Abstract
Systemic sclerosis (SSc) is a generalized connective tissue disorder, and SSc patients are at risk of developing pulmonary arterial hypertension (PAH). The aims of this study are to evaluate the right ventricular regional systolic function using two-dimensional speckle-tracking echocardiography (2D STE) and to determine the predictive ability of peak longitudinal systolic strain (PLSS) at the RV lateral wall for PAH in SSc patients. 80 SSc patients (mean age 51 ± 12 years) were included in the study. Echocardiography and 2D STE were performed at baseline and after 12 months. RHC was performed only in SSc patients with clinical indications. PLSS at the apical segment of the RV free wall was significantly impaired in PAH patients compared with non-PH patients (–14.6 ± 5.9 vs. − 22.2 ± 7.5%, p = 0.034). PLSS at the basal, mid, and apical segments of the RV free wall was lower in both groups at follow-up compared to baseline, but the drop in strain values was statistically significant only in the non-PH group (p < 0.05). Right atrial area (OR 1.758; p = 0.023), peak tricuspid regurgitation velocity (OR 24.23; p = 0.011) and PLSS at the apical segment of the RV lateral wall (OR 2.47; p = 0.005) were independent predictors of PAH. A cut-off value of − 14.48% PLSS at the apical segment of the RV lateral wall resulted in 100% specificity for predicting PAH in SSc patients. RV pressure overload affects RV systolic function as manifested by impaired RV longitudinal deformation. Evaluating RV regional systolic function with 2D STE could be useful as an additional echocardiographic parameter for screening PAH in SSc patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a multisystem disorder characterized by inflammation, widespread vascular lesions, and fibrosis involving various tissues and organs [1, 2]. SSc patients are at risk of developing pulmonary arterial hypertension (PAH), a debilitating, progressive condition of the pulmonary vasculature that leads to right heart failure and death [3, 4]. PAH is diagnosed when the mean pulmonary arterial pressure is ≥ 25 mmHg, pulmonary capillary wedge pressure (PCWP) is ≤ 15 mmHg and pulmonary vascular resistance (PVR) is > 3 Wood units (WU) as assessed by right heart catheterization (RHC) at rest in the absence of other causes of pre-capillary pulmonary hypertension [5, 6]. The current World Health Organization (WHO) classification of PH includes five distinct groups, four of which may occur in SSc patients [7, 8]. WHO Group I, also known as pulmonary arterial hypertension (PAH), is the most frequent type of PH in SSc patients [3]. PAH associated with SSc can lead to right ventricular (RV) systolic dysfunction [9]. Because RV systolic dysfunction is associated with poor prognosis in SSc patients, evaluation of RV function in these patients has gained importance in clinical practice [7].
Owing to the complex geometry of RV, conventional measurements of volumes and function are considered less accurate than those for the left ventricle [10]. Two-dimensional speckle-tracking echocardiography (2D STE) has been introduced for the evaluation of regional left ventricular and right ventricular function [11, 12]. 2D STE quantitatively analyzes the displacement and velocity of the myocardium by tracking myocardial movement from frame to frame throughout the cardiac cycle. This angle-independent method can reliably assess regional myocardial function [13, 14].
Therefore, we aimed to test the hypotheses that RV regional systolic function was reduced in SSc patients with PAH at baseline and that compared to baseline, peak longitudinal systolic strain (PLSS) at the RV lateral wall was diminished in all SSc patients after 12 months follow-up. We also hypothesized that PLSS at the RV lateral wall could predict PAH in SSc patients.
Methods
Study population
From May 2014 to May 2015, patients presenting to the rheumatology clinic of our tertiary university medical center and who fulfilled criteria for SSc, as proposed by the American College of Rheumatology, were approached for inclusion in the study [15]. Informed consent was obtained from all individual participants included in the study. From those granting informed consent, demographic data and co-morbidities were recorded. Clinical examinations and biochemical tests were performed on all participants. Echocardiography was performed as described below. All patients were evaluated by a rheumatologist and were classified as having diffuse or limited SSc according to the extent of cutaneous involvement [15]. From the SSc patients enrolled in the study, only patients with clinical indications were undergone RHC. Patients with documented coronary artery disease, diabetes mellitus, moderate to severe mitral or aortic stenosis or regurgitation, impaired left ventricular ejection fraction (< 50%), poor quality RV images, atrial fibrillation, pregnancy, or connective tissue disease other than systemic sclerosis were excluded from analysis. Systemic sclerosis patients who had different types of pulmonary hypertension other than PAH, who had a diagnosis of pulmonary hypertension confirmed by RHC or were on PH therapy prior to enrollment were also excluded from participating in the study.
