Abstract
In postmenopausal women with hormone receptor-positive early-stage breast cancer, the use of aromatase inhibitors (AIs) to suppress estrogen is associated with improved clinical outcomes compared with tamoxifen therapy. Women receiving such endocrine therapy may experience treatment-related side effects that negatively affect health-related quality of life (QoL) and adherence to therapy. In published clinical trials and in clinical practice, adverse events (AEs) constitute the main reason for nonadherence to endocrine treatment. Serious AEs are sometimes resolved by switching to a different agent, whereas other side effects can often be managed to allow patients to remain on therapy without sacrificing QoL. Across all adjuvant endocrine trials, regardless of the treatment received, vasomotor symptoms such as hot flashes are the most common side effects. Other frequently reported side effects, such as vaginal discharge, vaginal dryness, dyspareunia, and arthralgia, vary in prevalence between tamoxifen and AIs. Here we provide an overview of reported AEs of adjuvant endocrine therapy, focusing on those that are amenable to pharmacologic or nonpharmacologic management without treatment discontinuation. Also highlighted are specific management strategies that may improve patient QoL and thereby optimize adherence to therapy, which in turn might improve patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies of postmenopausal early breast cancer (EBC) demonstrate that adjuvant endocrine therapy using third-generation aromatase inhibitors (AIs) is associated with improved disease-free survival (DFS), compared with the previous standard of tamoxifen therapy. As shown in Table 1, these trials have explored the use of AIs as initial adjuvant treatment compared with tamoxifen [1–3], as sequential therapy following 2–3 years of tamoxifen [4–6], and as extended therapy following 5 years of tamoxifen [7, 8]. Thus, guidelines from both the American Society of Clinical Oncology (ASCO) Technology Assessment Status Report and the National Comprehensive Cancer Network (NCCN) recommend that AIs be included in adjuvant therapy to reduce the risk of disease recurrence in postmenopausal women with hormone receptor-positive (HR+) breast cancer [9, 10].
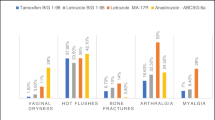
The recommended duration of initial adjuvant endocrine therapy is 5 years, and extended adjuvant therapy for an additional 5 years has proven beneficial for some patients. With such long treatment duration, adverse events (AEs) experienced during therapy must be addressed as they are more than a transient inconvenience for patients. Unless properly managed, AEs can become a major cause of morbidity and treatment discontinuation. Although some AEs, such as hot flashes, are not associated preferentially with one type of endocrine therapy over another, many AEs differ significantly in incidence depending on whether patients are treated with tamoxifen or AIs. These differences likely reflect the varied mechanisms of action of endocrine agents. For instance, tamoxifen therapy results in a higher incidence of vaginal bleeding and discharge, endometrial cancer, venous thromboembolism, and cerebrovascular events. In contrast, AI therapy results in a significantly higher incidence of musculoskeletal symptoms (including arthralgia, bone loss, and fractures), and vaginal dryness with dyspareunia [11].
In addition to their potential to exert a negative impact on quality of life (QoL), AEs may undermine necessary adherence to prescribed medication. Nonadherence, a relatively new focus of research interest in breast cancer, is a major contributing factor to variability in a drug’s intended therapeutic effect [12]. In a study of adherence to tamoxifen therapy in a clinical practice setting, nearly 25% of patients missed taking their oral dose on more than 20% of days during the first year of treatment, and overall adherence decreased to 50% by Year 4 [13]. Similarly, among 208 women surveyed regarding treatment preferences, approximately 50% admitted that they sometimes forgot or chose not to take their current oral breast cancer medication. Although 63% of women preferred daily oral medication over a monthly intramuscular (im) injection, the primary reason given for the im preference was to ensure adherence [14]. In a recent evaluation of 131 women receiving medication for breast cancer, 55% reported that they did not adhere to the medication plan, with a notable 16.7% of patients reporting that they intentionally chose not to take the medication [15].
Overall, the primary reported reason for nonadherence to prescribed tamoxifen is the development of side effects and disliking some aspect of taking medication, in particular unpleasant side effects, has been found to be significantly predictive of adherence to medication plans [15–17]. Limited data are available specifically on adherence to AIs. However, discontinuation rates were significantly higher for tamoxifen compared with anastrozole in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial (14.3% vs. 11.1%, respectively, P = 0.0002) [2]. In the Intergroup Exemestane Study (IES), more discontinuations because of AEs occurred in the exemestane group than in the tamoxifen group [4]. Further, withdrawal from the MA.17 trial because of toxicity was significantly more common in the letrozole arm compared with the placebo arm (4.9% vs. 3.6%, respectively, P = 0.019) [7].
No studies have yet investigated the degree of nonadherence to endocrine therapy that can be tolerated without compromising outcomes, although anecdotal experience supports the assumption that better adherence to a prescribed regimen leads to better outcomes. Overall, nonadherence to treatment represents a missed opportunity for health gain, and renders the interpretation of clinical trial data as potentially unrealistic in the absence of adherence data [15].
Assessing safety and tolerability of AI therapy
Established safety profile of AI therapy
Some of the key AEs reported by physicians on Case Report Forms (CRFs) in clinical trials of adjuvant endocrine therapy are summarized in Table 2. In general, AIs have exhibited a more favorable safety profile than tamoxifen when administered as initial adjuvant therapy or when taken after completion of 2–3 years of tamoxifen. Of the life-threatening AEs, venous thromboembolic events, ischemic cerebrovascular events, and endometrial cancer have been reported with a higher incidence in tamoxifen-treated compared with AI-treated patients, as seen in the ATAC trial and IES; statistically significant differences in the Italian Tamoxifen Anastrozole (ITA) trial were restricted to endometrial cancer. On the other hand, cardiac failure, other cardiovascular events, and hypercholesterolemia occurred with significantly higher incidence in letrozole-treated patients in the BIG 1-98 trial (with tamoxifen as the comparator), though hypercholesterolemia was not seen in the MA.17 trial (with placebo as the comparator) [3, 18]. In the ITA trial, the anastrozole group had a significantly higher incidence of lipid metabolism disorders, though similar incidence of cardiovascular disease, compared with the tamoxifen group [6]. Additionally, musculoskeletal disorders, including new-onset osteoporosis, fractures, and arthralgia, had a significantly higher incidence in AI-treated compared with tamoxifen-treated or placebo-treated patients in the ATAC, BIG 1-98, IES, and MA.17 trials, though statistically significant differences were not reported in the ITA trial [2–4, 6, 7].
