Abstract
The progesterone receptor (PR) has been increasingly well described as an important mediator of the pathogenesis and progression of breast cancer. The aim of this study was to assess the role of PR status as a prognostic factor in addition to other well-established prognostic factors. Data from five independent German breast cancer centers were pooled. A total of 7,965 breast cancer patients were included for whom information about their PR status was known, as well as other patient and tumor characteristics commonly used as prognostic factors. Cox proportional hazards models were built to compare the predictive value of PR status in addition to age at diagnosis, tumor size, nodal status, grading, and estrogen receptor (ER) status. PR status significantly increased the accuracy of prognostic predictions with regard to overall survival, distant disease-free survival, and local recurrence-free survival. There were differences with regard to its prognostic value relative to subgroups such as nodal status, ER status, and grading. The prognostic value of PR status was greatest in patients with a positive nodal status, negative ER status, and low grading. The PR-status adds prognostic value in addition to ER status and should not be omitted from clinical routine testing. The significantly greater prognostic value in node-positive and high-grade tumors suggests a greater role in the progression of advanced and aggressive tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, the treatment of patients with breast cancer and methods of predicting their prognosis and response to therapy have become increasingly based on molecular analyses. The aim is to individualize risk prediction and to tailor therapies individually to the patients and the tumors. Despite some major advances in treatment, approximately 17,000 patients still die of the disease in Germany every year [1]. Biomarkers used in clinical practice include tumor size, nodal status, grading, and estrogen receptor (ER) and HER2 receptor status as molecular markers [2]. However, other prognostic markers such as progesterone receptor (PR) status do not yet appear to have found a role in clinical practice. PR is usually used along with ER to assess the patient’s responsiveness to hormone therapy. Some retrospective analyses of clinical cohorts have shown that PR is also useful for assessing the prognosis independently of other prognostic factors, including ER, and this has been discussed in the framework of prospective randomized studies [3, 4].
The progesterone (P4) pathway has been investigated in both healthy breast tissue and in breast cancer tumors. In efforts to investigate the pathogenesis of breast cancer, there have been a variety of studies on the role of P4 in the proliferation and morphogenesis of breast epithelial cells. Less is known about the role of P4 in breast cancer tumors [5].
P4 and PR appear to be involved in proliferative mechanisms in normal breast epithelial cells and in breast cancer tumors. In healthy mammary tissue, PR-positive cells do not appear to be proliferative [6, 7], and proliferation seems to be mediated in a paracrine fashion. Receptor activator of nuclear factor-κB ligand (RANKL) and receptor activator of nuclear factor-κB (RANK) are candidate factors for the mediation of the P4 effect through PR-positive luminal cells to RANK-positive basal breast epithelial cells [8–12]. In breast cancer tumors, P4 is able to mediate the proliferation of breast cancer cells in a way that appears to be more independent of paracrine signaling [13, 14] and plays an important role in the pathogenesis and progression of breast cancer.
Anti-hormonal therapies have been developed to a high level of clinical relevance in the treatment of breast cancer tumors [15–17], but the usefulness of P4 is still not yet established. P4 is not always co-expressed with ER, and loss of PR has been discussed to have an unfavorable effect on the prognosis in some clinical cohorts [18]. In addition, it has been noted that metastases from PR-positive tumors frequently lose PR expression after systemic spread and that this change in the tumor phenotype is associated with a more unfavorable prognosis [19, 20].
Most reports published to date have not had large enough sample sizes to address subgroup analyses and questions involving interactions, or have lacked other relevant patient and tumor characteristics such as grading, which we consider to be of specific importance. The aim of the present study was therefore to investigate the role of PR and its interactions with other commonly used prognostic factors in a large, pooled analysis of data from several breast cancer centers in Germany.
Methods
The patients included in this retrospective study were recruited from cohort studies at five certified breast cancer centers in Germany (Erlangen, Freiburg, Heidelberg, Munich, Tübingen). Each of these centers contributed original data, which were pooled for the analysis. Thus, a total of 10,001 breast cancer cases were available, for which immunohistochemically assessed PR status was available. Study and patient characteristics for each study site are presented in supplementary Table 1. Further inclusion criteria for this analysis were histological proof of an invasive breast cancer, no evidence of distant metastasis at the time of the primary diagnosis, and information about overall survival, distant metastasis-free survival, and local recurrence-free survival. Applying these criteria, a total of 2,036 patients had to be excluded, resulting into a study population of 7,965 patients with primary, invasive breast cancer. Approval for the study was obtained from the local ethics committees at each university hospital.