Patients who were undergone RHC according to clinical indications were classified as either non-PH or WHO group 1 PH (PAH) according to current guidelines [6, 8]. Patients who had pulmonary hypertension due to left heart disease or due to lung disease/hypoxia as diagnosed by RHC were not included in the study. RHC was not performed to patients with peak tricuspid regurgitation velocity ≤ 2.8 m/s and no clinical signs of dyspnea, and these patients were also classified as the non-PH group.
Echocardiography and conventional Doppler measurements
Standard 2D echocardiographic measurements, tissue Doppler imaging (TDI) and 2D STE were performed at baseline and 12 months after initial presentation using a commercially available ultrasound transducer and equipment (M4S-RS probe, Vivid S6, GE Medical Systems, Horten, Norway). Images of all patients were obtained from the parasternal and apical views (parasternal long-axis and short axis views, apical 4-chamber, 2-chamber and apical long-axis views). Images were stored digitally for off-line analysis (EchoPAC, version BT13; GE, Norway). Peak tricuspid regurgitation (TR) velocity was measured by continuous-wave Doppler echocardiography from multiple standard views. The view that provided the highest peak velocity was used. Pulmonary artery systolic pressure was estimated from the peak continuous-wave Doppler velocity of the TR jet plus estimated central venous pressure [16]. Right atrium end-systolic area was calculated from the apical 4-chamber view. For conventional echocardiographic assessment of RV function, the tricuspid annular plane systolic excursion (TAPSE) and the tricuspid annular peak systolic velocity (s′) were measured from the apical 4-chamber view at the RV free wall level, as previously described [17]. RV end-diastolic diameters were determinated from the apical 4-chamber view [18]. RV end-diastolic and end-systolic areas were measured in the apical 4-chamber view by tracing the endocardial border of the RV and the tricuspid annular plane [18]. RV fractional area change (RVFAC) was calculated as follows: RV end-diastolic area minus RV end-systolic area divided by RV end-diastolic area [18].
The left ventricle ejection fraction (LVEF) was obtained according to the recommendations of the ASE [10]. The peak velocity of mitral E wave was measured from apical 4-chamber view by placing the sample volume at mitral leaflet tips. Peak early (e′) diastolic myocardial velocity of LV was measured in the apical 4-chamber view, placing the sample volume at the septal side of the mitral annulus and the E/e′ ratio was calculated to estimate LV filling pressures.
2-Dimensional speckle-tracking strain analysis
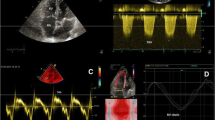
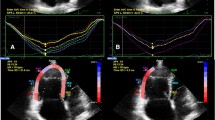
In each patient, 2-dimensional strain analysis was performed offline by use of a dedicated software package (EchoPAC, version BT13) which evaluates real-time tracking of natural acoustic markers during two consecutive 2-dimensional grayscale images taken at a frame rate of 60–80 frames/s. For assessment of RV peak longitudinal systolic strain, images taken at a frame rate of 60–80 frames/s were obtained from the apical 4-chamber view. Three consecutive cardiac cycles were acquired and digitally stored for offline analysis. The RV endocardial border was manually traced in the end-systolic frame. The region of interest (ROI) in each image was automatically generated. The position of the ROI and its width were adjusted manually when the speckle tracking appeared to be poor. The software then automatically tracked and accepted segments of good tracking quality and rejected poorly tracked segments. When all segments of RV were accepted, the regional longitudinal strain curves were obtained for six segments of RV. After that, segmental values of peak longitudinal systolic strain from the basal, mid, and apical segments of the RV free wall were recorded. Representative examples of regional longitudinal strain curves taken from the right ventricle by STE were shown in Figs. 1 and 2.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software package (IBM SPSS Statistics for Windows, Version 20.0., Armonk, NY, USA). Distribution of data was assessed by using one-sample Kolmogorov–Smirnov testing. Values displaying normal distribution were expressed as mean ± SD, whereas values not displaying normal distribution were expressed as median with interquartile range. Categorical variables were presented as number (n) and percentage (%). Comparison between groups was performed with the Student’s t test or the Mann–Whitney U test (non-normally distributed data). Chi square test was used to compare categorical variables. Correlation was tested by Pearson or Spearman’s correlation tests where appropriate. Statistical analysis for determining independent predictors of PAH was performed via a backward stepwise logistic regression model which included findings with p values under 0.25 among conventional, Doppler and 2-dimensional speckle-tracking echocardiographic results. The discriminatory performance of variables which could predict PAH in SSc patients was examined by receiver operating characteristic (ROC) curve analysis. For all analyses, a p value of less than 0.05 was considered statistically significant.