The different safety and tolerability profiles of tamoxifen and AIs support that treatment decisions must be tailored to the individual breast cancer patient, with attention to comorbidities and patient preferences.
Illuminating AI tolerability further through patient reported QoL assessment
In addition to clinician-reported AEs recorded for safety monitoring in clinical trials, data captured via patient self-report on validated QoL instruments may provide important complementary information about a drug’s tolerability [19]. Health-related QoL has been evaluated in parallel with efficacy and safety in several clinical trials of adjuvant endocrine therapy in postmenopausal women with EBC, using the Short Form 36-Item Health Survey (SF-36), the Functional Assessment of Cancer Therapy-Breast + Endocrine Subscale (FACT-B + ES), and the Menopause Specific Quality of Life (MENQOL) questionnaires.
Specifically, the SF-36 poses questions about overall health, including energy, fatigue, bodily pain, health-related limitations on physical and social activities, and emotional well-being. The FACT-B + ES is tailored for patients with breast cancer and includes specific questions related to endocrine therapy. The FACT-B Trial Outcome Index (TOI) combines the physical well-being (PWB) and functional well-being (FWB) subscales of the FACT-B with the breast cancer subscale (BCS). The MENQOL questionnaire and the FACT-B + ES contain questions specific to menopause. Thus these tools can provide important patient-reported information, which may be used to further illuminate the tolerability of AI therapy.
QoL was evaluated as a subprotocol of the ATAC trial, using the FACT-B + ES with 5 years of follow-up [20–22]. Both anastrozole and tamoxifen were associated with improvements from baseline in overall QoL, which did not differ significantly between the treatment groups, in spite of the different side effect profiles [20]. Endocrine symptom scores in both groups worsened by 3 months of therapy but subsequently stabilized (without returning to baseline). In a study of women with EBC who discontinued tamoxifen because of severe side effects, switching to anastrozole improved ES scores and patient QoL [23]. In IES, there were no meaningful changes from baseline in FACT-B scores for women receiving either exemestane or tamoxifen over 24 months.[24] However, mean ES scores improved over time in both treatment groups [24].
The MA.17 trial afforded an opportunity to assess the impact of AI therapy (letrozole) on patient QoL compared with placebo. Using the SF-36 and MENQOL questionnaires, the letrozole group, compared with the placebo group, had slight but statistically significant worsening of physical functioning, bodily pain, vitality, and sexual function by 1 or 2 years of therapy [25]. The improvement that occurred in the vasomotor domain was delayed in the letrozole group relative to the placebo group. Although overall QoL was not significantly different between these treatment groups, it should be noted that this was a highly selected patient cohort, who were disease-free after an initial 5 years of tamoxifen and who volunteered for an additional 5 years of AI therapy. Thus women who had found tamoxifen intolerable had already dropped out of treatment, suggesting this was a group of tamoxifen-tolerant participants. These and other circumstances of the study limit the ability to generalize its results to broader populations of patients with breast cancer.
Side effects of endocrine therapy that are not life-threatening but influence QoL deserve attention. Regardless of efficacy data, these effects can sway treatment decisions and determine adherence levels, which in turn may alter outcomes. It is also important to consider whether the events are physician- or patient-reported. In one assessment, treatment-related symptoms documented by clinicians in medical notes were compared to those that patients reported in an interview, and it was found that only 89% of women receiving hormonal therapy had side effects in their medical notes, compared with 99% who reported side effects in an interview [26]. Considerable discrepancy for symptoms in physician-completed CRFs and patient-reported questionnaires has also been observed in other hormonal therapy studies. For example, in the BIG 2-97 trial of tamoxifen and exemestane, discordance between physicians and patients was as high as 32% for some side effects; however, in this instance most cases of discordance were attributed to physician under-reporting [27]. In the ATAC trial, it was noted that physicians may under-report certain side effects of hormonal therapy, particularly those related to sexual function, as women may be uncomfortable volunteering this information. In contrast, patients were less likely than physicians to identify other side effects (e.g., hot flashes, vaginal discharge and bleeding, nausea) as significant [28].
In this review, we discuss the safety and tolerability reported in clinical trials of adjuvant endocrine therapies for postmenopausal women with HR + EBC to date; using both physician-reported events as well as available QoL analyses. Table 2 summarizes selected AEs reported by clinicians as part of the safety analysis of clinical trials of endocrine therapies, while Table 3 summarizes selected side effects as reported by patients in validated QoL assessments from some of these trials. Although there is little evidence that tamoxifen or an AI significantly affects overall QoL, it is necessary to examine specific symptoms to discover how the tolerability profiles of these endocrine agents differ and can be managed to achieve optimal patient outcomes. For each class of AEs, we will describe the specific pharmacologic and nonpharmacologic management strategies and evaluate their effectiveness.
