Abstract
Pulmonary arterial hypertension (PAH) is a serious and often fatal complication of connective tissue disease (CTD). Right atrial (RA) function is essential to maintaining adequate total right heart function in PAH. However, little is known about prognostic utility of RA function in CTD-PAH. RA longitudinal strain (LS) and strain rate (LSR) were evaluated in 53 consecutive patients (51 female, mean age 42 ± 15 years) with CTD-PAH, including systemic lupus erythematosus (SLE) (33.7%), mixed connective tissue disease (MCTD) (32.1%), primary Sjögren’s syndrome (pSS) (26.4%), and systemic sclerosis (SSc) (3.8%). At a mean follow-up of 19.3 ± 10.9 months, 20 patients (37.7%) were clinically worse. The group with clinical events had worse clinical conditions and poorer RA function at baseline compared with the group that had no clinical events. RA LS independently reflected World Health Organization functional class (WHO FC) after adjusting for RA area (RAA), tricuspid regurgitation (TR) grade, right ventricular (RV) global longitudinal strain (GLS), and pulmonary vascular resistance (PVR) (P = 0.006). Receiver operator characteristic (ROC) curve analysis indicated that RA LS < 22.9% was predictive of clinical worsening during follow-up (sensitivity = 80%; specificity = 87.9%; area under the curve (AUC) = 0.858), and the Kaplan–Meier curve confirmed that RA LS ≥ 22.9% was associated with more favorable long-term outcomes compared to RA LS < 22.9% (log-rank P < 0.01). On univariate Cox proportional hazards analysis, PVR, RVGLS, RAA, and RA LS were associated with long-term outcome, while RA LS was the only independent predictor in the multivariate analysis. Our findings suggest that RA LS measurements by speckle-tracking echocardiography (STE) can independently reflect the extent of right heart failure and predict clinical outcomes in patients with CTD-PAH. RA LS < 22.9% is associated with a higher risk of clinical worsening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a severe and often fatal complication of connective tissue disease (CTD) [1, 2]. CTD is a major cause of PAH, and CTD-associated PAH (CTD-PAH) comprises approximately one-fourth of the total PAH population [3]. PAH significantly worsens the prognosis of CTD and is a major cause of mortality. PAH leads to increased pulmonary vascular resistance (PVR), resulting in right ventricular (RV) dilation, dysfunction, and ultimately right heart failure. Mortality in PAH patients is associated with the extent of right-sided heart failure [4], and in the setting of RV failure, preserved right atrial (RA) function is essential for maintaining adequate total right heart function [5]. As such, accurate assessment of RA function is crucial in PAH. Two-dimensional speckle-tracking echocardiography (2D-STE) is an easily obtained, angle-independent technique for quantifying myocardial deformation that has proven to be reliable in the assessment of RV and RA function in PAH [6, 7]. RA longitudinal strain (LS) and strain rate (LSR) have been demonstrated to be predictive of right-sided heart failure, clinical deterioration, and mortality in patients with PAH [8, 9]. However, all of these studies were performed in populations with heterogeneous causes of PAH, and CTD-PAH has not been studied independently. CTD-PAH has unique characteristics compared with other PAH subtypes, including less favorable outcome, venous and cardiac involvement, and response to immunosuppression [10]. Immune and inflammatory mechanisms may play important roles in the development and progression of CTD-PAH, and early-stage CTD-PAH may be curable in some patients [11]. All of this suggests the importance of performing studies focusing on CTD-PAH. In the present study, RA function was evaluated in a CTD-PAH cohort. The aims were to assess RA LS and LSR in CTD-PAH using 2D-STE and to investigate the utility of RA strain for predicting adverse events in CTD-PAH.
Methods
Study population
We enrolled consecutive patients with CTD-PAH who were admitted to the Department of Rheumatology at the First Hospital of China Medical University (CMU) from July 1 2015 to June 31 2018. Patients underwent a complete echocardiography at the Department of Cardiovascular Ultrasound to assess heart structure and function and pulmonary artery pressure. Patients with peak tricuspid regurgitation velocity (TRV) on continuous-wave Doppler echocardiography > 3.4 m/s were included, and exclusion criteria were total lung capacity < 60% of predicted, severe interstitial lung disease, and congenital heart disease or left heart disease including cardiomyopathy, moderate or severe valvular disease, systolic dysfunction defined as LVEF < 52% in male or < 54% in female [12], and grade II or higher diastolic dysfunction [13]. Patients with poor acoustic echocardiographic windows and inadequate images for strain measurement were also excluded. CTD type at the time of enrollment was defined according to the American College of Rheumatology (ACR) criteria. Clinical data were collected simultaneously, including duration of disease, World Health Organization functional class (WHO FC), 6-min walk distance (6MWD), and plasma brain natriuretic peptide (BNP) level. Thirty healthy volunteers who were matched for age, sex, and body surface area (BSA) with the study patients were enrolled as a control group. All patients were evaluated regularly at 6-month intervals and all adverse events were recorded, with adverse events defined as death, initiation of prostanoid therapy, or worsening of PAH, as indicated by the occurrence of all three of the following: a decrease in 6MWD of at least 15% from baseline; worsening of PAH symptoms; and need for new PAH drug treatment. This study was approved by the Medical Ethics Committee of CMU and compliant with Ethics Committee requirements. All patients provided written informed consent before enrollment.