Intraobserver and interobserver variability were assessed in randomly selected 15 subjects (5 SSc patients with PAH and 10 SSc patients without PH), intraobserver variability was determined by having the same physician remeasurement after one month. Interobserver variability was assessed by a second observer who was blinded to the clinical and the STE findings. The absolute difference between two measurements divided by the average value of these two measurements was calculated. Interobserver and intraobserver reproducibility were calculated by intraclass correlation coefficient.
Results
During the study period, 96 SSc patients met the inclusion and exclusion criteria. During the image analysis, 3 SSc patients with PAH and 13 SSc patients without PH were excluded from the study because of poor image quality for optimal speckle tracking. Reliable measurements were obtained in 79% of SSc patients (11 of 14) with PAH and 84% of SSc patients (69 of 82) without PH.
Baseline characteristics
We included 80 SSc patients (11 males, mean age 51 ± 12 years) in our study. Of the 80 SSc patients enrolled, RHC was performed in 29 SSc patients according to clinical indications, and PAH was diagnosed in 11 of these. Demographic and clinical characteristics of the patients are shown in Table 1. Those with PAH were older and had had SSc diagnosed later in life than those without PH. The PAH and non-PH patients were not significantly different regarding gender, duration of disease, type of disease and incidence of hyperlipidemia and systemic hypertension. All patients were in sinus rhythm.
Of the SSc patients, 66.3% (53 of 80) had limited disease and 33.8% (27 of 80) had diffuse disease. The distribution of type of scleroderma between the PAH and non-PH groups was not statistically significant. The level of BNP was significantly higher in PAH than non-PH patients, but the incidence of positivity of ANA, anti-scl 70, and anti-centromere antibodies was similar in the two groups (Table 1).
Description of patient selection for RHC and hemodynamic findings of SSc patients
Patients who had peak tricuspid regurgitation velocity > 2.8 m/s and patients who had clinical signs of dyspnea and peak tricuspid regurgitant velocity ≤ 2.8 m/s were evaluated via RHC. PAH was diagnosed in 11 patients with SSc. Patients with peak tricuspid regurgitation velocity ≤ 2.8 m/s and no clinical signs of dyspnea were not evaluated via RHC. As expected, pulmonary artery systolic pressure (PASP), pulmonary artery diastolic pressure (PADP), mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR) were higher in PAH patients than in those without PH (Online Resource 1).
Conventional 2D and Doppler echocardiographic findings in SSc patients
Two-dimensional and Doppler echocardiographic parameters of the groups are compared in Table 2. Left ventricular ejection fraction (LVEF) was within normal range in both groups and was without a significant difference between the groups (p > 0.05). Patients with PAH had significantly larger RV diameters, reduced TAPSE and RVFAC values, larger right atrial area compared with non-PH patients (p < 0.05 for all). Peak tricuspid regurgitation velocity and PASP (as measured by TTE) was higher in PAH patients (p < 0.001).
Mitral E wave peak velocity was similar in PAH and non-PH patients (Table 2). Myocardial velocities as detected by TDI, did not differ significantly between the two groups except for RV s′, which was significantly lower in PAH patients (8.2 ± 1.7 vs. 12.4 ± 2.3 cm/s, p = 0.032). In addition, the septal E/e′ ratio, which is the predictor of left ventricular filling pressure, was not significantly different between PAH and non-PH patients (7.9 ± 1.6 vs. 8.2 ± 2.1, p = 0.748).