Treatment-related side effects and their management
Gynecologic symptoms
Investigators have reported that the incidence of vaginal bleeding or discharge is generally higher with tamoxifen than with AIs. Specifically, anastrozole and letrozole were each associated with significantly less vaginal bleeding than tamoxifen in the ATAC trial (5.4% vs. 10.2%, P < 0.0001) and in the BIG 1-98 trial (3.3% vs. 6.6%, P < 0.001), respectively [2, 3]. However, the Austrian Breast and Colorectal Cancer Study Group (ABCSG) trial 8/Arimidex-Nolvadex (ARNO) 95 trial combined analysis did not report a significant difference between the anastrozole and tamoxifen groups in the overall rate of vaginal bleeding plus discharge (18% vs. 17%, respectively) [5]. In the IES, vaginal bleeding was reported by more patients treated with tamoxifen than with a steroidal AI (exemestane) (5.5% vs. 4.0%, P = 0.05) [4]. Vaginal bleeding was also slightly more common in women receiving placebo compared with those taking letrozole in the MA.17 trial (8% vs. 6%, P = 0.005), suggesting inhibition of endometrial proliferation by the AI [7].
While vaginal bleeding is certainly a QoL issue, it is also a warning sign for possible endometrial hyperplasia or neoplasia after menopause and requires prompt investigation. In the ATAC trial, an excess of treatment-related endometrial cancer correlated with the significantly higher hysterectomy rate in the tamoxifen group compared with the anastrozole group (5% vs. 1%, P < 0.0001) [29]. Although tamoxifen reportedly elicits uterine abnormalities (endometrial cysts and polyps, enlargement of pre-existing fibroids) from as early as 3 months of therapy, AIs induce uterine atrophy and may even decrease uterine abnormalities due to a preceding course of tamoxifen [30]. Thus, breast cancer patients on tamoxifen who are experiencing vaginal bleeding and abnormal endometrial changes may benefit from switching to an AI.
Vaginal discharge, another event that may negatively impact QoL, is generally reported more often in patients receiving tamoxifen that those taking AIs. In the ATAC trial, significantly more tamoxifen-treated than anastrozole-treated patients experienced vaginal discharge (13.2% vs. 3.5%, P < 0.0001) and vaginal moniliasis (4% vs. 1%, P < 0.0001) [2, 29]. Significantly more patients in the tamoxifen group compared with the exemestane group were negatively affected by vaginal discharge in the IES (17.1% vs. 7.6%, P < 0.001), though this difference was not warranted for other endocrine symptoms [24].
Vaginal discharge usually reflects overgrowth of the healthy vaginal lactobacilli by normally under-represented microbial species, such as Gardnerella. It is promoted by postmenopausal estrogen deficiency, vaginal atrophy, and vaginal alkalinity, and is managed with antibiotics, antifungal agents, and avoidance of most douching products. However, a high rate of recurrent infection is not uncommon, and over-use of antibiotics or antifungals can lead to resistance. Strategies that maintain the normally acidic pH of the vagina generally discourage infection.
Patients with vaginal bleeding or discharge need to be treated individually, according to history, laboratory assessments, and severity of the symptoms. Therapy switching, when it occurs, is usually from tamoxifen to an AI.
Bone loss and osteoporosis
Estrogen is a key modulator of bone homeostasis, stimulating bone growth and inhibiting bone resorption and the normal postmenopausal loss of estrogen promotes bone loss. Tamoxifen exhibits tissue-specific estrogen-agonistic activity and is protective of bone [31]. Conversely, the systemic estrogen deprivation that results from AI therapy in postmenopausal women can result in bone resorption and osteoporosis, with higher fracture rates [2, 4, 7]. Therefore, minimizing bone loss is an important treatment issue for women receiving AI therapy.
Nonpharmacologic recommendations to help prevent bone loss include weight-bearing exercise, smoking cessation, and limitation of alcohol intake. Among the pharmacologic options for minimizing bone loss in those receiving adjuvant endocrine therapy for EBC, supplementation with calcium and vitamin D is widely recommended, though not yet adequately studied [32]. It has been reported that a majority of breast cancer patients with musculoskeletal symptoms have suboptimal levels of vitamin D, which regulates aromatase expression in osteoblasts and is essential for maintenance of bone mineral density (BMD) [33]. Results in a recent study of healthy postmenopausal women suggest that dietary calcium is significantly more effective than calcium supplements in maintaining BMD [34]. Further, excessive supplemental calcium is a risk factor for kidney stones.
Bisphosphonates, which inhibit osteoclastic bone resorption, have proven beneficial in the treatment of osteoporosis in postmenopausal women in general and appear to have a role in breast cancer. Zoledronic acid is a potent nitrogen-containing bisphosphonate, delivered by infusion, that has proven effective in reducing skeletal-related events and hypercalcemia of malignancy in metastatic breast cancer patients [35]. Preliminary results from the Zometa/Femara Adjuvant Synergy Trial (Z-FAST) suggest that lumbar spine BMD may actually increase when treatment with zoledronic acid is started sufficiently early during adjuvant letrozole therapy for postmenopausal women with EBC [36]. Breast cancer patients without metastases might elect treatment with one of the less potent oral bisphosphonates, such as alendronate or risedronate, which seem less likely to elicit AEs such as the rare but serious disorder osteonecrosis of the jaw. The Study of Anastrozole with the Bisphosphonate Risedronate (SABRE) trial is investigating the safety and efficacy of risedronate in combination with anastrozole in postmenopausal women with EBC [37]. New targeted therapies that inhibit bone loss (such as Fc-osteoprotegerin fusion protein and human anti-receptor activator of NF Kappa B ligand [RANKL] antibody) are still in preliminary clinical studies [38, 39].
Arthralgia
Arthralgia is a common complaint in the general population and has multiple associations with both diseases and treatments, including osteoarthritis, autoimmune diseases, infectious diseases, chemotherapy, and other types of drug therapy [40–43]. Prior chemotherapy and more advanced age are two major predisposing factors for joint pain in patients with breast cancer.
Arthralgia is considered a class effect of AIs, with a reported incidence 2–8% higher in patients treated with AIs versus those treated with tamoxifen [44, 45]. Musculoskeletal symptoms such as arthralgia that may appear during AI therapy are at least partly related to bone loss. Increased pain sensitivity due to estrogen deficiency might also be a factor in arthralgia, as estrogen is known to modulate neural processing of nociceptive input at the level of the central nervous system [46].