Conventional echocardiography
Standard complete transthoracic echocardiography was performed using a Philips EPIQ7 echocardiography machine equipped with a proprietary X5-1 probe (Philips Healthcare). Two-dimensional, M-mode, Doppler echocardiography measurements were performed according to the current recommendations of the American Society of Echocardiography (ASE) [12]. Right ventricular dimensions and function were evaluated as recommended by current guidelines [14]. RV end-diastolic area (RVEDA) and end-systolic area (RVESA) were calculated from the RV-focused view. RV fractional area change (FAC) was calculated as (RVEDA − RVESA)/RVEDA × 100%. Tricuspid annular plane systolic excursion (TAPSE) and tissue Doppler systolic tricuspid lateral annular systolic velocity (TA S′) were obtained from the RV-focused view. To assess RV diastolic function, Dopper velocities of transtricuspid flow (E wave and A wave) and tissue Doppler-derived velocities of tricuspid lateral annular (early diastolic velocity [TA E′] and late diastolic velocity [TA A′]) were measured, and Tricuspid E/A and E/E′ ratios were calculated. Right atrial area (RAA) was traced in the apical four-chamber view at the end of ventricular systole. Tricuspid regurgitation (TR) was evaluated qualitatively and graded according to the following scale: trace = 1; mild = 2; moderate = 3; severe = 4.
Hemodynamic parameters estimated by Doppler echocardiography
Systolic pulmonary artery pressure (PASP) was estimated according to the simplified Bernoulli equation as PASP = 4 × V2 (with V = peak velocity of tricuspid regurgitation) + RA pressure (RAP). RAP was estimated according to the diameter and respiratory variation in diameter of the inferior vena cava (IVC) on echocardiography. IVC diameter < 2.1 cm with > 50% collapse on forced inhalation was indicative of normal RAP (3 mmHg), and IVC diameter > 2.1 cm with < 50% collapse on forced inhalation, or < 20% collapse on quiet inspiration, was indicative of elevated RAP (15 mmHg). If the IVC diameter and collapse did not fit these criteria, an intermediate value of 8 mmHg was assigned [15]. Mean pulmonary artery pressure (mPAP) was calculated [16] as mPAP = 0.6 × PASP + 2 mmHg, and pulmonary vascular resistance (PVR) was calculated [17] as PVR = (PASP/right ventricular outflow tract velocity time interval [RVOTVTI]) + 3 (if notch present in pulse Doppler wave of the RVOT).
Speckle tracking echocardiography
Apical four chamber views were obtained and three consecutive heart cycles were stored during breath hold with stable ECG recording in order to achieve a better image for 2D-STE analysis. Standard 2D cine loops were recorded for offline analysis using a Phillips QLAB work station (Philips Healthcare). Strain analysis was performed in all studies with adequate image quality. Inadequate image quality was defined as poor visualization or tracking of two or more of six segments. The endocardial border was traced in the right ventricle at ventricular end-diastole and in the right atrium at ventricular end-systole for strain analyses. The region of interest was manually adjusted to the thickness of the myocardium. The wall of the RA or RV was automatically divided into six segments. The software then tracked speckles throughout the cardiac cycle and provided an average curve of the six segments. Peak RV global longitudinal strain (RV GLS) was measured with the average curve of the RV strain. RA LS and LSR were measured with the average curve of the RA strain and the strain rate. Three different values were derived for the LSR, as previously described [7]: (1) the peak LSR, which occurs at RV systole and indicates reservoir function; (2) the early LSR, which occurs with the RV E wave and indicates passive conduit function; and (3) the late LSR, which occurs with the RV A wave and indicates active contraction. All measurements were performed by a single investigator who was blinded to the patients’ clinical status.
Reproducibility
Intra- and interobserver variabilities for RA LS were examined in 20 randomly selected patients. To assess intraobserver variability, the same observer, blinded to the initial measurements, repeated the measurements after more than 4 weeks had elapsed. In addition, a second independent observer repeated the measurements twice to assess interobserver variability. One of the two observers was an expert in echocardiography.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Continuous data are presented as mean ± standard deviation (SD) and categorical variables as frequency (percentage). Normality plots with tests were performed using the Shapiro–Wilk test. Independent samples Student’s t test or Mann–Whitney U test was used, as appropriate, to compare differences in continuous variables between 2 groups, and categorical variables were compared using Chi square or Fisher exact test, as appropriate. Comparisons among three or more groups were assessed using one-way analysis of variance, and comparisons between groups were performed by post hoc analysis of variance using Scheffe’s method. A Pearson correlation was used to analyze the relationship between RA LS and the other echocardiographic and clinical parameters associated with cardiac function. A Spearmen correlation was used to analyze the relationship between the WHO FC and the echocardiographic and clinical parameters. Multivariable linear regression was used to adjust for confounding factors, and receiver-operator characteristic (ROC) analysis was performed for prediction of clinical outcomes based on RA strain parameters, with results expressed as areas under the curve (AUC). Event-free survival curves were determined by the Kaplan–Meier method with a comparison of cumulative event rates by log-rank test. An initial univariate Cox proportional hazards analysis was performed to identify univariate predictors of long-term events and was followed by a multivariate Cox proportional hazards model using stepwise selection, with P-values for entry from the model set at < 0.10. Intra- and interobserver variabilities for RA LS were examined in 20 randomly-selected patients using Bland–Altman analyses. For all tests, P < 0.05 (two-tailed) was considered statistically significant.