Compared to non-PH patients, SSc patients with PAH had significantly larger RV diameters and right atrial area, higher peak tricuspid regurgitation jet velocity and PASP, reduced RV systolic function by TAPSE, RV s′, RVFAC at follow-up (p < 0.05 for all). LVEF, mitral E wave peak velocity, septal e′ and the septal E/e′ ratio did not differ significantly between the PAH and the non-PH group at follow-up (Online Resource 2).
RV regional longitudinal function
PLSS values from the basal, mid, and apical segments of the right ventricular lateral wall are listed in Table 3. PLSS values from the apical segment of the RV free wall were significantly lower in the PAH group compared with the non-PH group at baseline (− 14.6 ± 5.9 vs. − 22.2 ± 7.5, p = 0.034). At the same visit, PLSS values from the basal and mid segments of the RV free wall were also lower in the PAH group, but these differences between the two groups were not statistically significant. PLSS at the basal, mid, and apical segments of the RV free wall were lower in both groups at follow-up compared to baseline, but the reduction of strain values was statistically significant only in the non-PH group (p < 0.05 for all).
The relationship between RV regional longitudinal function and hemodynamic parameters and predictors of PAH
PLSS at the basal, mid, and apical segments of the RV free wall correlated significantly with mean pulmonary artery pressure, as measured by RHC (r = − 0.456, − 0.627, − 0.604, respectively; p < 0.05) (Online Resource 3).
Using a logistic regression model, we found that right atrial area (RA area) (OR 1.758, p = 0.023), peak tricuspid regurgitation velocity (OR 24.23, p = 0.011) and PLSS at the apical segment of the RV lateral wall (OR 2.47; p = 0.005) were independent predictors of pulmonary arterial hypertension (Table 4).
Cut-off values of PLSS of the RV lateral wall for predicting PAH in SSc patients
Among strain values measured in the RV lateral wall, PLSS at the apical segment significantly discriminated SSc patients with PAH from patients without PH (p < 0.05). A PLSS value of − 14.48% in the apical segment of the RV lateral wall (RV Apex) was only 62% sensitive in predicting PAH but was 100% specific in ruling out PAH. PLSS values at the basal and mid segments of the RV lateral wall were not useful for discriminating SSc patients with PAH from patients without PH (Table 5).
Intraobserver and interobserver variability
Intraobserver variability of PLSS at the basal, mid, and apical segments of the RV free wall was 4.23, 6.71, 3.82%, respectively. Interobserver variability of PLSS at the basal, mid, and apical segments of the RV free wall was 7.93, 10.48, 6.42%, respectively. The intra-observer ICC for PLSS at the basal, mid, and apical segments of the RV free wall was 0.91, 0.88, 0.89, respectively. The interobserver ICC for PLSS at the basal, mid, and apical segments of the RV free wall was 0.84, 0.82, 0.86, respectively.
Discussion
In systemic sclerosis patients, we found that peak longitudinal systolic strain at baseline was significantly less in PAH patients (compared to non-PH patients) only at the apical segment of the RV free wall. Similarly, in the studies by Puwanant et al. and Li et al., PLSS from the RV lateral wall and interventricular septum (IVS) were decreased in patients with PAH compared to healthy controls [19, 20]. Also, Pirat et al. showed that PLSS at the RV free wall and IVS measured by velocity vector imaging was lower in patients with PAH than in normal controls [21].
Although others found that PLSS was lower in all segments of the RV free wall in patients with PAH, in our study we found that PLSS was reduced significantly only at the apical segment of the RV free wall in SSc patients with PAH. PLSS values from the basal and mid segments of the RV free wall were also lower in the PAH group, but these differences failed to reach statistical significance in our study. Results differing from previous studies may be due to our comparison of SSc patients with PAH and without PH. Unlike our study, previous studies had control groups made up of healthy subjects, who did not have any systemic diseases [19,20,21]. The reason we examined SSc patients (without PH) as the control group was due to the fact that subclinical myocardial involvement in SSc patients may cause a reduction of strain values [22,23,24].