Assessment of arthralgia and other musculoskeletal effects in clinical trials of adjuvant endocrine therapy has been hampered by the lack of uniform diagnostic criteria [46]. Thus, to better define these effects, measurements with QoL instruments are beginning to supplement more objective screening parameters, such as vitamin D metabolite levels and inflammatory and rheumatologic markers [47]. Further, distinguishing arthralgia from arthritis, which is characterized by swelling, synovitis, and elevated C-reactive protein (CRP) levels, may be important to allow rational treatment selection. Currently, treatment strategies for arthralgia and arthritis substantially overlap.
A number of nonpharmacologic and pharmacologic treatment strategies have been used for arthralgia; however, none of these has proven entirely satisfactory [47]. Nonpharmacologic approaches include weight loss, resistance exercise, physical or occupational therapy, and targeted heat. Of the available pharmacologic strategies, nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase-2 (COX-2) inhibitors may be contraindicated for long-term use due to potential AEs on the gastrointestinal tract, heart, and kidneys. Narcotic analgesics such as tramadol have the drawback of masking rather than curing ongoing destructive processes in the joints. Glucosamine is not of proven efficacy for arthralgia, and topical treatments such as capsaicin and methylsalicylate are temporarily palliative at best [47]. Other therapies undergoing testing, including high-dose vitamin D, overlap with treatments to maintain BMD. However, there is no evidence to suggest that bisphosphonate therapy to prevent osteoporosis also ameliorates symptoms of arthralgia. It has been speculated that treatment of sleep disturbance, which is frequently comorbid with arthralgia, might lessen arthralgia symptoms, although no studies have been done. When arthralgia is severe and unresponsive to available treatment options, patients may be switched from AI therapy to another endocrine therapy, such as tamoxifen. There are no clinical trial data that document reliable resolution of arthralgia symptoms after switching from an AI to tamoxifen.
Prospective studies are needed to better characterize and discover optimal treatments for arthralgia. While some of the therapies that inhibit bone loss may also ameliorate arthralgia, it is likely that new specific treatments will need to be developed to address this cluster of symptoms as current therapies are either inadequate or untested. Ultimately, effective treatment of these symptoms could potentially allow more AI-treated patients with breast cancer to adhere to their therapeutic regimens.
Hot flashes
One common side effect that has been observed in all clinical trials of adjuvant endocrine therapies is hot flashes. Hot flashes are characteristic of the perimenopausal period and have been observed with a relatively high incidence in all patients regardless of treatment with tamoxifen, AIs, or placebo [2–5, 7]. An exception was the incidence of cold sweats, which was lower with anastrozole than with tamoxifen in the ATAC trial [20].
Hot flashes are vasomotor symptoms involving vasodilatation and a drop in core body temperature, causing a sensation of intense warmth beginning in the chest and often progressing to the neck and face [48]. Estrogen withdrawal, rather than an absolute level of circulating estrogen, is believed to be the cause of hot flashes that occur with hormonal changes during menopause. Changes in estrogen levels may affect the functioning of the thermoregulatory centers in the hypothalamus [48].
In menopausal women, estrogen replacement therapy (ERT) with or without progestins is the most effective treatment for vasomotor symptoms. However, ERT is contraindicated in women with HR + breast cancer, as was demonstrated in the HABITS (Hormonal replacement therapy After Breast cancer—Is iT Safe?) trial, which showed an increased risk for new breast cancer events in women with previous breast cancer who received ERT [49]. Therefore, a number of alternative therapies may also be used to manage this effect.
Alternative steroid treatments for hot flashes include the progestins, such as medroxyprogesterone acetate (MPA) and megestrol, which in a comparative trial were equally effective in reducing hot flashes by nearly 90% [50]. There currently are no long-term data on the safety of progestins in women with a history of breast cancer [50]. Androgens also have been used to control hot flashes in women with breast cancer [51]. For example, dehydroepiandrosterone (DHEA) is an endogenous proandrogen produced by the adrenal glands and liver. In a small pilot study, DHEA reduced hot flashes by approximately 50% after 5 weeks of treatment in postmenopausal women with a history of breast cancer [51]. While conclusions from this study are limited by its short follow-up and lack of placebo control, these investigators reported no side effects beyond those found at baseline, and recorded improvements in overall QoL.
Norepinephrine and serotonin are the primary neurotransmitters involved in thermoregulation [48]. Selective serotonin reuptake inhibitors (SSRIs), including fluoxetine, paroxetine, venlafaxine, and citalopram, may reduce hot flash frequency and severity in postmenopausal women with or without a history of breast cancer [52–56]. Dry mouth, headache, nausea, and insomnia were among the reported side effects of SSRI treatment, especially at higher doses, although citalopram, an SSRI with low anticholinergic activity, may cause less dry mouth and sedation than the other SSRIs [56]. In a head-to-head comparison of MPA with venlafaxine for reduction of hot flashes, MPA was more effective, with hot flash scores reduced by 79% vs. 55%, respectively (P ≤ 0.0001) [57]. However, it should be noted that in breast cancer patients with the CYP2D6 genotype, the use of SSRIs and other drugs such as antidepressants which inhibit CYP2D6, may alter the effectiveness of tamoxifen. Therefore drug interactions should be considered in women treated with tamoxifen [58, 59].
Gabapentin, a medication for nerve pain, is yet another additional treatment option reported to reduce hot flashes in women with breast cancer [60]. Finally, there are several reports from randomized clinical trials showing the efficacy of acupuncture in reducing the hot flashes experienced by women during normal menopause. A recent report of acupuncture (including self-acupuncture) in 182 women with breast cancer documented long-term relief of vasomotor symptoms [61].
Of the other therapies that have shown efficacy, progestins, androgens, and gabapentin will benefit from additional safety/tolerability testing, while the usefulness of SSRIs may be limited by known side effects. Choosing the novel approach of acupuncture will likely be governed by patient preference.