Results
Patient characteristics
A total of 63 consecutive CTD-PAH patients were enrolled. After exclusion of 4 patients with suboptimal images because of poor echocardiographic windows, 4 patients with concomitant left heart disease, and 2 patients with congenital heart disease, 53 remaining CTD-PAH patients were enrolled in this study. Systemic lupus erythematosus (SLE) was the most common underlying CTD (22, 33.7%), followed by mixed connective tissue disease (MCTD) (17, 32.1%), primary Sjögren’s syndrome (pSS) (14, 26.4%), and systemic sclerosis (SSc) (2, 3.8%). The mean age was 42 ± 15 years, and 51 patients (96.2%) were female. At the time of enrollment, 37 patients (69.9%) had been treated with PAH-specific drugs and 16 were newly-diagnosed. The majority of patients were WHO FC III (50.9%) and IV (26.4%). PASP and PVR were 77.1 ± 11.5 mm Hg and 9.71 ± 3.11 WU. The baseline characteristics of the study population are summarized in Table 1 and the echocardiographic variables are presented in Table 2.
Comparison of baseline characteristics of patients with and without clinical worsening
At a mean follow-up of 19.3 ± 10.9 months, 20 patients (37.7%) exhibited clinical deterioration, specifically: worsening of PAH in 9 (17%); initiation of prostanoid therapy in 2 (3.8%); and death (9, 17%). Causes of death are summarized in Table 3. The patients were divided into two groups, one with and the other without clinical events, and these 2 groups showed different baseline clinical and echocardiographic characteristics. PASP, mPAP, and PVR were significantly higher in patients with events than in those without events (70.7 ± 12.3 vs. 87.5 ± 14.7, P < 0.001; 54.5 ± 8.8 vs. 44.4 ± 7.4, P < 0.001; 11.9 ± 3.6 vs. 8.39 ± 1.8, P < 0.001) and WHO FC and 6MWD were significantly worse in patients with events than in those without (FC IV, 45% vs. 15.2%, P = 0.025; 396.7 ± 79.4 vs. 323.0 ± 76.8, P = 0.019). The comparison of baseline characteristics of patients with and without clinical worsening is summarized in Table 1.
Comparison of RV remodeling in patients with and without worsening clinical conditions
RV remodeling was analyzed in 53 patients. Compared with controls, patients in both groups (with and without events) showed significantly enlarged RV sizes as reflected by enlarged RVEDA (14.8 ± 2.9 vs. 24.8 ± 5.6 and 33.2 ± 8.3, P < 0.001) and RVESA (7.6 ± 2.0 vs. 18.2 ± 5.5 and 26.8 ± 8.7, P < 0.001), and significant RV systolic function impairment of as reflected by lower RVFAC (59.3 ± 5.7 vs. 27.7 ± 8.4 and 20.2 ± 9.9, P = 0.005), TAPSE (21.1 ± 1.2 vs. 16.7 ± 3.6 and 14.3 ± 3.7, P = 0.026), and TA S′ (13.9 ± 2.5 vs. 10.6 ± 2.1 and 9.3 ± 1.8, P = 0.024). Patients with clinical events showed more significant RV size enlargements and RV systolic functional impairments compared with patients without events at baseline. Significant RV diastolic functional impairments were found in both groups (with and without events), as reflected by the lower tricuspid E/A (1.31 ± 0.31 vs. 0.74 ± 0.30 and 1.05 ± 0.65, P < 0.001). Compared with patients without events, patients with clinical events showed more significant impairments of RV diastolic function, as reflected by a higher tricuspid E/A (0.74 ± 0.30 vs. 1.05 ± 0.65, P < 0.01) and E/E′ (5.11 ± 1.98 vs. 6.96 ± 3.48, P < 0.01). TA A′, which represents the diastolic motion of tricuspid annular velocities resulted from active RA contraction, was higher in patients without events compared with that of the controls and patients with events (13.1 ± 3.3 vs. 10.1 ± 2.3 and 10.2 ± 3.9, P < 0.001). Comparisons among the echocardiographic RV parameters of patients with and without worsening clinical scenarios and healthy controls are summarized in Table 2.
Right atrial strain and strain rate
RA LS and LSR were analyzed in 53 patients and 30 healthy controls. Compared with controls, patients in both groups (with and without events) showed significant impairment of RA reservoir function, as reflected by impaired RA LS (42.41 ± 5.92 vs. 30.5 ± 8.4 and 18.6 ± 7.6, P < 0.001); significant impairment of RA peak LSR (3.7 ± 0.9 vs. 3.3 ± 1.0 and 2.1 ± 0.6, P < 0.001); and significant impairment of RA conduit function, as reflected by impaired RA early LSR (− 3.2 ± 0.9 vs. − 1.9 ± 1.1 and − 1.1 ± 0.6, P < 0.001). RA active contraction, as reflected by RA late LSR, was significantly lower in patients with events but significantly higher in patients without events compared with controls (− 3.4 ± 0.95 vs. − 4.4 ± 1.2 and − 2.8 ± 1.3, P < 0.001). Comparisons of RA echocardiographic parameters of patients with and without clinical worsening and healthy controls are summarized in Table 2, and Figs. 1 and 2 show representative imaging from patients with and without adverse events.