Previous studies showed that subclinical myocardial involvement in SSc patients could cause reduction of global longitudinal peak systolic strain of left ventricle [25, 26]. As a result, myocardial fibrosis in SSc patients can also affect the systolic function of the interventricular septum. Therefore we assume that right ventricular septum could be affected by myocardial involvement more severely than RV free wall in SSc patients because it has a greater amount of myocardial muscle mass compared to RV free wall. For the purpose of minimizing the effect of myocardial fibrosis on peak systolic strain values, right ventricular septum was excluded from analysis.
In previous studies of STE used to assess the effect of pulmonary hypertension on RV function, patients were not followed up for changes in RV function over a period of time [19,20,21]. In our study, however, patients were evaluated by STE at baseline and after at least 12 months to detect changes over time. Compared to baseline, decreases in PLSS at follow-up were significant only in the non-PH group. Of note, patients in the PAH group were given specific PAH therapy after the diagnosis of PAH. Initiation of approved PAH medications to SSc patients who were diagnosed with PAH could slow the progression of the disease and prevent further deterioration of the RV. Therefore STE measurements in the PAH group could remain stable at follow-up because of the initiation of specific PAH therapy.
We know that SSc patients are at risk of developing PAH, which is the leading cause of mortality in SSc [27]. Previous studies revealed that PLSS at the basal, mid, and apical segments of the right ventricular free wall were higher in patients with mild PAH than in patients with severe PAH, but were lower in patients with mild PAH than in healthy subjects [20]. Reduction of strain values in the RV free wall in non-PH patients at follow-up may be due to an increase in mean pulmonary arterial pressure and pulmonary vascular resistance in these patients. However, we can only speculate about long-term changes of these values in SSc patients without PH, because RHC was not performed in these patients at follow-up.
In our study, 29 SSc patients were referred to RHC based on their peak tricuspid regurgitation jet velocity and clinical status. PAH was not confirmed in 18 of these patients. So the proportion of RHC performed that did not confirm a diagnosis of PAH was 62% (18/29). Also, in the DETECT study, this ratio is found to be %65 (129/198) [28]. The proportion of RHC performed that did not confirm a diagnosis of PAH in our study was similar compared to the DETECT study. Pulmonary artery systolic pressure can be determined from peak tricuspid regurgitation jet velocity using the simplified Bernoulli equation [18]. Peak tricuspid regurgitation jet velocity is an estimate of systolic pulmonary artery pressure, not mean pulmonary artery pressure [18]. Therefore peak tricuspid regurgitation jet velocity can fail in diagnosing PAH.
In addition, failure to obtain systolic pulmonary artery pressure (PAP) has been reported in between 20 and 39% of patients in previous studies [5, 29,30,31,]. Doppler-derived pressure estimations show poor reliability in individual patients with both under- and overestimation of actual systolic PAP [30,31,32,33]. The prevalence of PAH in a population with tricuspid regurgitation (TR) jet ≤ 2.8 m/s is considered to be very low. However, the DETECT study showed that one-third of all SSc-PAH in a screening population is found in patients with a TR jet ≤ 2.8 m/s [28]. Therefore echocardiographic assessment of the estimated systolic PAP cannot always be relied on particularly in associated PAH where pressures are less elevated at diagnosis. So additional echocardiographic parameters are needed to rule out PAH especially in patients with TR jet velocity ≤ 2.8 m/s.
As far as we know our study is the first to determine the discriminative ability of PLSS at the RV lateral wall for the prediction of PAH in SSc patients. In this study, we found that PLSS from the apical segment of the RV lateral wall has a very high specificity for ruling out PAH in SSc patients. Therefore evaluating RV regional systolic function with 2D STE could be useful as an additional echocardiographic parameter for screening PAH in SSc patients.
Study limitations
Our study included a small number of SSc patients. Also, the follow-up period was relatively short. Furthermore, only patients with clinical indications were evaluated by RHC. Therefore future prospective studies of larger samples and longer follow-up periods are needed to determine the clinical utility of 2D STE for predicting PAH in SSc patients.
Conclusion
Our findings suggest that chronic RV pressure overload directly affects RV systolic function as manifested by impaired RV longitudinal deformation at the apical segment of the RV lateral wall in SSc patients with PAH. And 2D STE can identify these abnormalities in RV regional systolic function. Also in SSc patients, PLSS from the apical segment of the RV lateral wall has a very high specificity for ruling out PAH. Therefore we conclude that indices derived from 2D STE can be used as an adjunctive tool in the comprehensive evaluation of RV systolic function and the prediction of PAH in SSc patients.
