (2010)
X. Zheng, Y. Qian, Y. Wang, Catal. Commun. 11, 567 (2010)
Y. Qian, X. Zheng, Y. Wang, Eur. J. Org. Chem. 2010, 3672 (2010)
W. Chen, Y. Zhang, L. Zhu, J. Lan, R. Xie, J. You, J. Am. Chem. Soc. 129, 13879 (2007)
L. Alvey, S. Prado, V. Huteau, B. Saint-Jonis, S. Michel, M. Koch, S.T. Cole, F. Tillequin, Y.L. Janin, Bioorg. Med. Chem. 16, 8264 (2008)
T. Symeonidis, M. Chamilos, D.J. Hadjipavlou-Litina, M. Kallitsakis, K.E. Litinas, Bioorg. Med. Lett. 19, 1139 (2009)
L. Lakshmi, K. Pandey, A. Kapil, N. Singh, M. Samant, A. Dube, Phytomedicine 14, 36 (2007)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
Z.Q. Xu, K. Pupek, W.J. Suling, L. Enache, M.T. Flavin, Bioorg. Med. Chem. Lett. 14, 4610 (2006)
L. Alvey, S. Prado, B. Saint-Joanis, S. Michel, M. Koch, S.T. Cole, F. Tillequin, Y.L. Janin, Eur. J. Med. Chem. 44, 2497 (2009)
R. Ballini, G. Bosica, L.M. Conforti, R. Maggi, A. Mazzacani, P. Righi, G. Sartori, Tetrahedron 57, 1395 (2001)
R. Pratap, V.J. Ram, Chem. Rev. 114, 10476 (2014)
S. Maleki, R. Babaee, Tayebee. Appl. Organomet. Chem. 29, 408 (2015)
L. Chandrasekhara Rao, H.M. Meshram, N. Satish Kumar, N. Nageswara Rao, N. Jagadeesh Babu, Tetrahedron Lett. 55, 1127 (2014)
M. Kidwai, S. Saxena, M.K. Rahman Khan, S.S. Thukral, Bioorg. Med. Chem. Lett. 15, 4295 (2005)
M.R. Naimi-jamal, S. Mashkouri, A. Sharifi, Mol. Divers. 14, 473 (2010)
M. Abaszadeh, M. Seifi, Res. Chem. Intermed. 41, 7715 (2015)
K. Aswin, S.S. Mansoor, K. Logaiya, S.P.N. Sudhan, V.S. Malik, H. Ramadoss, Res. Chem. Intermed. 40, 2583 (2014)
F. Heidarizadeh, N. Taheri, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2247-3
F. Khorami, H.R. Shaterian, Res. Chem. Intermed. 41, 3171 (2015)
Y. Li, X. Meng, G. Cai, B. Du, B. Zhao, Res. Chem. Intermed. 40, 699 (2014)
M.T. Maghsoodlou, N. Hazeri, M. Lashkari, F.N. Shahrokhabadi, B. Naghshbandi, M.S. Kazemi-doost, M. Rashid, F. Mir, M. Kangani, S. Salahi, Res. Chem. Intermed. 41, 6985 (2015)
Z. Zeng, L. Wang, Y. Cao, Y. Luo, Res. Chem. Intermed. 38, 1751 (2012)
R. Sandaroos, S. Damavandi, Chem. Intermed. 39, 4167 (2013)
K. Rad-Moghadam, L. Yoseftabar-Miri, Tetrahedron 67, 5693 (2011)
Y. Peng, G. Song, Catal. Commun. 8, 111 (2007)
M.J. Khurana, B. Nand, P. Saluja, Tetrahedron 66, 5637 (2010)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron 69, 1074 (2013)
M. Keshavarz, N. Iravani, A. Ghaedi, A.Z. Ahmady, M. Vafaei-Nezhad, S. Karimi, SpringerPluss 2, 64 (2013)
J. Albadi, M. Keshavarz, Synth. Commun. 43, 2019 (2013)
Acknowledgments
The authors gratefully acknowledge the Research Council of Yasouj University for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keshavarz, M., Iravani, N., Ahmadi Azqhandi, M.H. et al. Ion-pair immobilization of l-prolinate anion onto cationic polymer support and a study of its catalytic activity as an efficient heterogeneous catalyst for the synthesis of 2-amino-4H-chromene derivatives. Res Chem Intermed 42, 4591–4604 (2016). https://doi.org/10.1007/s11164-015-2302-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2302-0