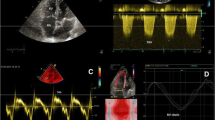
Representative image from a patient without adverse events. A 39 year-old man with SLE-PAH, WHO FC III, who was without adverse event after 28 months of follow-up. a, b Baseline echocardiography shows an enlarged RAA, measuring 33 cm2 (RAAi 16.5 cm2/m2), with mildly decreased RA LS and RA LSR. c, d Echocardiogram 28 months later shows significant reversal of right heart remodeling and increased RA LS and RA LSR
RA LS showed a passive correlation with RAA (r = − 0.68, P < 0.001), tricuspid E/A (r = − 0.34, P = 0.013), tricuspid E/E′ (r = − 0.39, P = 0.004), RV GLS (r = − 0.71, P < 0.001), PASP (r = − 0.42, P < 0.001), and PVR (r = − 0.66, P < 0.001). In addition, RA LS correlated well with clinical parameters, including WHO FC (r = − 0.70, P < 0.001), 6MWD (r = 0.58, P < 0.001), and BNP (r = −0.61, P < 0.001). Finally, RA LS independently predicted WHO FC after adjusting for RAA, TR grade, RV GLS, and PVR (P = 0.006). Correlations between RA LS, WHO FC and RAA, RV size and function, hemodynamic parameters, and clinical parameters are summarized in Table 4.
Association of right atrial strain and long-term outcomes
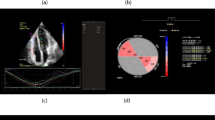
The ROC curve analysis showed that RA LS < 22.9% was predictive of clinical worsening during follow-up, with sensitivity = 80%, specificity = 87.9%, and AUC = 0.858 (95% confidence interval [CI] 0.750 to 0.966; P < 0.001) (Fig. 3), and the Kaplan–Meier curve indicated that patients with RA LS ≥ 22.9% had more favorable long-term outcomes compared to those with RA LS < 22.9% (log-rank P < 0.01) (Fig. 4). The univariate Cox proportional hazards analysis showed that PVR, RV GLS, RAA, and RA LS were associated with long-term outcome (hazard ratios and 95% CIs for each of these variables are shown in Table 5). Notably, RA LS was the only independent predictor of long-term outcomes on the multivariate analysis.
Reproducibility of right atrial longitudinal strain
Intra- and interobserver variability were assessed for RA LS in 20 patients chosen at random. The mean difference in intraobserver variability for RA LS was 3.4% (95% CI − 8.7 to 15.5%) and the mean difference in interobserver variability was 4.3% (95% CI − 12.1 to 20.7%).
Discussion
This prospective study evaluated RA function in patients with CTD-PAH by 2D-STE. The prognostic analysis demonstrated that RA LS can be a predictor of adverse events independently of RV strain and RA size. There has been no previous report about RA function in a homogeneous population of patients with CTD-PAH.
Characteristics of connective tissue disease-associated pulmonary arterial hypertension
CTD-PAH is the most common type of PAH. As a group, the survival of patients with CTD-PAH is poorer compared with patients with other types of PAH. However, most of the data are from cohort studies conducted in the United States [18] and Europe [19], with SSc-PAH as the most common CTD. In Asian cohort studies [20, 21], PAH is most commonly associated with SLE, followed by pSS and MCTD. The main CTDs in the present study were SLE, MCTD, and pSS. One of the most distinctive aspects of CTD-PAH compared to other PAH is its response to immunosuppression [10]. Inflammation and dysregulated immunity play major roles in the pathogenesis of CTD-PAH, and immunosuppressive therapy has resulted in clinical improvement in patients with CTD-PAH, except in SSc-PAH [22]. The CTD-PAH patients who responded to immunosuppressive therapy tended to have less severe disease at baseline [23], which suggests that these patients were at an earlier phase of the disease, whereas those with longstanding PAH had irreversible pathologic changes in their pulmonary vessels. PAH can also result from pulmonary vasculitis as part of systemic inflammation [24], and may be diagnosed at an early stage due to systemic manifestations. Some patients with CTD-PAH may be curable in the early stage [11]. In the present study, patients without adverse events also had less severe disease at baseline, presented with better WHO FC, and showed less right heart enlargement and lower PVR, and the patients with adverse events had a longer mean duration of PAH, although the difference did not achieve statistical significance. The right heart returned to near-normal size in some event-free patients after short-term therapy with PAH-specific and immunosuppressive drugs, which suggested that CTD-PAH patients without events were at an earlier phase of disease, had less severe hemodynamic abnormities and impairments of the right heart at baseline, and echocardiographic parameters that could assess disease severity and further predict prognoses.
Right atrial function in patients with connective tissue disease-associated pulmonary arterial hypertension
Mortality in patients with PAH is associated with the extent of right heart failure, and preserved RA function is essential in maintaining adequate total right heart function in the setting of RV failure. RA function, which can be easily evaluated by 2D-STE, was found to be predictive of right-sided heart failure, clinical deterioration, and mortality in patients with PAH [8, 9]. However, all of these studies were performed in populations with heterogeneous PAH causes, and CTD-PAH has not been studied independently. RA functional impairment has been found in SSc [25] and SLE [26] patients with normal pulmonary arterial pressure, which could affect the relationship between RA function and prognoses in patients with CTD-PAH. Our research evaluated RA function with 2D-STE, and further assessed the relationship between RA function, cardiac functional classes, and prognoses in patients with CTD-PAH.