Sexual dysfunction and vaginal dryness
Endocrine therapy for breast cancer is often associated with reduced QoL in the sexual domain. Vaginal dryness, which is a common postmenopausal complaint related to low estrogen levels, was reported significantly more often by AI-treated than tamoxifen-treated patients in the ATAC and MA.17 trials [20, 22, 24, 25]. Related to estrogen loss and vaginal dryness are two sexual function concerns: dyspareunia (painful intercourse) and loss of libido, which were reported with a significantly higher incidence in patients treated with anastrozole compared with tamoxifen in the ATAC trial [20, 22]. Significant worsening of the sexual domain of the MENQOL questionnaire was observed during therapy with letrozole compared with placebo in the MA.17 trial [25]. Dyspareunia and diminished libido were also reported in the IES, but without a significant difference in incidence between the AI and tamoxifen groups [24]. This cluster of symptoms affecting sexual function, while not life-threatening, can nonetheless diminish QoL and constitute a barrier to continuation of AI therapy in sexually active women.
To treat vaginal dryness and dyspareunia, water-based lubricants and moisturizers are available, but restoration of the vaginal epithelium from its atrophic state has not been achieved without some form of estrogen. Benefit has been reported from vaginal estrogen creams applied 2 or 3 times a week, and from vaginal rings and suppositories that locally release low-dose estrogen. These local therapies were allowed in the placebo-controlled MA.17 trial of letrozole as extended adjuvant therapy following 5 years of tamoxifen, without seeming to interfere with observed efficacy [11, 62]. However, the assumption of minimal systemic absorption with these vaginal estrogen preparations has recently been challenged. In a study of AI-treated patients with breast cancer who used vaginal estradiol tablets, investigators documented significant increases in systemic estradiol levels, at least in the short term (2–4 weeks), and therefore considered these preparations contraindicated for women with HR + breast cancer [63, 64]. Definitive recommendations on vaginal estrogen for AI-treated patients may have to await the results of randomized clinical trials.
To overcome the potential inadequacies of vaginal lubricant use, an ongoing trial is evaluating combining pelvic floor muscle relaxation exercises to prevent vaginismus, a vaginal moisturizer (Replens) to alleviate vaginal dryness, and olive oil as a lubricant during intercourse [65]. The effectiveness of this combination intervention will be prospectively assessed using self-administered questionnaires with validated measures of sexual functioning, QoL, and emotional outcomes.
Pharmacologic approaches can also be considered for restoring libido, although studies in women with breast cancer are limited. Transdermal formulations of testosterone have been shown to increase libido in clinical studies of women with surgically induced menopause or healthy premenopausal women [66–68] As mood has an impact on sexual activity, antidepressants have been used to boost libido. The antidepressant venlafaxine, compared with placebo, was reported to improve libido in at least one clinical trial in breast cancer survivors [55]. However, therapy with venlafaxine and other antidepressants (especially the SSRIs) has also been associated with sexual dysfunction in many reports [69]. As sexual problems are likely to be under-reported symptoms of breast cancer patients receiving adjuvant endocrine therapy, better patient/physician communication will probably be an integral part of the solution to these problems.
Cognitive dysfunction
Most studies of cognitive impairment in patients with breast cancer who receive adjuvant treatment have focused on chemotherapy. In retrospective and cross-sectional studies of chemotherapy-treated breast cancer survivors, compared with healthy women and patients who received only local therapy (i.e., surgery or radiation), impairment has been variably reported in speed of information processing, motor function, visual memory, language, attention and concentration, executive function, verbal memory, and visuospatial skill [70]. In a recent study with the advantage of a prospective, longitudinal design, significant cognitive decline (primarily in memory and concentration) from baseline to 18 months was measured in 18% of EBC patients treated with chemotherapy versus 11% of healthy controls [71]. Patients who had experienced treatment-induced menopause seemed more likely to show cognitive decline than those who were postmenopausal at baseline, although this difference was not significant (odds ratio [OR] = 2.6, 95% confidence interval [CI] 0.82–8.26, P = 0.084). However, the majority of patients were unaffected by adjuvant chemotherapy or even showed cognitive improvement during the study. Most of these study patients received relatively low-dose 5-fluorouracil, epirubicin, and cyclophosphamide (FEC). Others have reported a threefold higher risk of cognitive impairment in breast cancer with high-dose chemotherapy than with standard-dose chemotherapy [72].
Compared with chemotherapy, the effects of adjuvant endocrine therapy on cognitive function in patients with breast cancer have received limited attention. In retrospective studies of breast cancer survivors, some investigators found cognitive decline (especially in verbal learning, visuospatial functioning, and visual memory) to be greater in patients who received tamoxifen plus chemotherapy (compared with chemotherapy alone or surgery alone), while others reported that cognitive performance was independent of tamoxifen use [73, 74]. A pilot cross-sectional ATAC substudy reported impairments in verbal memory and processing speed in EBC patients receiving either anastrozole or tamoxifen, compared with healthy controls [75]. Cross-sectional brain imaging studies have also reached disparate conclusions, depending on the imaging techniques employed. By positron emission tomography (PET) and magnetic resonance imaging (MRI), breast cancer patients receiving tamoxifen, as compared with healthy postmenopausal women, exhibited hypometabolism in the inferior and dorsal lateral frontal lobes, smaller hippocampal volumes, and significantly lower semantic memory scores [76]. In contrast, results of proton magnetic resonance spectroscopy suggested that tamoxifen might actually be neuroprotective for elderly breast cancer survivors [77, 78]. From the vantage point of a prospective, longitudinal study, EBC patients receiving one or more nonchemotherapy adjuvant treatments (endocrine, radiotherapy, trastuzumab) experienced significant cognitive decline from baseline (primarily in memory and concentration) in 26% and 14% of the cohort at 6 and 18 months of therapy, respectively, versus 18% and 11% of healthy controls [71]. With the caveat that practice effects could explain some improvement on the cognitive tests over time, stable or improved cognitive performance was observed for the majority of treated patients.
