Abstract
Estrogen receptor status in breast cancer is associated with response to hormonal therapy and clinical outcome. The additional value of progesterone receptor (PR) has remained controversial. We examine the value of PR for prognosis and response to tamoxifen on a population-based series of 4,046 invasive early stage breast cancer patients. Clinical information for age at diagnosis, stage, pathology, treatment and outcome was assembled for the study cohort; the median follow-up was 12.4 years. PR status was determined by immunohistochemistry using a rabbit monoclonal antibody on tissue microarrays built from breast tumor surgical excisions. Survival analyses, Kaplan–Meier functions and Cox proportional hazards regression models were applied to assess the associations between PR and breast cancer specific survival. Progesterone receptor was positive in 51% of all cases and 67% of estrogen receptor positive (ER+) cases. Survival analyses for both the whole cohort and ER+ cases given tamoxifen therapy showed that patients with PR+ tumors had 24% higher relative probability for breast cancer specific survival as compared to PR− patients, adjusted for ER, HER2, age at diagnosis, grade, tumor size, lymph node status and lymphovascular invasion covariates. Higher PR expression showed stronger association with patient survival. Log-likelihood ratio tests of multivariate Cox proportional hazards regression models demonstrated that PR was an independent statistically significant factor for breast cancer specific survival in both the whole cohort and among ER+ cases treated with tamoxifen. PR adds significant prognostic value in breast cancer beyond that obtained with estrogen receptor alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant breast tumors are composed of a number of biological subtypes, displaying a variety of pathological and clinical features [1, 2]. About 70–75% of breast cancers are estrogen receptor positive (ER+), and half express the progesterone receptor (PR) [3, 4]. Immunohistochemical assessment of ER is part of the standard clinical workup of newly diagnosed breast carcinomas. ER status predicts response to hormonal therapies such as tamoxifen and aromatase inhibitors [5], but is a relatively weak prognostic biomarker, as it is related to tumor histology and grade [6]. Problems have been reported in clinical implementation of ER testing, including false negatives—which have led to major problems in patient care [7].
PR expression is activated by ER. Low PR is associated with up-regulated growth factor signaling and aggressive tumors [8]. PR expression may define a subpopulation of breast cancer patients having a stronger dependence on hormone receptor-associated biological growth, and therefore superior response to hormone therapy [9]. A study of 500 patients in a clinical trial evaluated hormone receptors and the effect of adjuvant tamoxifen in premenopausal breast cancer patients, and demonstrated that PR is in fact a stronger marker for predicting treatment response than ER [10]. Molecular profiling research suggests that both ER and PR could have prognostic value for breast cancer clinical outcomes, using a population-based gene signature approach [11]. Olivotto et al. [12] in contrast, reported in 2004 that PR testing did not add diagnostic information nor have therapeutic impact for breast cancer patients, and suggested that PR testing may be redundant. Meanwhile Banerjee’s [13] data found that PR was an independent prognostic indicator for disease-free survival, regardless of the type of adjuvant therapy. These discussions stirred debate about of the value of PR testing in breast cancer management [14].
Thus it has remained controversial as to the extent to which PR adds value when ER status is known. In this study we assess whether or not PR status, determined using a rabbit monoclonal antibody, has significant value as a prognostic factor for patient survival, in a large population-based cohort of breast cancer patients with long term follow-up and known adjuvant treatments.
Materials and methods
Study population
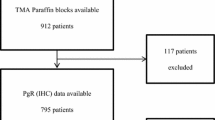
The study cohort included 4,046 female patients with invasive breast cancer in British Columbia newly diagnosed between 1986 and 1992. Mean age at diagnosis was 58.9 years (23–95 years), with a median follow-up of 12.4 years, with detailed information kept on date and cause of death. In British Columbia, most breast cancer patients are treated according to provincial guidelines developed by the British Columbia Cancer Agency, based on age at diagnosis, tumor size, lymphovascular invasion (LVI), nodal and ER status [15]. During the time period of this study cohort, patients were defined as high risk if their lymph nodes were positive, or (if node negative) there was presence of LVI or if the tumor was both >2 cm and ER negative at the time of diagnosis. These high risk patients were given adjuvant systemic therapy (AST), including tamoxifen, chemotherapy or both depending on age and menopausal status. Patients defined as low risk at time of diagnosis were not given any AST. The baseline clinical information of the study population includes age at diagnosis; histology; grade; tumor size; number of involved axillary nodes; LVI; type of local and initial adjuvant systemic therapy (AST); and dates of diagnosis, first local, regional, or distant recurrence, death and cause of death (breast cancer vs. other). This study was approved by the Clinical Research Ethics Board of the University of British Columbia and BC Cancer Agency.