The RA has three different functions during the cardiac cycle: a reservoir function, assessed by RA LS and RA peak LSR, a passive conduit function, assessed by RA early LSR, and an active contractile function, assessed by RA late LSR. The RA reservoir function is 4 times more important than its contractile function because it has a distensible chamber with its dilatation amplitude reflected in the potential energy stored in the atrium that contributes to an atrial kick [27]. The conduit function depends on RV relaxation properties. The contractile function depends on the intrinsic contractility and is affected by RA compliance. It has been previously reported that the reservoir function and conduit function were impaired in PAH patients [7, 9, 28, 29]. In the present study, CTD-PAH patients were divided into two groups based on whether or not adverse clinical events occurred during the follow-up period, and baseline RA functions were compared. We also found that the RA reservoir and conduit functions were impaired in both groups, with or without events. We further found that the group with events showed more seriously impaired RA and RV functions at baseline and that RA functional impairments were associated with RV diastolic and systolic dysfunctions. In the setting of PAH and RV pressure overloading, RV systolic function was initially preserved, but diastolic dysfunction occurred as a result of myocardial compensatory hypertrophy [30]. Elevated RV pressures and diastolic dysfunctions induced RA expansion. The RA reservoir and conduit functions were impaired as the result of RA expansion and reduced RV compliance. Gaynor et al. [31] found that the RA can dynamically adjust its phase function and that the conduit-to-reservoir ratio is inversely related to cardiac output. Increases in compensatory conduit functions were found in patients with atrial failure and without ventricular dysfunction [32]. Sato et al. [33] evaluated RA function using cardiac magnetic resonance (CMR) imaging in patients with PH and found decreased reservoir and increased conduit functions, in which study, conduit function was quantified as conduit volume. However, work by our laboratory and others [7, 28, 29] evaluating RA function with 2D-STE or 3DE, assessed conduit function by early diastolic strain rate or passive emptying fraction, found that both reservoir and conduit functions were decreased. Differences in the parameters for conduit functions between CMR and echocardiography could account for the difference in conclusions, because in the setting of RA enlargement, even if the conduit volume increases, the passive emptying fraction may be reduced.
Compared with healthy controls, RA active systolic function in CTD-PAH patients without events was enhanced, and tricuspid annular late-diastolic velocity was increased, which represented an increase in the contribution of RA active contractions to RV diastolic fillings. According to the Frank-Starling mechanism [34], an enlarged RA can compensate for ventricular dysfunction and maintain cardiac output by augmenting active contractile function. However, when RV pressure-overload progresses further, and the RA functional reserves reach a limit, RA compensation for RV dysfunction is lost, leading to decreased cardiac output with the onset of severe RHF and rapid deterioration until death occurs. Preserved RA active function in patients without events could indicate preserved right heart function, while seriously impaired active RA function in patients with events might signal severe right heart dysfunction and an imminent worsening of clinical conditions.
In the three-phase functions, RA reservoir function is considered to have particular importance as it provides energy needed for atrial kick [27]. Querejeta et al. [7] found that RA reservoir function independently reflected RV failure and overload. Liu et al. [9] found that RA reservoir function was valuable for predicting patient functional status and exercise capacity. In the present study, RA reservoir function, as reflected by RA LS, was significantly correlated with RV GLS, BNP, and WHO FC. The RA LS correlated better with WHO FC than RV GLS, which could have been because the symptoms of serious right heart failure are associated with significantly elevated RAP, which is more directly reflected by decreased RA function. We found a better correlation between RA LS and WHO FC than RAA, which suggest that RA LS might be a more sensitive indicator of severe heart failure than RAA. A similar result was reported by Liu et al. [9], who found that RA size was similar in PAH patients with WHO FC III and IV, but that RA LS was significantly decreased in patients with WHO FCIV. It should be noted that RAA might not accurately reflect the size of the RA. Patel et al. [35] found that there was only a modest correlation between RAA and three-dimensional (3DE)-derived RA volumes. The lack of a strong correlation could be due to a foreshortening of the 2DE images and the limitation of the single view for RA size assessment. RT-3DE has been proven to be a reliable method for assessing cyclic RA volume and function [28, 35]. However, the analysis process is relatively complicated, and accuracy largely depends on optimal full volume image quality. In contrast, RA LS provides a simple, fast, and reliable noninvasive measure of RA function. In addition, we found that RA LS independently reflects WHO FC after adjusting for RAA, TR grade, RV GLS, and PVR, which suggests that the RA LS could be a reliable indicator of right heart dysfunction severity in CTD-PAH.
Prognostic utility of right atrial function in connective tissue disease-associated pulmonary arterial hypertension
The prognostic utility of RA function in PAH has recently been of interest. Sato et al. [36] evaluated right heart function in patients with pre-capillary pulmonary hypertension (PH) by CMR and found that RA volume and reservoir function combined with RVEF are novel predictors of clinical worsening. Darsaklis et al. [37] demonstrated that decreased RAEF at CMR in PH patients is independently associated with worse survival after adjustment for other risk factors. Compared with CMR, 2D-STE, which produces reliable measurements of RA function, is more economical and accessible. Brunner et al. [38] have reported that low active and total RAEF values measured were associated with mortality in patients with treatment-naïve group 1 PAH. Bhave et al. [8] evaluated RA function in patients with group 1 PAH by 2D-STE and found that RA strain is predictive, but not independently predictive, of clinical outcomes. D’Alto et al. [29] found that RA function also has prognostic value in idiopathic PAH, and that poor RA function, as explored by strain and strain rate analysis, is associated with a worse outcome. However, the prognostic value of RA function in CTD-PAH has not been reported until now. In the present study, we demonstrated that RA LS is predictive of clinical events in CTD-PAH, independent of RAA, RV GLS, WHO FC, and hemodynamic parameters derived from Doppler echocardiography. The Kaplan–Meier curve showed that patients with lower RA LS tended to have a significantly higher incidence of adverse events during a relatively short follow-up time. The RAA is an established poor prognostic risk factor, but our study demonstrated that RA LS more sensitively reflects cardiac function statues and predicts the prognosis than RAA in patients with CTD-PAH. RA reservoir function has reportedly been associated with poor clinical outcomes, which was presumably due to a significant link between atrial reservoir function and functional capacity or overall cardiac function, as we have previously demonstrated. We showed that the prognostic effect of RA function in CTD-PAH patients was similar to that in patients with other PAH types. Compared with the results of Bhave et al. [8], our research demonstrated the RA LS predicted poor prognoses independent of RV function in CTD-PAH patients, which could imply that RA function has a particularly important prognostic role in the CTD-PAH disease process. Compared with the research of PH patients by Fukuda et al. [39], we found a lower cutoff value for predicting clinical worsening in CTD-PAH patients, which suggests that a lower cutoff value should be used to assess prognoses due to the influence of CTD in RA function. RV function plays a key role in PAH prognoses, but we could not confirm an independent predictive use of RV strain in determining clinical outcomes, which could be because the majority of patients were classified into the WHO FC III and IV (77.3%) groups with significantly impaired RV function. RA function might be a more sensitive reflection of disease severity in regard to RV failure. In the present study, the patients with adverse events had worse functional status and significantly larger RA size at baseline and the adverse events occurred after a relatively short follow-up period. In such a cohort, RA LS may have greater value as an independent predictor of clinical deterioration.