References
Liakouli V, Cipriani P, Marrelli A, Alvaro S, Ruscitti P, Giacomelli R (2011) Angiogenic cytokines and growth factors in systemic sclerosis. Autoimmun Rev 10(10):590–594. https://doi.org/10.1016/j.autrev.2011.04.019
Bhattacharyya S, Wei J, Varga J (2011) Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol 8(1):42–54. https://doi.org/10.1038/nrrheum.2011.149
Yaqub A, Chung L (2013) Epidemiology and risk factors for pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 15(1):302. https://doi.org/10.1007/s11926-012-0302-2
Gashouta MA, Humbert M, Hassoun PM (2014) Update in systemic sclerosis-associated pulmonary arterial hypertension. Presse Med 43(10 Pt. 2):e293–e304. https://doi.org/10.1016/j.lpm.2014.06.007
Denton CP, Cailes JB, Phillips GD, Wells AU, Black CM, Bois RM (1997) Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol 36(2):239–243
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB (2013) Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 62(25 Suppl):D42–D50. https://doi.org/10.1016/j.jacc.2013.10.032
Sweiss NJ, Hushaw L, Thenappan T, Sawaqed R, Machado RF, Patel AR, Gomberg-Maitland M, Husain AN, Archer SL (2010) Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep 12(1):8–18. https://doi.org/10.1007/s11926-009-0078-1
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37(1):67–119. https://doi.org/10.1093/eurheartj/ehv317
Chaisson NF, Hassoun PM (2013) Systemic sclerosis-associated pulmonary arterial hypertension. Chest 144(4):1346–1356. https://doi.org/10.1378/chest.12-2396
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18(12):1440–1463. https://doi.org/10.1016/j.echo.2005.10.005
Jamal F, Bergerot C, Argaud L, Loufouat J, Ovize M (2003) Longitudinal strain quantitates regional right ventricular contractile function. Am J Physiol Heart Circ Physiol 285(6):H2842–H2847. https://doi.org/10.1152/ajpheart.00218.2003
Pellerin D, Sharma R, Elliott P, Veyrat C (2003) Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart 89(Suppl 3):iii9–i17
Artis NJ, Oxborough DL, Williams G, Pepper CB, Tan LB (2008) Two-dimensional strain imaging: a new echocardiographic advance with research and clinical applications. Int J Cardiol 123(3):240–248. https://doi.org/10.1016/j.ijcard.2007.02.046
Perk G, Tunick PA, Kronzon I (2007) Non-Doppler two-dimensional strain imaging by echocardiography–from technical considerations to clinical applications. J Am Soc Echocardiogr 20(3):234–243. https://doi.org/10.1016/j.echo.2006.08.023
Masi AT, Subcommittee For Scleroderma Criteria of the American Rheumatism Association Diagnostic, Therapeutic Criteria Committee (1980) Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23:581–590. https://doi.org/10.1002/art.1780230510
Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA (2002) Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 15(2):167–184
Kaul S, Tei C, Hopkins JM, Shah PM (1984) Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J 107(3):526–531
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010 (quiz 786–688)
Puwanant S, Park M, Popovic ZB, Tang WH, Farha S, George D, Sharp J, Puntawangkoon J, Loyd JE, Erzurum SC, Thomas JD (2010) Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation 121(2):259–266. https://doi.org/10.1161/CIRCULATIONAHA.108.844340
Li Y, Xie M, Wang X, Lu Q, Fu M (2013) Right ventricular regional and global systolic function is diminished in patients with pulmonary arterial hypertension: a 2-dimensional ultrasound speckle tracking echocardiography study. Int J Cardiovasc Imaging 29(3):545–551. https://doi.org/10.1007/s10554-012-0114-5
Pirat B, McCulloch ML, Zoghbi WA (2006) Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol 98(5):699–704. https://doi.org/10.1016/j.amjcard.2006.03.056
Deswal A, Follansbee WP (1996) Cardiac involvement in scleroderma. Rheum Dis Clin N Am 22(4):841–860
Follansbee WP, Miller TR, Curtiss EI, Orie JE, Bernstein RL, Kiernan JM, Medsger TA Jr (1990) A controlled clinicopathologic study of myocardial fibrosis in systemic sclerosis (scleroderma). J Rheum 17(5):656–662
