Abstract
Purpose of Review
The article reviews the consequences of estrogen deprivation during endocrine therapy for breast cancer and provides an update on alternative therapies for the management of symptoms.
Recent Findings
Endocrine therapy has progressed substantially in recent years, and its use is recommended for all breast cancer patients expressing hormone receptors. The main adverse events of this treatment can be controlled with medications and nonpharmacological measures. Antidepressants are effective in controlling vasomotor symptoms. Vaginal discomfort can be treated with local lubricants and pelvic floor physiotherapy, which may help in sexual dysfunction. Pathophysiological mechanisms of musculoskeletal symptoms during aromatase inhibitors treatment are not well understood, but some studies evaluating treatment with duloxetine, yoga, and acupuncture have shown some benefits. For prevention of bone loss, patients with risk factors should be offered bisphosphonates or denosumab.
Summary
Individualization of treatment is crucial. Consideration should be given to therapy effects on quality of life, and strategies for controlling associated symptoms should be offered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 80% of diagnosed breast cancer tumors express estrogen receptors (ERs), and 90% of these patients present with stage I to III disease [1]. Estradiol is important for breast cancer development and progression. Tamoxifen is a selective estrogen receptor modulator that competitively inhibits estrogen’s binding to ER and is effective in both pre- and postmenopausal women. Aromatase inhibitors (anastrozole, exemestane, and letrozole) decrease estradiol concentration by inhibiting conversion of androgens to estrogen and are effective only in postmenopausal women (including those who are postmenopausal because of medical ovarian suppression or oophorectomy).
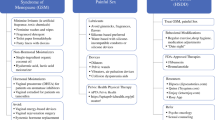
Endocrine therapy prevents the deleterious effects of estradiol in breast tissue reducing the recurrence and mortality rates. However, androgen-deprivation therapy may also have significant side effects including vasomotor symptoms, arthralgia, nausea, weight gain, and vaginal dryness, among others, and these may affect quality of life and are important contributors to nonadherence to therapy (Fig. 1) [2]. In patients with HR+ breast cancer, the risk of recurrence occurs in a period from 5 to 20 years after finishing adjuvant treatment. In 2005, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) described a reduction in the breast cancer mortality rate by 31% with 5 years of adjuvant tamoxifen; in 2015, a meta-analysis showed an additional benefit of AIs in comparison to tamoxifen [3]. The improvement of 2–4% in disease-free survival with 5 years of AI treatment after an initial 5 years of tamoxifen (with no differences in overall survival) was demonstrated in three trials: ABCSG-6a (anastrozole), MA.17 (letrozole), and NSABP B33 (exemestane) [4]. In the MA.17R trial, women were randomized to receive letrozole or placebo for another 5 years within 2 years after completing treatment with an AI; there was almost a doubling in the rate of osteoporosis (11 vs 6%) and a 3.5% absolute increase in fracture rates [5]. A recent systematic review of 16,349 women showed increased odds of fracture (odds ratio (OR) 1.34; 95% CI 1.16–1.55) with AI treatment beyond 5 years [6].
Extended duration therapy is associated with adverse effects and may affect quality of life or increase the risk of other health problems. For these reasons, and because the absolute benefits of risk reduction with extended therapy are modest in average-risk patients, the extension of treatment must be individualized and based on cancer stage and risk of late recurrence [9••].
This review will discuss the common effects of endocrine therapy and the management of symptoms related to hormone deprivation in patients with cancer.
Vasomotor Symptoms
Hot flashes and night sweats are associated with hormone depletion, occurring in approximately 75% of women during the menopausal transition and are related with all endocrine treatments, especially tamoxifen and the AIs [10]. Hot flashes have been defined as a sensation of intense warmth in the chest area, neck, and face and may be accompanied by perspiration, chills, heart palpitations, and feelings of anxiety. Women who have had treatments that cause early menopause may have hot flashes that are more severe and last longer [11]. The frequency and intensity of hot flashes can cause fatigue, difficulty in concentration, irritability, poorer health status, and sleep disturbances that diminish quality of life and reduce adherence with the prescribed therapies. Hot flash reductions have been reported with antidepressants in breast cancer survivors and women without a history of breast cancer [12,13,14]. Agents that increase serotonin and norepinephrine transmission (SSRIs/SNRIs) are efficacious in the treatment of vasomotor symptoms (VMS). Venlafaxine was the first antidepressant agent that demonstrated clinical efficacy in the treatment of hot flashes [12]. A 4-arm placebo-controlled randomized trial revealed that venlafaxine 75 mg per day decreased hot flashes by 60% [15].