Clinical implications
RV strain has been suggested to be an important predictor of clinical worsening [40, 41]. However, previous studies have seldom included RA strain. Darsaklis et al. [37] demonstrated that the CMR-determined RA emptying fraction was directly associated with the RVEF and inversely associated with death independently of RVEF and other risk factors, which implied that RA function could have a particularly important prognostic role in the disease process. D’Alto et al. [29] first showed that RA function has prognostic value in idiopathic PAH. However, no previous CTD-PAH research has been performed. The association of RA function in the prognosis of patients with CTD-PAH appears more complicated because RA functional impairments have been found in SSc [25] and SLE [26] patients with normal pulmonary pressures. Our study found RA LS independently reflected WHO FCs, which implied that markedly reduced RA LS was the sign of a severe right heart functional impairment. We further demonstrated that markedly-reduced RA function independently suggested a poor prognosis in spite of RA functional injury due to underlying, coexisting CTD disease. RA function could have a particularly critical prognostic role in CTD-PAH. Campo et al. [42] examined the outcomes of hospitalized patients with right heart failure and pulmonary arterial hypertension and found that in-hospital mortality was significantly higher and the 12-month post-discharge clinical outcomes were significantly worse in patients with CTD-PAH compared with those with IPAH. Inadequate RV adaptation to increased afterloads could contribute to rapidly progressive right heart failure and death in CTD-PAH patients. Markedly reduced RA function is a sign of RV maladaptations and functional decompensations that can predict clinical worsening and sudden death. Fukuda et al. [39] found that patients with an RA LS < 30.2% were more likely to develop clinical worsening. In contrast, we found a lower cutoff (RA LS < 22.9%) in CTD-PAH patients, which might be because RA LS is lower in CTD patients than healthy people, which further decreases after PAH occurs. This finding implies that a lower predictive value should be used in CTD-PAH; however, disparities among the different analytic software should also be considered. In conclusion, severe impairments of RA function are poor prognostic predictors in patients with CTD-PAH, and early detection could guide decisions regarding more aggressive therapies, which include the consideration of lung transplantations.
Limitations
The present study has several limitations. Firstly, not all of the patients were diagnosed by right heart catheterization, which is the gold standard for PAH. However, according to the guideline [15], the probability of PAH is high when TRV exceeds 3.4 m/s. In addition, our strict exclusion criteria may have further improved the diagnostic accuracy for PAH. The lack of hemodynamic parameters derived from right heart catheterization is another limitation. Hemodynamic parameters of PAH, including pulmonary arterial pressure and resistance, can be estimated by Doppler echocardiography [15,16,17], but their reliability has not been widely recognized, and further comparative studies with right heart catheterization are needed. Next, RA function was measured using LV-specific software because no RA-specific software was available. However, previous studies have shown the validity of such software in the analysis of RA strain [43]. Not using 3D-speckle tracking echocardiography (STE) for RA assessments is another limitation of our study. 3D-STE merges the benefits of 3D-echocardiography and STE and assesses the volume and strain of heart chambers concurrently from the same 3D echocardiographic dataset and can calculate global strain of all segments [44, 45]. 3D-STE can provide more comprehensive information, which should be used in future research. Finally, the sample size was relatively small, and the follow-up time was relatively short. Further studies should expand the sample size and extend the follow-up period.
Conclusion
In conclusion, our data suggest that patients with more severe CTD-PAH and worse RA function at baseline are more likely to have worse outcomes. STE-derived RA LS measurements can independently reflect the extent of right heart failure and predict the clinical outcome, and patients with RA LS < 22.9% have a higher risk of clinical worsening.