Methodological issues have complicated the interpretation of the studies referenced above. While most studies have been cross-sectional or retrospective in design, therapy-associated cognitive decline is most accurately assessed in a prospective, longitudinal study with baseline and follow-up evaluations. Though the interpretation of neuropsychological tests has not been standardized, these tools are considered more valid than self-reported cognitive function [72–74, 79]. The most consistent predictors of neuropsychological performance appear to be age, intelligence, and education [71, 79]. A chronic health problem and menopausal status are secondary predictors [79]. Separating the cognitive effects of endocrine therapy from those of chemotherapy is difficult, as few breast cancer patients receive one treatment without the other and almost none receive neither treatment [71]. Other confounders include the cognitive sequelae of surgery with general anesthesia and the cognitive impact of cancer-induced stress and depression [76]. Several large prevention studies are currently recruiting women at high risk of developing breast cancer to be randomized to anastrozole or placebo (The International Breast Cancer Intervention Study II [IBIS II]) or to exemestane or placebo (MAP3) [80]. Subprotocols longitudinally measuring cognitive function up to 5 years should provide a better window on the cognitive effects of AIs, as the subjects will all be healthy women not previously treated with surgery, radiotherapy, or chemotherapy. Cognitive decline associated with AI therapy might contraindicate use of AIs for prevention.
Potential mechanisms for cognitive impairment due to systemic adjuvant therapy have not been well investigated. The cytotoxic and cytostatic chemotherapy drugs have effects in the central nervous system as well as other parts of the body. Estrogen deficiency is another mechanism that might be common to chemotherapy and AIs. High-dose chemotherapy has irreversible effects on ovarian function and can lead to a premature menopause, while damage due to standard-dose chemotherapy may be reversible. As cognitive decline has been measured in association with normal menopause, age-matched healthy controls are essential to interpret the cognitive effects of adjuvant therapy.
There has been limited attention to developing treatments for cognitive decline in patients with breast cancer. One proposed treatment is recombinant human erythropoietin (epoetin alfa), which is used to correct anemia as a result of cancer and its treatments (chiefly chemotherapy and radiotherapy) [81]. While the standard treatment for severe anemia is blood transfusion, epoetin alfa is an effective, albeit expensive, alternative that circumvents the risks associated with transfusion. Reports that epoetin alfa has beneficial effects on QoL and cognitive function are consistent with the detection of erythropoietin and its receptor in the central nervous system [82]. Patients randomized to epoetin alfa, compared with placebo, exhibited higher mean hemoglobin levels, better executive control function, and less fatigue at 12 weeks of adjuvant anthracycline-based chemotherapy, though group differences were not significant at 6 months [83]. There are no reports of erythropoietic agent use in conjunction with tamoxifen or AIs, and use of such agents in patients with hemoglobin levels above 12 g/dl is questionable given increased risk of thromboembolic events. Venous thromboembolism is a risk of erythropoietic therapy in the presence of elevated hemoglobin values [84]. This concern plus cost considerations will probably restrict erythropoietic agent use to anemic women (Hb < 12 g/dl) for the foreseeable future.
Conclusions
As previously discussed, postmenopausal women with HR + EBC typically receive adjuvant endocrine therapy that includes either tamoxifen or an AI, and clinical studies have shown that AIs are associated with improved clinical outcomes compared with tamoxifen [2–8]. However, all endocrine agents have some inherent side effects. As survival of patients with EBC and recommended duration of endocrine therapy are being extended, management of these effects is becoming increasingly important because these side effects can adversely impact both patient QoL and adherence to prescribed treatments.
While much additional progress is needed, some effective options, as reviewed in Table 4, are available to manage side effects associated with endocrine therapy. For example, vitamin D and bisphosphonates have shown promise in the prevention and reversal of bone loss associated with estrogen deficiency [36], and a variety of treatments, including progestins, androgens, SSRIs, and acupuncture have shown efficacy in reducing hot flashes [50, 51, 57, 61]. Other side effects have fewer options available for management. For example, clear recommendations on how to address sexual side effects in patients with breast cancer are needed as systemic estrogen therapy is effective but mostly contraindicated in these women [56]. Also, local estrogen therapy has been demonstrated, but emerging data have shown the potential for increased systemic estradiol levels. Effective long-term management of arthralgia will probably require additional research and novel strategies. Management of cognitive impairment associated with endocrine therapy represents another important area that has just begun to be studied.
The extent and impact of treatment-related side effects also needs to be understood and incorporated into overall patient-management decisions. Physician-recorded events in the safety analysis of clinical trials need to be expanded upon using self-reported QoL evaluations to gain the full picture for a given patient, as these can differ significantly. A better understanding of patient QoL and preferences is fundamental to the shared model of medical decision-making, acknowledged as the preferred practice in determining treatment [14].
Despite the current efforts at management of treatment-related side effects and ongoing research to understand these events and expand the management options available, in most cases current practices may not completely eliminate symptoms. In addition to the need for more prospective studies, improved QoL instruments will likely be required for both the clinical trial and clinical practice settings to gain a better picture for patient management. Supplementing QoL determinations with more objective measurements, such as serum estradiol, bone density, rheumatologic markers, and noninvasive brain imaging, might hasten the development of effective treatments and allow earlier intervention for serious conditions, such as osteoporosis.
Overall, adjuvant endocrine therapy poses the challenge of emerging and significant side effects in patients who ideally should undergo the recommended long-term therapy. Patients risk inadequate clinical benefit due to poor adherence [14], and the DFS advantages gained with the use of adjuvant endocrine therapies may even be compromised. It is expected that better management of treatment-related side effects will have a cascade effect, starting with better QoL, which should lead to better adherence, resulting in better patient outcomes. An abundant supply of research needs related to these issues have been outlined in this article.