Kahan A, Coghlan G, McLaughlin V (2009) Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 48(Suppl 3):iii45–i48. https://doi.org/10.1093/rheumatology/kep110
Piccione MC, Zito C, Bagnato G, Oreto G, Di Bella G, Bagnato G, Carerj S (2013) Role of 2D strain in the early identification of left ventricular dysfunction and in the risk stratification of systemic sclerosis patients. Cardiovasc Ultrasound 11(1). https://doi.org/10.1186/1476-7120-11-6
Spethmann S, Rieper K, Riemekasten G, Borges AC, Schattke S, Burmester GR, Hewing B, Baumann G, Dreger H, Knebel F (2014) Echocardiographic follow-up of patients with systemic sclerosis by 2D speckle tracking echocardiography of the left ventricle. Cardiovasc Ultrasound 12:13. https://doi.org/10.1186/1476-7120-12-13
Aithala R, Alex AG, Danda D (2017) Pulmonary hypertension in connective tissue diseases: an update. Int J Rheum Dis. https://doi.org/10.1111/1756-185x.13001
Coghlan JG, Denton CP, Grunig E, Bonderman D, Distler O, Khanna D, Muller-Ladner U, Pope JE, Vonk MC, Doelberg M, Chadha-Boreham H, Heinzl H, Rosenberg DM, McLaughlin VV, Seibold JR (2014) Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 73(7):1340–1349. https://doi.org/10.1136/annrheumdis-2013-203301
Kowal-Bielecka O, Avouac J, Pittrow D, Huscher D, Behrens F, Denton CP, Foeldvari I, Humbert M, Matucci-Cerinic M, Nash P, Opitz CF, Rubin LJ, Seibold JR, Strand V, Furst DE, Distler O (2010) Echocardiography as an outcome measure in scleroderma-related pulmonary arterial hypertension: a systematic literature analysis by the EPOSS group. J Rheumatol 37(1):105–115. https://doi.org/10.3899/jrheum.090661
Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM (2009) Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 179(7):615–621. https://doi.org/10.1164/rccm.200811-1691OC
Mukerjee D, St George D, Knight C, Davar J, Wells AU, Du Bois RM, Black CM, Coghlan JG (2004) Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology 43(4):461–466. https://doi.org/10.1093/rheumatology/keh067
Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, Maitre B, Yaici A, Hajji L, O’Callaghan DS, Clerson P, Girot R, Galacteros F, Simonneau G (2011) A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 365(1):44–53. https://doi.org/10.1056/NEJMoa1005565
Selby VN, Scherzer R, Barnett CF, MacGregor JS, Morelli J, Donovan C, Deeks SG, Martin JN, Hsue PY (2012) Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS (London England) 26(15):1967–1969. https://doi.org/10.1097/QAD.0b013e3283579653
Acknowledgements
There were no external sources of funding obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of ınterest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Ethics Committee of the Hacettepe University Faculty of Medicine Hospital (11.DEC.2013/477-33). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Research involving animal rights
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Right heart catheterization findings (mean±SD) of systemic sclerosis patients. Supplementary material 1 (XLSX 9 KB)
Online Resource
2 . Conventional 2-dimensional and Doppler echocardiographic variables of systemic sclerosis patients with (PAH) and without (non-PH) pulmonary hypertension at follow-up (Visit 2) Supplementary material 2 (XLSX 8 KB)
Online Resource 3
Correlation between peak systolic strain from the apical, mid, and basal segments of the RV free wall and mean pulmonary artery pressure (mPAP), as measured by right heart catheterization. Supplementary material 3 (XLSX 10 KB)
Rights and permissions
About this article
Cite this article
Hekimsoy, V., Kaya, E.B., Akdogan, A. et al. Echocardiographic assessment of regional right ventricular systolic function using two-dimensional strain echocardiography and evaluation of the predictive ability of longitudinal 2D-strain imaging for pulmonary arterial hypertension in systemic sclerosis patients. Int J Cardiovasc Imaging 34, 883–892 (2018). https://doi.org/10.1007/s10554-018-1299-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1299-z