Tissue microarray, immunohistochemistry and scoring system
The Vancouver General Hospital’s centralized provincial estrogen receptor laboratory retained single archival blocks from each patient. This material had been frozen before fixation in neutral buffered formalin. Slides from these blocks, stained with hematoxylin and eosin, were reviewed by two pathologists to identify areas of invasive breast carcinoma. 17 tissue microarrays (TMAs) from the samples of the 4,046 patients were constructed, and immunohistochemistry for PR, ER, and HER2 was performed as described [16, 17]. Following technical assessment of several commercial antibodies, PR staining was performed using rabbit monoclonal antibody 1E2 (prediluted by supplier = Ventana). ER staining was done using ER rabbit monoclonal antibody SP1 (1:250; LabVision, Fremont, CA). For HER2, rabbit monoclonal antibody SP3 (1:100 LabVision) was used for immunostaining, and fluorescent in situ hybridization (FISH) was performed as described [17, 18]. Primary antibody was omitted in negative controls. External controls were slides from breast cancers with previously documented PR, ER, and HER2 expression. Stained TMA slides were digitally scanned and linked to a relational database [19].
All the stained TMAs were scored visually by two pathologists, blinded to the clinical characteristics and outcomes of the patients. IHC status of PR and ER was determined by the percentage of tumor cell nuclear positivity, and scored as negative (<1%); positive 1 + (1–25%); positive 2 + (>25–75%); or positive 3 + (>75%). For most analyses, IHC scores are dichotomized at ≥1% = ER or PR positive. HER2 status was determined by both IHC and FISH. Tumors were positive if scored as 3+ based on HercepTest criteria, or amplification ratio ≥2.0 by FISH when the IHC score was 2+.
Statistical analysis
Statistical analyses are performed using SPSS version 13.0 (SPSS Inc, Chicago, IL) and R 2.1.1 (www.r-project.org). Quality of data was examined for any missing data and unusual outliers that might reflect errors in measurement or data entry. Histogram and data summary were used to check the distribution, including mean, minimum and maximum of the outcome variable (breast cancer disease specific survival time) in groups with different PR status. Because the distributions of the outcome variable were not normal, nonparametric Wilcoxon testing was used to check the bivariate relationship between breast cancer specific survival and PR status, and other potential confounders including age at diagnosis, grade, tumor size, involvement of lymph nodes, LVI, ER, and HER2 status. Chi-square testing was used to check the relationship between PR and potential confounders.
For survival analysis, the event under study was death from breast cancer. Breast cancer specific survival time was defined as the number of years between the date of diagnosis of breast cancer and the date of death attributable to breast cancer. Survival time was censored at the time a patient died from another cause, or the follow-up period ended. For univariate survival analyses, the Kaplan–Meier function was applied to estimate probabilities of breast cancer specific survival. Log-rank testing was used to assess differences in survival among breast cancer subtypes. For multivariate survival analyses, Cox proportional hazards regression models were built to estimate hazard ratios of PR status adjusted by the above potential confounding variables, based on the maximum likelihood estimation. Smoothed, rescaled Schoenfeld residuals plots were performed to test proportional hazards assumptions. Only cases with sufficient information for all covariates were included in the analysis. Log-likelihood ratio testing was used to check the significance of the model, against a null hypothesis that all coefficients are 0. Wald statistics were used to test the significance of individual coefficients. Collinearity between PR and status of ER or HER2 was checked by observing the change in standard error of the coefficient of PR after adding ER or HER2 into the Cox regression model. Interactions among PR, ER, and HER2 were checked by building Cox regression models for positive and negative ER or HER2 status, and comparing the coefficient of PR. All the tests were two-sides at significant level of 0.05.