Data collection
All of the participating breast centers are certified by the German Cancer Society and by the German Society for the Study of Breast Diseases (Deutsche Gesellschaft für Senologie). To obtain certification, a breast centre has to document each breast cancer case prospectively, including patient and tumor characteristics, treatment data, and epidemiological data. As part of the certification process, it is checked whether the treatment decisions taken are those recommended in accordance with the German guidelines for the treatment of breast cancer. This ensures fairly homogeneous treatment of breast cancer patients across several institutions. Follow-up information has to be provided for up to 10 years after the primary diagnosis. In addition, all histological data have to be documented—such as tumor size, axillary lymph-node status, grading, ER status, PR status, and HER2/neu status. As part of the continuous certification process, the quality of the data is audited and re-audited annually. Data obtained from this process were used in the analysis presented here.
PR status was assessed according to the centers’ protocols in routine clinical immunohistochemical protocols. The interpretation of whether or not a stain was positive was left to the discretion of the investigators at each study site. However, the patients were recruited at a time when 10 % was the cut-off value for positivity. In case neoadjuvant chemotherapy was given, hormone receptor assessment as well as grading had to be available for the pretherapeutic core biopsy.
Statistical considerations
The primary objective was to study whether PR status information is a prognostic factor for overall survival (OS), distant metastasis-free survival (DMFS), and local recurrence-free survival (LRFS) in addition to well-known prognostic factors. For this purpose, Cox proportional hazards regression analyses as described below were carried out with OS, DMFS, and LRFS, respectively, as outcome and the following predictors: PR (positive vs. negative), age at diagnosis (continuous), pT (ordinal), ER (positive vs. negative), grading (ordinal), nodal status (positive vs. negative), chemotherapy (yes vs. no), and anti-hormone therapy (yes vs. no).
Patients with missing outcome and patients with missing PR information were excluded. Missing predictor values were imputed using single “best guesses” (median value of continuous or integer predictors, the most common value of categorical or ordinal predictors) based on non-missing data across all subjects. Continuous predictors were used as natural cubic spline functions to describe non-linear effects [21]. The number of degrees of freedom (between one and four) of each predictor was determined by first fitting several simple cubic spline Cox regression models which differ from each other by the number of degrees of freedom, and then choosing the number of degrees of freedom which optimizes the Akaike information criterion (AIC). The AIC was used because it measures goodness of fit and also takes over-fitting into account by penalizing complex models.
The main survival analysis started with a Cox regression model with the well-known prognostic factors described above but without PR (based model). Next, another Cox model was fitted containing PR, the prognostic factors from the base model and the interactions of these prognostic factors with PR (full model). Both models were compared using the likelihood ratio test. A significant test results means that PR information improved the survival prognosis additionally to the considered well-known prognostic factors either across all patients or at least within one of the subgroups defined by the interaction terms. In case of a non-significant result, no further analyses were carried out to avoid false-positive results. If, however, the p value was significant, then the following variable selection procedure was performed to identify predictors which are associated with PR regarding survival: 1,000 bootstrap samples of the same size as the original dataset were taken with replacement from the original dataset. On each bootstrap sample, the full Cox model as defined above was fitted. A backward stepwise variable selection which kept all the predictors of the base model was carried out to obtain the best model in accordance with the AIC. The retained variables from each bootstrap sample were recorded, and a final variable selection was made by applying a procedure proposed by Sauerbrei and Schumacher [22]. In this procedure, the most frequently selected (>70 %) variables were chosen, and, to address correlation among variables, the variable with the larger frequency out of each highly frequent variable pair (>90 %) was chosen. A Cox regression model with these finally selected variables was fitted to the original dataset (the final model). Due to the selection conditions, the final model necessarily contained all the predictors of the base model and additionally, it possibly contained PR but no interaction term or possibly PR and at least one interaction term. Repetitive variable selections were carried out to get a stable stepwise regression result [23].