References
Steen VD, Medsger TA (2007) Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 66(7):940–944. https://doi.org/10.1136/ard.2006.066068
Fei Y, Shi X, Gan F, Li X, Zhang W, Li M, Hou Y, Zhang X, Zhao Y, Zeng X, Zhang F (2014) Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin Rheumatol 33(1):57–63. https://doi.org/10.1007/s10067-013-2383-3
McGoon MD, Miller DP (2012) REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev 21(123):8–18. https://doi.org/10.1183/09059180.00008211
McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, Ahearn G (2004) Prognosis of pulmonary arterial hypertension: aCCP evidence-based clinical practice guidelines. Chest 126(1 Suppl):78S–92S. https://doi.org/10.1378/chest.126.1_suppl.78S
Sivak JA, Raina A, Forfia PR (2016) Assessment of the physiologic contribution of right atrial function to total right heart function in patients with and without pulmonary arterial hypertension. Pulm Circ 6(3):322–328. https://doi.org/10.1086/687767
Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC (2011) Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest 139(6):1299–1309. https://doi.org/10.1378/chest.10-2015
Querejeta Roca G, Campbell P, Claggett B, Solomon SD, Shah AM (2015) Right atrial function in pulmonary arterial hypertension. Circ Cardiovasc Imaging 8(11):e003521. https://doi.org/10.1161/circimaging.115.003521 (discussion e003521)
Bhave NM, Visovatti SH, Kulick B, Kolias TJ, McLaughlin VV (2017) Right atrial strain is predictive of clinical outcomes and invasive hemodynamic data in group 1 pulmonary arterial hypertension. Int J Cardiovasc Imaging 33(6):847–855. https://doi.org/10.1007/s10554-017-1081-7
Liu W, Wang Y, Zhou J, Bai H, Wang F, Wang J (2018) The association of functional capacity with right atrial deformation in patients with pulmonary arterial hypertension: a study with two-dimensional speckle tracking. Heart Lung Circ 27(3):350–358. https://doi.org/10.1016/j.hlc.2017.02.029
Kato M, Atsumi T (2018) Pulmonary arterial hypertension associated with connective tissue diseases: a review focusing on distinctive clinical aspects. Eur J Clin Invest. https://doi.org/10.1111/eci.12876
Huang C, Zhang S, Tian Z, Li M, Zeng X (2014) Could pulmonary arterial hypertension be an active index of systemic lupus erythematosus? A successful case of SLE-PAH cured by methylprednisolone pulse therapy. Lupus 23(14):1533–1536. https://doi.org/10.1177/0961203314552461
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270. https://doi.org/10.1093/ehjci/jev014
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010 (quiz 786-688)
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Group ESCSD (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37(1):67–119. https://doi.org/10.1093/eurheartj/ehv317
Chemla D, Castelain V, Humbert M, Hebert JL, Simonneau G, Lecarpentier Y, Herve P (2004) New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest 126(4):1313–1317. https://doi.org/10.1378/chest.126.4.1313
Opotowsky AR, Clair M, Afilalo J, Landzberg MJ, Waxman AB, Moko L, Maron BA, Vaidya A, Forfia PR (2013) A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol 112(6):873–882. https://doi.org/10.1016/j.amjcard.2013.05.016
Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, Miller DP, Nicolls MR, Zamanian RT (2010) Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 138(6):1383–1394. https://doi.org/10.1378/chest.10-0260
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G (2006) Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173(9):1023–1030. https://doi.org/10.1164/rccm.200510-1668OC
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M (2012) Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a single-centre cohort. Rheumatology (Oxford, England) 51(10):1846–1854. https://doi.org/10.1093/rheumatology/kes140
Hao YJ, Jiang X, Zhou W, Wang Y, Gao L, Wang Y, Li GT, Hong T, Huo Y, Jing ZC, Zhang ZL (2014) Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J 44(4):963–972. https://doi.org/10.1183/09031936.00182813
Miyamichi-Yamamoto S, Fukumoto Y, Sugimura K, Ishii T, Satoh K, Miura Y, Tatebe S, Nochioka K, Aoki T, Do EZ, Shimokawa H (2011) Intensive immunosuppressive therapy improves pulmonary hemodynamics and long-term prognosis in patients with pulmonary arterial hypertension associated with connective tissue disease. Circ J 75(11):2668–2674
Sanchez O, Sitbon O, Jais X, Simonneau G, Humbert M (2006) Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest 130(1):182–189. https://doi.org/10.1378/chest.130.1.182
Johnson SR, Granton JT (2011) Pulmonary hypertension in systemic sclerosis and systemic lupus erythematosus. Eur Respir Rev 20(122):277–286. https://doi.org/10.1183/09059180.00003811
D’Andrea A, D’Alto M, Di Maio M, Vettori S, Benjamin N, Cocchia R, Argiento P, Romeo E, Di Marco G, Russo MG, Valentini G, Calabro R, Bossone E, Grunig E (2016) Right atrial morphology and function in patients with systemic sclerosis compared to healthy controls: a two-dimensional strain study. Clin Rheumatol 35(7):1733–1742. https://doi.org/10.1007/s10067-016-3279-9
Ge XY, Shao L, Zheng ZL (2016) Assessment right atrial function in patients with systemic lupus erythematosus by speckle tracking and three-dimensional echocardiography. Zhonghua yi xue za zhi 96(47):3815–3818. https://doi.org/10.3760/cma.j.issn.0376-2491.2016.47.010
Rai AB, Lima E, Munir F, Faisal Khan A, Waqas A, Bughio S, ul Haq E, Attique HB, Rahman ZU (2015) Speckle tracking echocardiography of the right atrium: the neglected chamber. Clin Cardiol 38(11):692–697. https://doi.org/10.1002/clc.22438