Analyses were repeated using relapse-free survival as an endpoint, and are presented as supplemental information at: http://www.gpec.ubc.ca/index.php?content=papers/PR.php.
Results
Clinicopathologic characteristics of the study population
Among the 4,046 patients with breast cancer shown in Table 1, mean age at diagnosis was 58.9 years (range: 23–95 years old). More than two-thirds were postmenopausal at referral. About half were poorly differentiated tumors (grade 3) and just over 40% were node positive. 58% received adjuvant systemic therapy, with either chemotherapy or tamoxifen or both, including 32% who received tamoxifen as their only AST. Among interpretable cases, 51.2% of the whole cohort was PR+, 69.5% were ER+, and 13.1% were HER2+. Integrating PR and ER, 47.1% were ER+/PR+, 23.3% ER+/PR−, 4.1% ER−/PR+, and 25.5% ER−/PR−.
Prognostic values of progesterone receptor immunohistochemistry in the whole cohort and in groups with different ER status
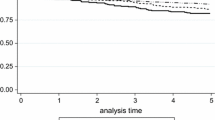
To test the prognostic value of PR in breast cancer patients, we assessed the survival functions of PR status associated with breast cancer specific survival (BCSS) in the whole cohort. Patients with PR+ tumors had significantly better survival. By grouping positive cases by the fraction of tumor nuclei immunostaining for PR, patients with the strongest PR expression had greatest BCSS (Fig. 1a). The results from cases stratified by both ER and PR status also demonstrated differences in disease specific survival, with the greatest survival in the ER+/PR+ group and lowest in the ER−/PR− group (Fig. 1b). Patients with either ER+/PR− or ER−/PR+ tumors (the latter comprising 4% of cases) had intermediate outcomes. Among the ER+ cases, those additionally positive for PR had significantly longer breast cancer specific survival. 10-year BCSS (95% CI) was 79% (76–81%) for PR+, and 68% (65–72%) for PR− patients. A significant difference was also observed between PR+ and PR− groups among the ER− cases, with a 5-year BCSS of 79% (72–87%) for PR+ and 67% (64–70%) for PR− patients. However, this difference in BCSS was no longer statistically significant at 10-year follow-up: 68% (58–77%) in PR+ and 63% (60–66%) in PR− cases.
Predictive values of progesterone receptor immunohistochemistry in different treatment groups
About 42% of patients in the cohort did not receive any adjuvant systemic therapy and were, as a group, low risk with relatively few events and good outcomes. We nevertheless observed significant differences in breast cancer specific survival between PR+ and PR− patients in this no adjuvant systemic therapy subgroup, as well as in the group of patients who received tamoxifen as their sole adjuvant systemic therapy, and in the group receiving combined tamoxifen and chemotherapy (Fig. 2). Even limiting the analysis to only the ER+ cases, significant differences in breast cancer specific survival between PR+ and PR− patients were still evident in all of these treatment groups (Fig. 3).
Multivariable analyses using Cox proportional hazards regression models
Cox proportional hazards regression models were built to estimate the raw and adjusted hazard ratio (HR) for PR status, considering potential confounders (age at diagnosis, tumor grade and size, positive axillary lymph nodes, LVI, HER2, and ER status). Smoothed, rescaled Schoenfeld residuals plots showed that PR, ER, and HER2 followed the assumptions well during the first 10 years follow-up, but varied slightly during longer follow-up; all other covariates followed proportional hazards assumptions.
Table 2 shows the estimates of raw univariate and adjusted multivariate hazard ratios for breast cancer specific survival, for the whole study cohort. PR remained a strong positive prognostic indicator even in multivariate analysis, with an adjusted hazard ratio of 0.76 (95% CI = 0.65, 0.88). The probability of breast cancer specific survival among PR+ patients was 24% (1–0.76) higher than among PR− patients, adjusted by age at diagnosis, tumor grade, size, nodal status, LVI, HER2, and ER. Results of Wald statistics for individual coefficients showed that PR and HER2 immunohistochemical status, tumor grade and size, nodal status and LVI each had significant effects on breast cancer specific survival. We observed a consistent association between the level of PR expression and patient survival, with lower hazard ratios in groups having higher PR expression.