References
ATAC Trialists’ Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) randomised trial. Lancet 359:2131–2139
Howell A, Cuzick J, Baum M et al (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60–62
Thürlimann B, Keshaviah A, Coates AS et al, for the Breast International Group (BIG) 1-98 Collaborative Group (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757
Coombes RC, Hall E, Gibson LJ et al, for the Intergroup Exemestane Study (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Jakesz R, Jonat W, Gnant M et al, on behalf of the ABCSG and the GABG (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366:455–462
Boccardo F, Rubagotti A, Puntoni M et al (2005) Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole trial. J Clin Oncol 23:5138–5147
Goss PE, Ingle JN, Martino S et al (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97:1262–1271
Jakesz R, Samonigg H, Greil R et al (2005) Extended adjuvant treatment with anastrozole: results from the Austrian Breast and Colorectal Cancer Study Group Trial 6a (ABCSG-6a). J Clin Oncol 23:526 (abstract)
Winer EP, Hudis C, Burstein HJ et al (2005) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol 23:619–629
National Comprehensive Cancer Network (2005) Breast cancer: clinical practice guidelines in oncology – v.2.2005. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. Cited 14 June 2005
Whelan TJ, Pritchard KI (2006) Managing patients on endocrine therapy: focus on quality-of-life issues. Clin Cancer Res 12:1056s–1060s
Partridge AH (2006) Non-adherence to endocrine therapy for breast cancer. Ann Oncol 17:183–184
Partridge AH, Wang PS, Winer EP et al (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21:602–606
Fallowfield L, Atkins L, Catt S et al (2006) Patients’ preference for administration of endocrine treatments by injection or tablets: results from a study of women with breast cancer. Ann Oncol 17:205–210
Atkins L, Fallowfield L (2006) Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer 42:2271–2276
Lash TL, Fox MP, Westrup JL et al (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220
Grunfeld EA, Hunter MS, Sikka P et al (2005) Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns 59:97–102
Wasan KM, Goss PE, Pritchard PH et al (2005) The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L). Ann Oncol 16:707–715
Sloan JA, Frost MH, Berzon R et al (2006) The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer 14:988–998
Fallowfield L, Cella D, Cuzick J et al (2004) Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 22:4261–4271
Cella D, Fallowfield L, on behalf of the ATAC Trialists’ Group (2005) Five-year quality of life (QOL) follow-up of adjuvant endocrine therapy for postmenopausal women in the arimidex (A), tamoxifen (T), alone or in combination (ATAC) trial. J Clin Oncol 23:577 (abstract)
Cella D, Fallowfield L, Barker P et al (2006) Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 100:273–284
Massacesi C, Zepponi L, Rocchi MB et al (2006) Tamoxifen-related endocrine symptoms in early breast cancer patients are relieved when it is switched to anastrozole. J Clin Oncol 24:10597 (abstract)
Fallowfield LJ, Bliss JM, Porter LS et al (2006) Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol 24:910–917
Whelan TJ, Goss PE, Ingle JN et al (2005) Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 23:6931–6940
Fellowes D, Fallowfield LJ, Saunders CM et al (2001) Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for ‘well-tolerated’ treatments?. Breast Cancer Res Treat 66:73–81
Coombes C, Bliss J, Hall E et al (2003) Under-reporting of symptoms in patients with early breast cancer (EBC) who have received tamoxifen treatment for 2–3 years. Proc Am Soc Clin Oncol 22:13 (abstract 48)
Fallowfield L, Cella D, on behalf of the ATAC Trialists’ Group (2002) Assessing the quality of life (QOL) of postmenopausal women randomized into the ATAC (‘arimidex’, tamoxifen, alone or in combination) adjuvant breast cancer trial. Poster presented at: American Society of Clinical Oncology 38th Annual Meeting; May 18–21, 2002; Orlando, FL
Buzdar A, Howell A, Cuzick J et al (2006) Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 7:633–643
Morales L, Neven P, Paridaens R (2005) Choosing between an aromatase inhibitor and tamoxifen in the adjuvant setting. Curr Opin Oncol 17:559–565
Powles TJ, Hickish T, Kanis JA et al (1996) Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol 14:78–84
Viale PH (2005) Aromatase inhibitor agents in breast cancer: evolving practices in hormonal therapy treatment. Oncol Nurs Forum 32:343–353
Taylor M, Rastelli A, Civitelli R et al (2004) Incidence of 25-OH vitamin D deficiency in patients with a history of breast cancer who have musculoskeletal symptomatology. Abstract presented at the 27th Annual San Antonio Breast Cancer Symposium; December 8–11, 2004; San Antonio, TX
Thompson JN, Napoli N, Civitelli R et al (2006) Effects of dietary calcium vs. calcium supplements on estrogen metabolism and bone density. Osteoporos Int 17:S205 (abstract P696MO)
Rosen LS, Gordon DH, Dugan W Jr et al (2004) Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 100:36–43
Brufsky A (2006) Management of cancer-treatment-induced bone loss in postmenopausal women undergoing adjuvant breast cancer therapy: A Z-FAST update. Semin Oncol 33:S13–S17
Anastrozole bisphosphonate study in postmenopausal women with hormone-receptor-positive early breast cancer. Available at: http://www.clinicaltrials.gov/ct/show/NCT00082277. Cited 10 October 2006
McClung MR (2006) Inhibition of RANKL as a treatment for osteoporosis: preclinical and early clinical studies. Curr Osteoporos Rep 4:28–33
Hamdy NAT (2005) Osteoprotegerin as a potential therapy for osteoporosis. Curr Osteoporos Rep 3:121–125
Harrington L, Schneider JI (2006) Atraumatic joint and limb pain in the elderly. Emerg Med Clin N Am 24:389–412
Sarzi-Puttini P, Atzeni F, Capsoni F et al (2005) Drug-induced lupus erythematosus. Autoimmunity 38:507–518