A systematic review and meta-analysis of 11 randomized controlled trials (RCTs) evaluated the effectiveness of five antidepressants (paroxetine, citalopram, escitalopram, fluoxetine, and sertraline) and showed a significant decrease in the number of daily hot flashes at 4 to 8 weeks, when compared to placebo [14]. The doses supported by International Menopause Societies include paroxetine 10–15 mg, escitalopram 10–20 mg, citalopram 10–20 mg, desvenlafaxine 100–150 mg, and venlafaxine 37.5–150 mg per day. Caution is needed for women using tamoxifen, since some antidepressants may interfere with tamoxifen metabolism by inhibiting the CYP2D6 enzyme (most notably fluoxetine and paroxetine).
Gabapentin and pregabalin are structural analogs of the neurotransmitter gamma-aminobutyric acid (GABA), and studies have suggested these 2 drugs as nonhormonal treatment options for vasomotor symptoms [16]. A recent systematic review and meta-analysis provided evidence of the positive efficacy of gabapentin in alleviating hot flash in women receiving hormone deprivation therapy [17]. The evidence of pregabalin for the management of VMS was limited and needs further investigation.
Clonidine is an adrenergic agonist used for treating hypertension. In women with breast cancer, significant decreases in hot flash frequency and severity were seen after 4 weeks of transdermal clonidine (equivalent to 0.1 mg/d orally). However, side effects were significant and included dry mouth, constipation, and drowsiness. Only 48% of the 89 participants indicated a preference for clonidine over placebo at the end of the study [18].
A recent phase II, double-blind, placebo-controlled trial tested a new neurokinin-3 receptor antagonist (MLE4901) 40 mg orally twice daily. MLE4901 significantly led to a reduction of the total weekly number of hot flashes by 45% compared with placebo [19].
Hormone therapy is the gold standard for relief of hot flashes and night sweats during menopause, but it is generally contraindicated in women after breast cancer due to their potential proliferative effect on breast [20].
Table 1 shows a summary of pharmacological strategies for vasomotor symptoms in women with breast cancer.
Acupuncture could regulate temperature control through increasing beta-endorphin levels and subsequent inhibition of GnRH. Preliminary studies have demonstrated the effect of acupuncture on hot flashes for breast cancer survivors. However, in the previous studies, hot flash treatment efficacy did not differ between acupuncture and the pharmacologic treatment [21]. To confirm these findings, larger comparative effectiveness trials in broader populations are needed. Hypnosis is characterized by a state of deep relaxation and heightened focus, with improved awareness and suggestibility allowing improved concentration on specific feelings, images, thoughts, and sensations, and is used primarily to relieve stress, anxiety, and pain. Hypnosis has been studied in small pilot trials of breast cancer survivors. Two RCTS have demonstrated improvements in vasomotor symptoms with weekly hypnosis sessions for 5 weeks. A 56% reduction in hot flush scores compared to baseline was demonstrated in 187 postmenopausal women, and a reduction of 68% was shown in women with a history of breast cancer [22].
A systematic review of yoga for menopausal symptoms found insufficient evidence to suggest that yoga was an effective intervention for hot flashes [23], although yoga may have other benefits on sleep, mood, or quality of life.
Cognitive behavioral therapy (CBT) has been shown to reduce VMS problem ratings, but not VMS frequency, in 2 randomized controlled trials [24••]. However, CBT can be considered to alleviate low mood or anxiety that arises as a result of menopause.