To assess the value of PR status in the usual clinical scenario of hormonal treatment, further Cox proportional hazards regression models were established to estimate the hazard ratio of PR, adjusted by potential confounders, among ER+ cases treated with tamoxifen as their sole adjuvant systemic therapy. The multivariate analysis (Table 3) showed that the adjusted hazard ratio of PR was still 0.76 (95% CI = 0.59, 0.98). Thus, among ER+ patients treated with tamoxifen, the probability of breast cancer specific survival remained 24% (1–0.76) higher in PR+ patients than among those who are PR−. PR thus adds independent and significant information to predict improved patient outcome among ER positive patients receiving adjuvant tamoxifen. For the two Cox proportional hazards regression models built above, the number of events per variable was 346 for the whole cohort, and 101 for the ER+ cases treated with tamoxifen.
Discussion
The value of assessing progesterone receptor status in patients with breast cancer has been heavily debated over the past decade, with no clear consensus regarding the extent to which PR status has independent value. PR status is not currently playing an obligatory role in determining hormonal therapy in many jurisdictions. Problems in estrogen receptor testing [7] have lent renewed urgency to the question of whether PR status adds value for predicting the need for hormonal treatment.
Our results show that, in a cohort of approximately 4,000 patients, 51% are PR positive. When ER status is factored in, just under half are ER/PR double positive, about a quarter are double negative, just under a quarter are ER+/PR−, and 4% are ER−/PR+. Our results from both the whole cohort and among ER+ patients demonstrate that patients with PR+ status have significantly improved breast cancer specific survival. The association between patient survival and the positivity and level of PR expression is consistent with observations recently reported by Dowsett et al. [20] on the Arimidex, Tamoxifen, Alone or in Combination (ATAC) clinical trial.
In the late 1980s, only patients in our cohort with high risk characteristics were treated with adjuvant systemic hormonal therapy. However, in current practice, all hormone receptor positive patients will likely be offered some form of hormonal therapy. Among the patients treated with adjuvant hormonal therapy in our study, PR status still demonstrated a significant association with breast cancer specific survival, and even among the ER+ cases, patients treated with tamoxifen have better survival if their tumors are also PR+.
In 1990s, it was reported that immunohistochemically determined PR status was an independent prognostic factor associated with overall survival, and an independent factor for disease-free [21] and metastasis-free survival in multivariate analyses including patient’s age, menopausal status, tumor size, grade, and ER status [22]. Study 1–98 reported by the Breast International Group in 2007 showed that centrally assessed ER and PR had prognostic value, but PR status did not predict the efficacy of hormonal therapy [23]. Results from a small group of breast cancer patients suggested that response and overall survival was associated with ER expression, rather than with PR [24]. Use of different cutpoints may explain some discrepancies, as tumors with as few as 1–10% of cells positive for PR by immunohistochemistry included a subset of patients (15%) who still benefited from hormonal therapy, so those interpreting PR results using a higher cut off of 10% positive tumor cells may misclassify up to 15% of breast cancer patients as PR negative [25]. Although some studies have shown that the survival benefit of ER positivity is moderated by PR expression, and a multi-marker model worked better for predicting survival than traditional guidelines [26], other studies have reported that lack of PR expression was only associated with early breast cancer relapse, with no observed association between PR status and disease relapse after 3 years of tamoxifen treatment [27]. Our larger study, using new antibody reagents, strongly demonstrates that PR status has not only an independent and significant but also a long term (extending to at least 15-years’ follow-up) association with patient survival.