Deng Y, Guo SL, Wu WF, Wang Q, Su HY, Tan Z, Wang F, He QY (2016) Right atrial evaluation in patients with pulmonary hypertension: a real-time 3-dimensional transthoracic echocardiographic study. J Ultrasound Med 35(1):49–61. https://doi.org/10.7863/ultra.15.01028
D’Alto M, D’Andrea A, Di Salvo G, Scognamiglio G, Argiento P, Romeo E, Di Marco GM, Mattera Iacono A, Bossone E, Sarubbi B, Russo MG (2017) Right atrial function and prognosis in idiopathic pulmonary arterial hypertension. Int J Cardiol 248:320–325. https://doi.org/10.1016/j.ijcard.2017.08.047
Gan CT, Holverda S, Marcus JT, Paulus WJ, Marques KM, Bronzwaer JG, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A (2007) Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest 132(1):11–17. https://doi.org/10.1378/chest.06-1263
Gaynor SL, Maniar HS, Prasad SM, Steendijk P, Moon MR (2005) Reservoir and conduit function of right atrium: impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol 288(5):H2140–2145. https://doi.org/10.1152/ajpheart.00566.2004
Hoit BD, Gabel M (2000) Influence of left ventricular dysfunction on the role of atrial contraction: an echocardiographic-hemodynamic study in dogs. J Am Coll Cardiol 36(5):1713–1719
Sato T, Tsujino I, Oyama-Manabe N, Ohira H, Ito YM, Yamada A, Ikeda D, Watanabe T, Nishimura M (2013) Right atrial volume and phasic function in pulmonary hypertension. Int J Cardiol 168(1):420–426. https://doi.org/10.1016/j.ijcard.2012.09.133
Anwar AM, Geleijnse ML, Soliman OI, Nemes A, ten Cate FJ (2007) Left atrial Frank-Starling law assessed by real-time, three-dimensional echocardiographic left atrial volume changes. Heart 93(11):1393–1397. https://doi.org/10.1136/hrt.2006.099366
Patel AR, Alsheikh-Ali AA, Mukherjee J, Evangelista A, Quraini D, Ordway LJ, Kuvin JT, Denofrio D, Pandian NG (2011) 3D echocardiography to evaluate right atrial pressure in acutely decompensated heart failure correlation with invasive hemodynamics. JACC Cardiovasc Imaging 4(9):938–945. https://doi.org/10.1016/j.jcmg.2011.05.006
Sato T, Tsujino I, Ohira H, Oyama-Manabe N, Ito YM, Yamada A, Ikeda D, Watanabe T, Nishimura M (2015) Right atrial volume and reservoir function are novel independent predictors of clinical worsening in patients with pulmonary hypertension. J Heart Lung Transplant 34(3):414–423. https://doi.org/10.1016/j.healun.2015.01.984
Darsaklis K, Dickson ME, Cornwell W 3rd, Ayers CR, Torres F, Chin KM, Matulevicius S (2016) Right atrial emptying fraction non-invasively predicts mortality in pulmonary hypertension. Int J Cardiovasc Imaging 32(7):1121–1130. https://doi.org/10.1007/s10554-016-0883-3
Brunner NW, Haddad F, Kobayashi Y, Hsi A, Swiston JR, Gin KG, Zamanian RT (2015) Prognostic utility of right atrial emptying fractions in pulmonary arterial hypertension. Pulm Circ 5(3):473–480. https://doi.org/10.1086/682218
Fukuda Y, Tanaka H, Ryo-Koriyama K, Motoji Y, Sano H, Shimoura H, Ooka J, Toki H, Sawa T, Mochizuki Y, Matsumoto K, Emoto N, Hirata K (2016) Comprehensive functional assessment of right-sided heart using speckle tracking strain for patients with pulmonary hypertension. Echocardiography 33(7):1001–1008. https://doi.org/10.1111/echo.13205
Shukla M, Park JH, Thomas JD, Delgado V, Bax JJ, Kane GC, Howlett JG, White JA, Fine NM (2018) Prognostic value of right ventricular strain using speckle-tracking echocardiography in pulmonary hypertension: a systematic review and meta-analysis. Can J Cardiol 34(8):1069–1078. https://doi.org/10.1016/j.cjca.2018.04.016
da Costa Junior AA, Ota-Arakaki JS, Ramos RP, Uellendahl M, Mancuso FJ, Gil MA, Fischer CH, Moises VA, de Camargo Carvalho AC, Campos O (2017) Diagnostic and prognostic value of right ventricular strain in patients with pulmonary arterial hypertension and relatively preserved functional capacity studied with echocardiography and magnetic resonance. Int J Cardiovasc Imaging 33(1):39–46. https://doi.org/10.1007/s10554-016-0966-1
Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Lechtzin N, Chami H, Girgis RE, Hassoun PM (2011) Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J 38(2):359–367. https://doi.org/10.1183/09031936.00148310
Peluso D, Badano LP, Muraru D, Dal Bianco L, Cucchini U, Kocabay G, Kovacs A, Casablanca S, Iliceto S (2013) Right atrial size and function assessed with three-dimensional and speckle-tracking echocardiography in 200 healthy volunteers. Eur Heart J Cardiovasc Imaging 14(11):1106–1114. https://doi.org/10.1093/ehjci/jet024
Nemes A, Domsik P, Kalapos A, Kormanyos A, Ambrus N, Forster T (2018) Three-dimensional speckle-tracking echocardiography detects different patterns of right atrial dysfunction in selected disorders: a short summary from the MAGYAR-Path Study. Quant Imaging Med Surg 8(2):182–186. https://doi.org/10.21037/qims.2018.01.11
Vitarelli A, Mangieri E, Gaudio C, Tanzilli G, Miraldi F, Capotosto L (2018) Right atrial function by speckle tracking echocardiography in atrial septal defect: prediction of atrial fibrillation. Clin Cardiol 41(10):1341–1347. https://doi.org/10.1002/clc.23051
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bai, Y., Yang, J., Liu, J. et al. Right atrial function for the prediction of prognosis in connective tissue disease-associated pulmonary arterial hypertension: a study with two-dimensional speckle tracking. Int J Cardiovasc Imaging 35, 1637–1649 (2019). https://doi.org/10.1007/s10554-019-01613-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01613-w