Vulvovaginal Symptoms
Vulvovaginal atrophy (VVA) is a common consequence of estrogen deficiency. Symptoms of VVA include vaginal dryness, irritation, itching, infection, discomfort, and dyspareunia. Vaginal dryness and discomfort with sexual activity occur in nearly half of postmenopausal women and in more than 60% of breast cancer survivors [25]. A study to investigate the prevalence of urogenital symptoms and vaginal atrophy in postmenopausal breast cancer patients demonstrated that vaginal dryness and pain or discomfort with intercourse were more common in AI users. The only vaginal atrophy symptom that tended to be more common among tamoxifen-treated patients than among AI users was moderate to severe vaginal discharge [26].
Nonprescription treatments like vaginal moisturizers and lubricants are recommended as first-line treatment [27]. A prospective study suggested that use of lidocaine was associated with 90% of patients reporting comfortable penetration and 85% of abstainers from intercourse being able to resume comfortable intercourse [28]. According to the American College of Obstetricians and Gynecologists, the use of low-dose vaginal estrogens does not result in sustained serum estrogen levels. However, for women with a history of estrogen-dependent breast cancer, the use should be individualized and reserved for patients who are unresponsive to nonhormonal treatments [29].
The oral SERM, ospemifene, is not recommended for the treatment of refractory vaginal symptoms as there is no evidence about safety in women with breast cancer, although preclinical studies suggest a neutral effect on breast tissue [24••].
Small studies of laser therapy have demonstrated an improvement in vaginal symptoms in breast cancer survivors, but RCTs are still needed [30].
Sexual Dysfunction
Sexual functioning is an important component of quality of life for many patients with cancer and cancer survivors. For a variety of cancer types, estimates of sexual dysfunction after treatment range from 40 to 100% and involve physical and psychological causes. Sexual dysfunction can cause emotional distress by reinforcing negative body image, disrupting relationships, and reminding patients of their cancer experience [31].
In addition to the treatment of vaginal dryness, women may benefit from a multidisciplinary approach to sexual dysfunction that incorporates a psychologist or sex therapist, particularly if there are relationship concerns [31]. Therapist trained in the management of pelvic floor disorders can be helpful for the management of tight, tender pelvic floor muscles and to promote relaxation of the pelvic floor muscles, direct dilator therapy, and decrease fear of penetration.
Musculoskeletal Symptoms
Musculoskeletal symptoms are among the most common side effects of aromatase inhibitors (AI) and include joint pain and stiffness, muscle pain and weakness, and carpal tunnel syndrome. This group of symptoms is currently called aromatase inhibitor associated musculoskeletal syndrome (AIMSS). To date, there are no clinical and radiological criteria that define the diagnosis of AIMSS. The reported prevalence of AIMSS ranges from 20 to 74% in clinical trials [32]. The pathophysiological mechanism is still not well understood. Reduction of estrogen levels with the resulting changes in immune system, cytokines, and pain sensitivity in the central nervous system is the most accepted theory [33].
A recent Cochrane review of 7 RCTs evaluated the effectiveness of physical exercise on the prevention and management of AIMSS, but no improvements in symptoms, quality of life, or adherence to AI could be demonstrated [34•]. Due to the positive effects of physical activity on general health, it should still be recommended to all patients. In an RCT using a yoga modality (YOCAS © ®) in breast cancer patients on endocrine therapy (AI or tamoxifen), there was a reduction in musculoskeletal symptoms such as general pain, muscle pain, and physical discomfort [35].
In regard to acupuncture, one systematic review and meta-analysis of 5 RCTs did not show statistically significant beneficial effects [36]. In a more recent RCT, not included in that meta-analysis, there was a statistically significant difference between the groups (true acupuncture, sham acupuncture, waitlist) favoring the true acupuncture group. However, given the small magnitude of the difference, the authors question the clinical relevance of this finding [37].