Although ER status is believed to be the most important indicator of response to hormonal therapy, not all ER+ tumors are responsive to anti-estrogen agents, and 30–40% of ER+ breast cancers will relapse or develop distant metastases despite tamoxifen treatment [28]. Since PR is induced by ER, it has been considered a surrogate marker of functional ER activation of the associated biological pathway influencing breast cell growth, and thereby potentially adds value to ER for predicting response to hormonal therapy [29, 30]. A recent study using molecular profiling in ER+ patients treated with tamoxifen showed that, aside from identified multigene clusters, PR gene expression status is the only other independent predictor (HR = 0.49, P = 0.02) of response to hormonal therapy, with histological grade, tumor size, nodal status and HER2 not significant in multivariate Cox regression analysis for time to distant metastases [28]. When taking both ER and PR status into account, ER+/PR+ tumors have a better prognosis than those with ER+/PR− or ER−/PR+ [9, 31]. Although ER−/PR+ tumors only represent 4% of breast cancers in our cohort, PR testing can not only identify these potentially hormone-responsive patients who are immunohistochemically negative for ER, but also segregates two types of ER+ tumors, where ER+/PR+ tumors are more likely to respond to hormonal therapies than ER+/PR− tumors [32, 33].
Several potential limitations of our study should be mentioned. First, the cohort is obtained from a specific regionally based population. However, an earlier study confirmed that this cohort is comparable with the North American population [17, 34]. Second, PR, ER, and HER2 do not completely satisfy the assumption of proportional hazards after 10 years follow-up. Third, the study was conducted on a tissue microarray platform using old archival specimens which had been frozen prior to fixation, whereas new cases are assessed on whole slides from freshly fixed tumor tissue.
In conclusion, our study provides strong evidence that PR status is an independent factor associated with improved survival in breast cancer patients. Assessment of PR status, using the 1E2 rabbit monoclonal antibody and a 1% cutoff for positive cells, has significant additional value in predicting patient survival and determining hormone therapy strategies, beyond that obtained by estrogen receptor alone. Furthermore the identification of the ER−/PR+ cohort allows them the option for hormonal therapy.
References
Nguyen PL, Taghian AG, Katz MS et al (2008) Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 26:2373–2378. doi:10.1200/JCO.2007.14.4287
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 295:2492–2502. doi:10.1001/jama.295.21.2492
Nadji M, Gomez-Fernandez C, Ganjei-Azar P et al (2005) Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol 123:21–27. doi:10.1309/4WV79N2GHJ3X1841
Ibrahim M, Dodson A, Barnett S et al (2008) Potential for false-positive staining with a rabbit monoclonal antibody to progesterone receptor (SP2): findings of the UK National external quality assessment scheme for immunocytochemistry and FISH highlight the need for correct validation of antibodies on introduction to the laboratory. Am J Clin Pathol 129:398–409. doi:10.1309/2YXRLEQVPPNRWHGA
ASCO Tumor Marker Expert Panel (1996) Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol 14:2843–2877
Anderson WF, Chatterjee N, Ershler WB et al (2002) Estrogen receptor breast cancer phenotypes in the surveillance, epidemiology, and end results database. Breast Cancer Res Treat 76:27–36. doi:10.1023/A:1020299707510
Hede K (2008) Breast cancer testing scandal shines spotlight on black box of clinical laboratory testing. J Natl Cancer Inst 100:836–837. doi:10.1093/jnci/djn200
Cui X, Zhang P, Deng W et al (2003) Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol 17:575–588. doi:10.1210/me.2002-0318 (erratum appears in Mol Endocrinol. 2003 Sep;17(9):1892)
Cui X, Schiff R, Arpino G et al (2005) Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23:7721–7735. doi:10.1200/JCO.2005.09.004
Stendahl M, Ryden L, Nordenskjold B et al (2006) High progesterone receptor expression correlates to the effect of adjuvant tamoxifen in premenopausal breast cancer patients. Clin Cancer Res 12:4614–4618. doi:10.1158/1078-0432.CCR-06-0248
Ma Y, Qian Y, Wei L et al (2007) Population-based molecular prognosis of breast cancer by transcriptional profiling. Clin Cancer Res 13:2014–2022. doi:10.1158/1078-0432.CCR-06-2222