Hazard ratios (HR) with 95 % confidence intervals based on the final model were calculated. An overall HR representing the average prognostic effect of PR across all subgroups as well as HRs representing the average prognostic effect of PR within subgroups was shown. The fact that the study was multi-centric was taken into account by stratifying the models by study center, i.e., different baseline hazard functions were estimated for each study center. Interesting findings were illustrated using Kaplan–Meier curves.
The proportional hazards assumptions were checked using the method of Grambsch and Therneau [24]. If the proportional hazards assumptions had not been fulfilled, the analysis was repeated separately for survival time up to 5 years and from 5 years on.
The performance of the final Cox models in terms of discrimination and calibration (“goodness of fit”) was measured with the area under the receiver operator curve (AUC) for survival data [25] and the modified Hosmer–Lemeshow statistic for survival data proposed by Gronnesby and Borgan [26]. The AUC ranges from 0.5 (no discrimination at all) to 1 (perfect discrimination at any time point between patients with event already and patients without event then). Following Gronnesby and Borgan, the observed number of events and the model-based estimated number of events within deciles of predicted risk were compared using a goodness of fit χ2 test. A large p value indicates a satisfactory calibration.
All of the tests were two sided, and a p value <0.05 was regarded as statistically significant. Calculations were carried out using the R system for statistical computing (version 2.13.1; R Development Core Team, Vienna, Austria, 2011).
Results
A total of 7,965 patients were included in the analysis. The percentage of missing values in each variable was below 2 % except of grading (6.7 %). The missing values were imputed as described above. At the time of diagnosis, their average age was 57 years (±12 years). The PR status was positive in 5,275 patients (66.2 %), and the ER status was positive in 5,860 (73.7 %) patients. Most of the patients had an early tumor stage and were node negative, with a grade of two. Chemotherapy was administered in 38.4 % of the cases (n = 3,969). Patients with a positive PR status were older and more often had a lower tumor stage, a lower grade, and more often a positive ER status. Detailed patient characteristics are shown in Table 1. The median follow-up time was 5.1 years for OS, and 4.1 years for DMFS and LRFS, respectively.
Survival analyses
The survival analyses had to be carried out separately for the first 5 years and the second 5 years of the follow-up period for overall survival and for distant metastasis-free survival, as the proportional hazards assumptions were fulfilled for the whole follow-up period only for local recurrence-free survival. The preliminary survival analysis showed that the continuous predictor age was fitted best as a cubic spline variable with 2, 2, and 3 degrees of freedom for OS, DMFS, and LRFS, respectively. The performance of the final models, which were discussed below, seemed to be quite good with AUC values between 0.72 and 0.77 for OS and DMFS, and 0.64 for LRFS. The p-values of the Hosmer–Lemeshow tests ranged from 0.22 to 0.70 indicating satisfactory calibrations.
Overall survival
In relation to the first 5 years of follow-up, the multiple Cox survival analysis showed that the prognosis can be improved overall by taking PR into account (p < 0.001, likelihood ratio test comparing the base model with the full interaction model). The most frequent selected interactions at the repetitive variable selection process were PR by age (89 %) and PR by nodal status (75 %) resulting in a final Cox regression model with PR, these interactions and the prognostic factors from Table 1. All other interaction terms were selected in less than 60 % of all bootstrap samples. The percent frequencies are listed in Table 2. These frequency values may be regarded as a measure of variable importance.
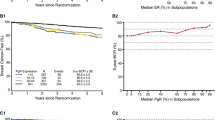
On average—i.e., without examining specific subgroups—patients with PR-positive tumors had a better overall survival prognosis than PR-negative patients (HR 0.61; 95 % CI, 0.48–0.76). However, the benefit differed between patient groups. Younger patients and node-positive patients benefited more than their counterparts (Table 3, Figs. 1, 2). We could not show that PR has prognostic value after 5 years of follow-up (p = 0.06, likelihood ratio test).