For pain control, simple analgesics like paracetamol and nonsteroidal anti-inflammatory drugs can be used, based on expert opinions, however, with no RCT validation [38]. Omega 3 was evaluated in a placebo-controlled RCT with 249 breast cancer patients using AI; although initial results showed no improvement in pain scores [39], a post hoc evaluation showed reduction in arthralgias in patients with body mass index (BMI) ≥ 30 kg/m2 [40].
Vitamin D was evaluated in 3 RCTs in patients with AIMSS. One of them used high doses of vitamin D weekly (50,000 IU), showing some improvement in pain after 2 months of use; 4 of 28 patients enrolled in the intervention group, however, developed hypercalciuria [41]. In other 2 RCTs, there was no benefit of vitamin D in pain relief [42, 43]. In clinical practice, this approach still needs efficacy validation.
Duloxetine efficacy was assessed in the SWOG S1202 study, a randomized placebo-controlled trial [44••]. There was an improvement in AIMSS symptoms after a 12-week observation period in the general population; a post hoc analysis showed that the benefit in pain was statistically improved only in the obese population (BMI ≥ 30kg/m2) [45].
The strategy of exchanging one AI for another can help to improve the compliance in some patients. However, most of them continue to complain of musculoskeletal symptoms after starting the new medication [46]. Periodic AI treatment interruptions of short durations seem to be safe and to have no effect on clinical outcomes, representing an option in patients with more severe symptoms [47]. The switch from an AI to tamoxifen could also be considered in patients with AIMSS.
Bone Health
Endocrine therapy in breast cancer can alter the basal bone metabolism. Estrogen deficiency causes an imbalance in bone remodeling, leading to loss of bone density [48]. Medications as AI usually decrease bone mass by reducing estrogen levels. Tamoxifen has different actions depending on menopausal status: in postmenopausal women, it acts as a partial agonist, increasing bone mass, and in premenopausal patients, it acts as a partial antagonist, competing with estrogen and reducing bone density. In postmenopausal women treated with AI, it is expected a 17% higher risk of bone fracture in comparison with general population and a 33% increase in fractures when compared to patients using tamoxifen [49].
A group of societies and specialists from different countries recently published a systematic review on the management of bone loss in patients with breast cancer using aromatase inhibitors [50]. Two guidelines from societies involved in oncology have been published in recent years regarding bone health of cancer patients (American Society of Clinical Oncology and European Society of Clinical Oncology) [51, 52]. Among the measures cited are those of bone loss control for patients with non-metastatic breast cancer using endocrine therapy.
Among the most important nonpharmacological recommendations are smoking cessation and regular physical exercise [50,51,52]. The orientation is that all patients perform diversified physical activities, mainly involving resistance and strength, although there are no studies showing an increase in bone density with this approach alone. In a systematic review in patients without neoplasia, there was a tendency to increase bone mass with physical exercise in postmenopausal women [53]. In two recent meta-analyses on the impact of physical activity on cancer patients (most of the selected studies included breast cancer patients, many of them using endocrine therapy), there was no significant increase in bone mass in postmenopausal patients with exercises; only one single study demonstrated an increase in bone density in premenopausal patients with this approach [54, 55].
Daily consumption of 1000 to 1200mg of calcium and at least 800UI of vitamin D is recommended for all patients. Measurement of serum vitamin D should be performed routinely, and replacement of higher doses should be indicated as needed [50,51,52].
Dual-energy X-ray absorptiometry (DXA) is the most widely used diagnostic test for bone loss. Based in bone mineral density (BMD) obtained, medications such as bisphosphonates or denosumab are indicated. According to the guidelines, patients using aromatase inhibitors and with a T score ≤2 should receive treatment with those drugs. For those patients using medications that lead to loss of bone mass and that present two or more fracture risk factors (T score ≤1.5, > 65 years, BMI <20kg/m2, family history of hip fracture, personal history of fracture, use of corticosteroids > 6 months, and smoking), the use of bone antiresorptive therapy is also indicated [50,51,52].