Olivotto IA, Truong PT, Speers CH et al (2004) Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol 22:1769–1770. doi:10.1200/JCO.2004.99.251
Banerjee M, George J, Song EY et al (2004) Tree-based model for breast cancer prognostication. J Clin Oncol 22:2567–2575. doi:10.1200/JCO.2004.11.141
MacGrogan G, de Mascarel I, Sierankowski G et al (2005) Time for reappraisal of progesterone-receptor testing in breast cancer management. J Clin Oncol 23:2870–2871. doi:10.1200/JCO.2005.05.241 (author reply 2871)
Olivotto A, Coldman AJ, Hislop TG et al (1997) Compliance with practice guidelines for node-negative breast cancer. J Clin Oncol 15:216–222
Cheang MC, Treaba DO, Speers CH et al (2006) Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol 24:5637–5644. doi:10.1200/JCO.2005.05.4155
Cheang MC, Voduc D, Bajdik C et al (2008) Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 14:1368–1376. doi:10.1158/1078-0432.CCR-07-1658
Chia S, Norris B, Speers C et al (2008) Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 26:5697–5704. doi:10.1200/JCO.2007.15.8659
Ng TL, Gown AM, Barry TS et al (2005) Nuclear beta-catenin in mesenchymal tumors. Mod Pathol 18:68–74. doi:10.1038/modpathol.3800272
Dowsett M, Allred C, Knox J et al (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol 26:1059–1065. doi:10.1200/JCO.2007.12.9437
Castagnetta LA, Traina A, Liquori M et al (1999) Quantitative image analysis of estrogen and progesterone receptors as a prognostic tool for selecting breast cancer patients for therapy. Anal Quant Cytol Histol 21:59–62
MacGrogan G, Soubeyran I, de Mascarel I (1996) Immunohistochemical detection of progesterone receptors in breast invasive ductal carcinomas: a correlative study of 942 cases. Appl Immunohistochem 4:9
Viale G, Regan MM, Maiorano E et al (2007) Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol 25:3846–3852. doi:10.1200/JCO.2007.11.9453
Singh M, Zaino RJ, Filiaci VJ et al (2007) Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol 106:325–333. doi:10.1016/j.ygyno.2007.03.042
Mohsin SK, Weiss H, Havighurst T et al (2004) Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol 17:1545–1554. doi:10.1038/modpathol.3800229 [erratum appears in Mod Pathol. 2005 Mar;18(3):461 Note: Qian, Zho (corrected to Qian, Zhang)]
Linke SP, Bremer TM, Herold CD et al (2006) A multimarker model to predict outcome in tamoxifen-treated breast cancer patients. Clin Cancer Res 12:1175–1183. doi:10.1158/1078-0432.CCR-05-1562
Tovey S, Dunne B, Witton CJ et al (2005) Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer? Clin Cancer Res 11:4835–4842. doi:10.1158/1078-0432.CCR-05-0196
Loi S, Haibe-Kains B, Desmedt C et al (2008) Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genomics 9:239. doi:10.1186/1471-2164-9-239
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467. doi:10.1016/S0140-6736(97)11423-4
Ravdin PM, Green S, Dorr TM et al (1992) Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest oncology group study. J Clin Oncol 10:1284–1291
Ciocca DR, Elledge R (2000) Molecular markers for predicting response to tamoxifen in breast cancer patients. Endocr 13:1–10. doi:10.1385/ENDO:13:1:1
Creighton CJ, Kent Osborne C, van de Vijver MJ et al. (2008) Molecular profiles of progesterone receptor loss in human breast tumors. Breast Cancer Res Treat. doi:10.1007/s10549-008-0017-2
Osborne CK, Yochmowitz MG, Knight WAIII et al (1980) The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer 46:2884–2888. doi:10.1002/1097-0142(19801215)46:12+<2884::AID-CNCR2820461429>3.0.CO;2-U
Olivotto IA, Bajdik CD, Ravdin PM et al (2005) Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 23:2716–2725. doi:10.1200/JCO.2005.06.178
Acknowledgments
This research was supported by a Canadian Breast Cancer Research Alliance Translation Acceleration Grant and the National Cancer Institute Strategic Partnering to Evaluate Cancer Signatures program (UO1-CA114722). Torsten O. Nielsen is a Michael Smith Foundation for Health Research Senior Scholar. The Genetic Pathology Evaluation Centre is supported by an unrestricted educational grant from sanofi-aventis. We thank Blake Gilks, Allen Gown, and David Huntsman for assistance during assay development (staining and scoring).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, S., Chia, S.K., Mehl, E. et al. Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat 119, 53–61 (2010). https://doi.org/10.1007/s10549-009-0318-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0318-0