Overall survival prognosis in patients with progesterone negative and progesterone-positive tumors relative to their age at diagnosis. The results (in terms of log hazard ratio with PR- and 56 years as reference) are based on the final Cox regression model with age as cubic spline function adjusted for the prognostic factors in Table 1. The higher the value on the y-axis, the better the survival prognosis. The log hazard ratio (HR) for PR-positive versus PR-negative for patients with a specific age can be read by fixing the age value and subtracting the associated y-values on the black and blue curves
Distant metastasis-free survival
PR proved to be a significant additional predictor both in the first period and in the second period, according to likelihood ratio tests comparing base models and full models (p < 0.00001 and p = 0.02). An overall effect could be shown in the first period (HR 0.49; 95 % CI, 0.39–0.63) but not in the second period (HR 0.79; 95 % CI, 0.51–1.25). In both periods, the prognostic effect differed between patient groups defined by grading and nodal status. (Tables 2, 3). A higher grading is associated with decreasing benefit for patients with PR-positive tumors compared to PR-negative patients throughout the whole follow-up time. Within the first five years, the survival difference between PR-positive and PR-negative patients is larger in ER-negative patients than in ER-positive patients. After five years, however, there were no differences anymore in ER-positive patients, whereas the differences increased in ER-positive patients (Table 3, Fig. 3).
Local recurrence-free survival
PR significantly improved the prognosis throughout the total observation time (p = 0.02, likelihood ratio test): Young patients with PR-negative tumors seemed to live longer local recurrence free than young patients with PR-positive tumors. This effect might be reversed with increasing age. A relevant prognostic effect of PR information could be shown in nodal positive but not in nodal-negative patients (Table 3, Fig. 4).
Discussion
This retrospective study shows that data on PR status can improve the prediction of each survival outcome (crude OS, DMFS, and LRFS) in addition to the commonly established prognostic factors. Specifically, the effect of PR status on prognosis was different among the subgroups of nodal status, grade, and ER status. It appeared that the protective prognostic effect of PR status was greatest in patients with node-positive disease, a low-grade tumor, and negative ER status.
A large study by Bardou et al. reported a better disease-free survival, with a HR of 0.62 (95 % CI, 0.49–0.80) and a better overall survival with a HR of 0.67 (95 % CI, 0.54–0.82) for patients with a positive PR status [3]. Liu et al. reported a HR of 0.76 (95 % CI, 0.59–0.98), but in a group that only included ER-positive patients [27]. The present study found HRs that were lower than those in Bardou et al., but the HR for ER-positive patients was very similar to that in Liu et al. Similar effects have also been reported in other studies [28–30]. Some of these studies analyzed survival in subgroups relative to the interaction between ER status and PR status [3, 29, 30], but without taking further interactions into consideration. The other studies did not analyze for any interactions [27, 28].
The present analysis was modeled in relation to the question of whether or not the addition of PR status, and its interaction terms is able to improve the prediction of the prognosis over and above the commonly established factors, including ER. In addition to an interaction with ER, interactions with nodal status and grading were identified, as well as evidence suggesting that the progression of breast cancer is a process, for the understanding of which more—probably still unkown—factors have to be taken into consideration.
There have been several studies on the involvement of P4 and PR in the proliferation of benign, premalignant, and malignant breast cells [5]. Proliferation is part of the grading assessment, which showed an interaction with PR status relative to the prognosis in the present study. As mentioned, PR-positive benign breast epithelial cells are not highly proliferative [6, 7], but during carcinogenesis, the proportion of proliferating PR-positive cells appears to increase [31–34], and this has been linked to an increased risk of progression to malignant breast cancer cells [35]. In invasive breast cancer tumors, there are both PR-positive cells with a high grading or proliferation and PR-positive cells with a low grading or proliferation. In some breast tumors, therefore, it appears that there is a link between PR signaling and proliferation, while in others there is not. In preclinical models, the progesterone–PR pathway appears to lead to a transient proliferation of breast cancer cells, although resulting in subsequent cell cycle arrest [5, 35–38]. In some breast cancers, however, this pathway is considerably altered [39].
In the present study, PR status was best able to differentiate prognostic groups in low-grade tumors. This effect appeared to become smaller from low-grade to high-grade tumors. The HR (DMFS) for low-grade tumors was 0.34 (95 % CI, 0.22–0.54); this increased to 0.70 (95 % CI, 0.53–0.93). Grading and proliferation play an important role in the differentiation of intrinsic molecular types of breast cancer [40–42]. Proliferation as assessed by Ki-67, for example, is helpful for distinguishing between luminal A and luminal B breast cancers. Luminal B breast cancers have been reported to show a 53 % rate of grade three tumors, whereas only 19 % of luminal A breast cancers were grade three [40]. Similarly, in the present study only 41 % of tumors with a high grade were PR-positive, in comparison with 81 % of grade one tumors. It appears that the loss of the PR has a relatively greater impact on the prognosis in this subgroup than in tumors with a high grade, in which other molecular pathways may also contribute to the unfavorable outcome.