Bisphosphonates act by inhibiting osteoclasts, leading to an increase in BMD. Zoledronic acid is the most studied medication in this class, and in randomized studies, it has shown improvement in BMD in patients with breast cancer using endocrine therapy [56, 57]. A meta-analysis of 13 randomized clinical trials (RCTs) evaluated the use of zoledronic acid in preventing bone loss after 12 months in patients with initial breast cancer receiving adjuvant therapy. The most frequent dose described was 4 mg every 6 months. Regardless of menopausal status, there was an improvement in BMD, being statistically significant in premenopausal women in the lumbar spine (LS) and in postmenopausal women in the LS and total hip [58]. In addition, in postmenopausal patients, the use of zoledronic acid is associated with a reduced risk of bone metastasis (28%) and a reduction of breast cancer mortality (18%) as demonstrated in the EBCTCG meta-analysis [59]. Oral bisphosphonates can also be used in the prevention of bone loss, as RCTs with risedronate, ibandronate, and alendronate have already demonstrated a gain in BMD [50]. Table 2 shows the main RCTs available on the use of bisphosphonates for breast cancer patients with a follow-up of 5 years for outcomes.
Denosumab is a RANKL inhibitor and acts by preventing its interaction with RANK, diminishing osteoclasts function. In one RCT, denosumab 60mg subcutaneous twice per year demonstrated a 50% reduction in risk of fracture in patients with breast cancer using aromatase inhibitors, becoming a treatment option in these patients [64]. Its use is not recommended to reduce the risk of bone metastasis, since studies such as D-CARE have not demonstrated a clinical benefit of this medication [65].
Ongoing Clinical Trials
Personalized medicine and treatments based on individual patient characteristics will become the key points in making therapeutic decisions in the future. Understanding the mechanisms of action of medications used in endocrine therapy for BC and how they interact with the genetic characteristics of each patient is the subject of some studies [66, 67]. In a trial of the SWOG/National Cancer Institute in the USA, genetic polymorphisms in ESR1, TCL1A, and CYP191 are being evaluated in a prospective cohort to analyze its correlation with adherence to AI treatment in patients affected with AIMSS (NCT01824836).
New options for managing the side effects of endocrine therapy are essential for patients to maintain adherence to treatment and to not compromise its clinical results. To date, most trials of nonpharmacological interventions have been conducted in this field.
Acupuncture has shown conflicting results in the treatment of some of the symptoms associated with endocrine therapy, and therefore, it is not routinely indicated for these patients. Two randomized studies are recruiting patients to evaluate acupuncture for breast cancer patients: NCT04511832 is studying the effectiveness of acupuncture versus nonsteroidal anti-inflammatory drugs in the management of AIMSS, and NCT03783546 compares acupuncture to usual care for hot flashes.
Laser therapy for treating vaginal symptoms is another point of controversy that needs to be better clarified in clinical studies. In a safety communication in 2018, the US Food and Drug Administration warned against use of energy-based devices for treatment of vaginal and urinary symptoms, based on the lack of robust evidence of benefits and risks of this therapy [68]. Four randomized trials about efficacy of laser therapy are being conducted for genitourinary syndrome of menopause in breast cancer survivors; different kinds of laser will be tested: 3 trials with fractional CO2 and one with hybrid fractional (NCT03647189). Two of the trials are analyzing laser against placebo or sham therapy (NCT03738605, NCT03238053), and one has 3 arms of comparison (laser, microablative radiofrequency and intravaginal estriol) (NCT04081805).
Exercises are recommended for all BC patients treated with endocrine therapy, although no clear evidence of improvement in side effects of treatment has been proved yet. Two new RCTs are evaluating benefit of exercises in AIMSS and bone health (NCT04457895, NCT03953157).
Conclusion
Adequate management of adverse effects related to the use of endocrine therapy in patients with BC is essential to maintain adherence to treatment and to avoid serious events, such as bone fractures. Since adjuvant endocrine treatment is a long-term treatment in non-metastatic disease, maintaining patients’ quality of life should always be prioritized when indicating a specific therapy.