Abu-Khalaf MM, Windsor S, Ebisu K et al (2005) Five-year update of an expanded phase II study of dose-dense and -intense doxorubicin, paclitaxel and cyclophosphamide (ATC) in high-risk breast cancer. Oncology 69:372–383
Formenti SC, Volm M, Skinner KA et al (2003) Preoperative twice-weekly paclitaxel with concurrent radiation therapy followed by surgery and postoperative doxorubicin-based chemotherapy in locally advanced breast cancer: a phase I/II trial. J Clin Oncol 21:864–870
Donnellan PP, Douglas SL, Cameron DA et al (2001) Aromatase inhibitors and arthralgia. J Clin Oncol 19:2767
McCloskey E (2006) Effects of third-generation aromatase inhibitors on bone. Eur J Cancer 42:1044–1051
Felson DT, Cummings SR (2005) Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum 52:2594–2598
Plourde P, Locker G, Hoctin-Boes G et al (2005) Arthralgia in postmenopausal breast cancer patients on adjuvant endocrine therapy: a risk-benefit analysis. Poster presented at: 7th Annual Lynn Sage Breast Cancer Symposium; October 6–9 2005; Chicago, Illinois
Mom CH, Buijs C, Willemse PHB et al (2006) Hot flushes in breast cancer patients. Crit Rev Oncol Hematol 57:63–77
Holmberg L, Anderson H, for the HABITS Steering, Data Monitoring Committees (2004) HABITS (hormonal replacement therapy after breast cancer—is it safe?), a randomised comparison: trial stopped. Lancet 363:453–455
Bertelli G, Venturini M, Del Mastro L et al (2002) Intramuscular depot medroxyprogesterone versus oral megestrol for the control of postmenopausal hot flashes in breast cancer patients: a randomized study. Ann Oncol 13:883–888
Barton DL, Loprinzi C, Atherton PJ et al (2006) Dehydroepiandrosterone for the treatment of hot flashes: a pilot study. Support Canc Ther 3:91–97
Loprinzi CL, Sloan JA, Perez EA et al (2002) Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 20:1578–1583
Stearns V, Isaacs C, Rowland J et al (2000) A pilot trial assessing the efficacy of paroxetine hydrochloride (Paxil) in controlling hot flashes in breast cancer survivors. Ann Oncol 11:17–22
Stearns V, Beebe KL, Iyengar M et al (2003) Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 289:2827–2834
Loprinzi CL, Kugler JW, Sloan JA et al (2000) Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 356:2059–2063
Barton DL, Loprinzi CL, Novotny P et al (2003) Pilot evaluation of citalopram for the relief of hot flashes. J Support Oncol 1:47–51
Loprinzi CL, Levitt R, Barton D et al (2006) Phase III comparison of depomedroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol 24:1409–1414
Jin Y, Desta Z, Stearns V et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Stearns V, Johnson MD, Rae JM et al (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Pandya KJ, Morrow GR, Roscoe JA et al (2005) Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet 366:818–824
Filshie J, Bolton T, Browne D et al (2005) Acupuncture and self acupuncture for long term treatment of vasomotor symptoms in cancer patients—audit and treatment algorithm. Acupunct Med 23:171–180
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802
Schover LR (2005) Sexuality and fertility after cancer. Hematology (Am Soc Hematol Educ Program) 1:523–527
Kendall A, Dowsett M, Folkerd E et al (2006) Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 17:584–587
Mok K, Mireskandari S, Juraskova I et al (2006) OVER (Olive oil Vaginal Exercises and Replens) Come. Psycho-Oncology 15:S332 (abstract 778)
Shifren JL, Braunstein GD, Simon JA et al (2000) Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 343:682–688
Goldstat R, Briganti E, Tran J et al (2003) Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 10:390–398
Mathias C, Cardeal MC, Ponde de SE et al (2006) An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol 17:1792–1796
Gregorian RS, Golden KA, Bahce A et al (2002) Antidepressant-induced sexual dysfunction. Ann Pharmacother 36:1577–1589
Jansen CE, Miaskowski C, Dodd M et al (2005) Chemotherapy-induced cognitive impairment in women with breast cancer: a critique of the literature. Oncol Nurs Forum 32:329–342
Jenkins V, Shilling V, Deutsch G et al (2006) A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 94:828–834
van Dam FS, Schagen SB, Muller MJ et al (1998) Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210–218
Castellon SA, Ganz PA, Bower JE et al (2004) Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26:955–969
Ahles TA, Saykin AJ (2002) Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer 3(Suppl 3):S84–S90
Jenkins V, Shilling V, Fallowfield L et al (2004) Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psycho-Oncology 13:61–66
Eberling JL, Wu C, Tong-Turnbeaugh R et al (2004) Estrogen- and tamoxifen-associated effects on brain structure and function. NeuroImage 21:364–371
Ernst T, Chang L, Cooray D et al (2002) The effects of tamoxifen and estrogen on brain metabolism in elderly women. J Natl Cancer Inst 94:592–597
Ganz PA, Castellon SA, Silverman DH (2002) Estrogen, tamoxifen, and the brain. J Natl Cancer Inst 94:547–549
Cimprich B, So H, Ronis DL et al (2005) Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psycho-Oncology 14:70–78
Howell A, Cuzick J (2005) Vascular effects of aromatase inhibitors: data from clinical trials. J Steroid Biochem Mol Biol 95:143–149
Cella D (2006) Quality of life and clinical decisions in chemotherapy-induced anemia. Oncology (Williston Park) 20:25–28
Leyland-Jones B, O’Shaughnessy JA (2003) Erythropoietin as a critical component of breast cancer therapy: survival, synergistic, and cognitive applications. Semin Oncol 30:174–184
O’Shaughnessy JA, Vukelja SJ, Holmes FA et al (2005) Feasibility of quantifying the effects of epoetin alfa therapy on cognitive function in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Clin Breast Cancer 5:439–446
Finelli PF, Carley MD (2000) Cerebral venous thrombosis associated with epoetin alfa therapy. Arch Neurol 57:260–262
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cella, D., Fallowfield, L.J. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 107, 167–180 (2008). https://doi.org/10.1007/s10549-007-9548-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9548-1