The present study has several strengths and weaknesses. Strengths include its large sample size and multicenter nature. The findings were obtained after a stratified analysis of data from the participating study centers and were found consistently for the several outcomes of OS, DMFS, and LFRS. However, the multicenter design also involves some weaknesses. The study is retrospective, and the data were therefore collected heterogeneously in all the participating hospitals (detailed data in supplementary Table 1). This might be of particular importance in relation to immunohistochemical assessment not only of PR, but also ER. ER and PR status were defined in the study according to the local assessment at the participating study site, but a cut-off value of 10 % positively stained cells was usually used to determine the hormone receptor status. It should be borne in mind that other studies have reported that up to 20 % of assessments were inaccurate [43]. In addition, the cut-off value for hormone responsiveness is currently regarded as lying at 1 % rather than 10 %, further limiting the generalizability of the data. With regard to molecular subgroups, it should be pointed out that no proliferation markers or HER2 status were available for all study centers. It has to be kept in mind that the analysis was performed in a heterogeneously treated patient population. Therefore, the described effects are most probably the result of both, a prognostic effect and an effect which translates into prognosis due to different effectiveness of chemotherapy and anti-hormone therapy. However, antihormone therapy and chemotherapy were both taken into account in our analysis. As no interaction variables between PR status and therapy were significant, it can be assumed that the effects for the prediction of prognosis are similar in treated and untreated patients.
Conclusions
The study shows that data on the PR status of patients with breast cancer are capable of improving prognostic predictions over and above other commonly used prognostic factors. However, the value of the PR status appeared to differ in different subgroups including nodal status, grading, and ER status. Assessment of the PR status should be a mandatory part of assessing the prognosis of breast cancer patients.
References
Eisemann N, Waldmann A, Katalinic A (2013) Epidemiology of Breast Cancer - Current Figures and Trends. Geburtsh Frauenheilk 73(2):130–135. doi:10.1055/s-0032-1328075
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. doi:10.1093/annonc/mdt303
Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM (2003) Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21(10):1973–1979. doi:10.1200/JCO.2003.09.099
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 29(32):4273–4278. doi:10.1200/JCO.2010.31.2835
Obr AE, Edwards DP (2012) The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol 357(1–2):4–17. doi:10.1016/j.mce.2011.10.030
Ismail PM, Li J, DeMayo FJ, O’Malley BW, Lydon JP (2002) A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16:2475–2489
Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S (2002) Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol 80:137–148
Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, Pasparakis M, Khokha R, Ormandy CJ, Widschwendter M, Schett G, Penninger JM (2010) Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468(7320):98–102. doi:10.1038/nature09387
Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R (2010) Progesterone induces adult mammary stem cell expansion. Nature 465(7299):803–807. doi:10.1038/nature09091
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE (2010) Control of mammary stem cell function by steroid hormone signalling. Nature 465(7299):798–802. doi:10.1038/nature09027
Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C (2010) Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA 107(7):2989–2994. doi:10.1073/pnas.0915148107
Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103(1):41–50
Medina D, Kittrell FS, Shepard A, Stephens LC, Jiang C, Lu J, Allred DC, McCarthy M, Ullrich RL (2002) Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J 16:881–883
Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J (2003) Hormone dependence in premalignant mammary progression. Cancer Res 63:1067–1072
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28(3):509–518. doi:10.1200/JCO.2009.23.1274
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. doi:10.1016/S0140-6736(11)60993-8
Luftner D, Lux MP, Maass N, Schutz F, Schwidde I, Fasching PA, Fehm T, Janni W, Kummel S, Kolberg HC (2012) Advances in Breast Cancer - Looking Back over the Year. Geburtsh Frauenheilk 72(12):1117–1129. doi:10.1055/s-0032-1328084
Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M (2005) Retrospective Analysis of Time to Recurrence in the ATAC Trial According to Hormone Receptor Status: an Hypothesis-Generating Study. J Clin Oncol 23:7512–7517
Hoefnagel LD, Moelans CB, Meijer SL, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, van der Valk P, van Gils CH, van der Wall E, van Diest PJ (2012) Prognostic value of estrogen receptor alpha and progesterone receptor conversion in distant breast cancer metastases. Cancer. doi:10.1002/cncr.27518
Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, van der Valk P, van der Groep P, de Vries EG, van der Wall E, van Diest PJ (2010) Receptor conversion in distant breast cancer metastases. Breast Cancer Res 12(5):R75. doi:10.1186/bcr2645
Harrell FE Jr, Lee KL, Pollock BG (1988) Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 80(15):1198–1202
Sauerbrei W, Schumacher M (1992) A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 11(16):2093–2109
Simon R, Altman DG (1994) Statistical aspects of prognostic factor studies in oncology. Br J Cancer 69(6):979–985
Grambsch PM, Therneau TM (1994) Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 81(3):515–526
Chambless LE, Diao G (2006) Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med 25(20):3474–3486. doi:10.1002/sim.2299
Gronnesby JK, Borgan O (1996) A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal 2(4):315–328
Liu S, Chia SK, Mehl E, Leung S, Rajput A, Cheang MC, Nielsen TO (2010) Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat 119(1):53–61. doi:10.1007/s10549-009-0318-0
Yang LH, Tseng HS, Lin C, Chen LS, Chen ST, Kuo SJ, Chen DR (2012) Survival benefit of tamoxifen in estrogen receptor-negative and progesterone receptor-positive low grade breast cancer patients. J Breast Cancer 15(3):288–295. doi:10.4048/jbc.2012.15.3.288
Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, Nicholson RI, Lee AH, Robertson JF, Ellis IO (2007) Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol 25(30):4772–4778. doi:10.1200/JCO.2007.12.2747
Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, Montagna E, Iorfida M, Mazza M, Balduzzi A, Veronesi P, Luini A, Intra M, Goldhirsch A, Colleoni M (2012) Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol. doi:10.1093/annonc/mds430
Rosen JM (2003) Hormone receptor patterning plays a critical role in normal lobuloalveolar development and breast cancer progression. Breast Dis 18:3–9
Brisken C, Rajaram RD (2006) Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia 11(3–4):239–248. doi:10.1007/s10911-006-9026-0
Brisken C (2002) Hormonal control of alveolar development and its implications for breast carcinogenesis. J Mammary Gland Biol Neoplasia 7(1):39–48. doi:10.1023/A:1015718406329
Anderson E (2002) The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4(5):197–201
Shoker BS, Jarvis C, Clarke RB, Anderson E, Munro C, Davies MP, Sibson DR, Sloane JP (2000) Abnormal regulation of the oestrogen receptor in benign breast lesions. J Clin Pathol 53(10):778–783
Sutherland RL, Prall OW, Watts CK, Musgrove EA (1998) Estrogen and progestin regulation of cell cycle progression. J Mammary Gland Biol Neoplasia 3(1):63–72. doi:10.1023/A:1018774302092
Skildum A, Faivre E, Lange CA (2005) Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol 19(2):327–339. doi:10.1210/me.2004-0306
Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB (1997) Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol 11(11):1593–1607
Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL (2009) DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 150(7):3318–3326. doi:10.1210/en.2008-1630
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360(8):790–800. doi:10.1056/NEJMra0801289
Strehl JD, Wachter DL, Fasching PA, Beckmann MW, Hartmann A (2011) Invasive Breast Cancer: recognition of Molecular Subtypes. Breast Care (Basel) 6(4):258–264. doi:10.1159/000331339
Schmidt M, Fasching PA, Beckmann MW, Kolbl H (2012) Biomarkers in Breast Cancer–an Update. Geburtsh Frauenheilk 72(9):819–832. doi:10.1055/s-0032-1315340
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. doi:10.1200/JCO.2009.25.6529
Acknowledgments
This work was partly funded by the ELAN programme of the University Hospital Erlangen.
Conflict of Interest
AH declares a consultant role for Medac, PAF declares a consultant role for Novartis and research funding by Novartis, WJ declares research funding by Pfizer, AstraZeneca and Novartis. All other authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jessica Salmen and Julia Neugebauer have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salmen, J., Neugebauer, J., Fasching, P.A. et al. Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res Treat 148, 143–151 (2014). https://doi.org/10.1007/s10549-014-3130-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3130-4