New therapies have been studied to control hormone suppression symptoms in postmenopausal patients without neoplasms. It is important that these treatments are validated in clinical trial for patients with breast cancer undergoing endocrine therapy to ensure their safety and effectiveness. In addition, the mechanisms that generate these different adverse events must be better understood biologically so that they can be treated more effectively.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84. https://doi.org/10.1016/S0140-6736(11)60993-8.
Moon Z, Moss-Morris R, Hunter MS, Carlisle S, Hughes LD. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence. 2017;11:305–22. https://doi.org/10.2147/PPA.S126651.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet Lond Engl. 2005;365(9472):1687–717.
Pistilli B, Paci A, Ferreira AR, Di Meglio A, Poinsignon V, Bardet A, et al. Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. J Clin Oncol. 2020;38(24):2762–72. https://doi.org/10.1200/JCO.19.01758.
Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, et al. Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005;23(28):6931–40. https://doi.org/10.1200/JCO.2005.11.181.
Lemieux J, Brundage MD, Parulekar WR, Goss PE, Ingle JN, Pritchard KI, et al. Quality of life from Canadian cancer trials group MA.17R: a randomized trial of extending adjuvant letrozole to 10 years. J Clin Oncol. 2018;36(6):563–71. https://doi.org/10.1200/JCO.2017.75.7500.
Regan MM, Price KN, Giobbie-Hurder A, Thürlimann B, Gelber RD, International Breast Cancer Study Group and BIG 1-98 Collaborative Group. Interpreting breast international group (BIG) 1-98: a randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive, early breast cancer. Breast Cancer Res. 2011;13(3):209. https://doi.org/10.1186/bcr2837.
Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Austrian breast and colorectal cancer study group. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99(24):1845–53. https://doi.org/10.1093/jnci/djm246.
•• Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Oncol Pract. 2019;15(2):106–7. https://doi.org/10.1200/JOP.18.00617This is a systematic review of randomized clinical trials from 2012 to 2018 on adjuvant endocrine therapy. The study demonstrated that extension of aromatase inhibitors treatment is associated with increased adverse effects that may affect quality of life. Extension of adjuvant endocrine treatment should be based on considerations of recurrence risk using established prognostic factors.
Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–35. https://doi.org/10.2105/AJPH.2005.066936.
Goldfarb S, Mulhall J, Nelson C, Kelvin J, Dickler M, Carter J. Sexual and reproductive health in cancer survivors. Semin Oncol. 2013;40(6):726–44. https://doi.org/10.1053/j.seminoncol.2013.09.002.
Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, LaVasseur BI, Barton DL, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomized controlled trial. Lancet. 2000;356(9247):2059–63. https://doi.org/10.1016/S0140-6736(00)03403-6.
Johns C, Seav SM, Dominick SA, Gorman JR, Li H, Natarajan L, et al. Informing hot flash treatment decisions for breast cancer survivors: a systematic review of randomized trials comparing active interventions. Breast Cancer Res Treat. 2016;156(3):415–26. https://doi.org/10.1007/s10549-016-3765-4.
Shams T, Firwana B, Habib F, Alshahrani A, Alnouh B, Murad MH, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204–13. https://doi.org/10.1007/s11606-013-2535-9.
Condorelli R, Vaz-Luis I. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther. 2018;18(11):1101–12. https://doi.org/10.1080/14737140.2018.1520096.
Pandya KJ, Morrow GR, Roscoe JA, Zhao H, Hickok JT, Pajon E, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005;366(9488):818–24. https://doi.org/10.1016/S0140-6736(05)67215-7.
Shan D, Zou L, Liu X, Shen Y, Cai Y, Zhang J. Efficacy and safety of gabapentin and pregabalin in patients with vasomotor symptoms: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;222(6):564–579.e12. https://doi.org/10.1016/j.ajog.2019.12.011.
Sica DA, Grubbs R. Transdermal clonidine: therapeutic considerations. J Clin Hypertens (Greenwich). 2005;7(9):558–62. https://doi.org/10.1111/j.1524-6175.2005.04133.x.
Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809–20. https://doi.org/10.1016/S0140-6736(17)30823-1.
Pinkerton JV, Santen RJ. Managing vasomotor symptoms in women after cancer. Climacteric. 2019;22(6):544–52. https://doi.org/10.1080/13697137.2019.1600501.
Tran S, Hickey M, Saunders C, Ramage L, Cohen PA. Nonpharmacological therapies for the management of menopausal vasomotor symptoms in breast cancer survivors. Support Care Cancer. 2020;29:1183–93. https://doi.org/10.1007/s00520-020-05754-w.
McCormick CA, Brennan A, Hickey M. Managing vasomotor symptoms effectively without hormones. Climacteric. 2020;22:1–7. https://doi.org/10.1080/13697137.2020.1789093.
Cramer H, Peng W, Lauche R. Yoga for menopausal symptoms-a systematic review and meta-analysis. Maturitas. 2018;109:13–25. https://doi.org/10.1016/j.maturitas.2017.12.005.
•• Marsden J, Marsh M, Rigg A, British Menopause Society. British Menopause Society consensus statement on the management of estrogen deficiency symptoms, arthralgia and menopause diagnosis in women treated for early breast cancer. Post Reprod Health. 2019;25(1):21–32. https://doi.org/10.1177/2053369118824920This guidance provides an overview of the management strategies of estrogen deficiency symptoms associated with breast cancer treatment.
Santen RJ, Stuenkel CA, Davis SR, Pinkerton JV, Gompel A, Lumsden MA. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102(10):3647–61. https://doi.org/10.1210/jc.2017-01138.
Baumgart J, Nilsson K, Stavreus-Evers A, Kask K, Villman K, Lindman H, et al. Urogenital disorders in women with adjuvant endocrine therapy after early breast cancer. Am J Obstet Gynecol. 2011;204(1):26.e1–7. https://doi.org/10.1016/j.ajog.2010.08.035.
Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91(8):1133–46. https://doi.org/10.1016/j.mayocp.2016.04.009.
Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33(30):3394–400. https://doi.org/10.1200/JCO.2014.60.7366.
American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127(3):e93–6. https://doi.org/10.1097/AOG.0000000000001351.
Knight C, Logan V, Fenlon D. A systematic review of laser therapy for vulvovaginal atrophy/genitourinary syndrome of menopause in breast cancer survivors. Ecancermedicalscience. 2019;13:988. https://doi.org/10.3332/ecancer.2019.988.
Flynn KE, Reese JB, Jeffery DD, Abernethy AP, Lin L, Shelby RA, et al. Patient experiences with communication about sex during and after treatment for cancer. Psychooncology. 2012;21(6):594–601. https://doi.org/10.1002/pon.1947.
Beckwée D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Support Care Cancer. 2017;25(5):1673–86. https://doi.org/10.1007/s00520-017-3613-z.
Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16(3):223–34. https://doi.org/10.1016/j.breast.2007.01.011.
• Roberts KE, Rickett K, Feng S, Vagenas D, Woodward NE. Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst Rev. 2020;1(1):CD012988. https://doi.org/10.1002/14651858.CD012988.pub2In this systematic review and meta-analysis of 7 studies, authors have not found any clear evidence of benefit for exercise therapies for preventing or treating women with breast cancer and aromatase inhibitor-induced musculoskeletal symptoms.
Peppone LJ, Janelsins MC, Kamen C, Mohile SG, Sprod LK, Gewandter JS, et al. The effect of YOCAS©® yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy. Breast Cancer Res Treat. 2015;150(3):597–604. https://doi.org/10.1007/s10549-015-3351-1.
Chien TJ, Liu CY, Chang YF, Fang CJ, Hsu CH. Acupuncture for treating aromatase inhibitor-related arthralgia in breast cancer: a systematic review and meta-analysis. J Altern Complement Med. 2015;21(5):251–60. https://doi.org/10.1089/acm.2014.0083.
Hershman DL, Unger JM, Heather Greenlee ND, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer - a randomized clinical trial. JAMA. 2018;320(2):167–76. https://doi.org/10.1001/jama.2018.8907.
Gupta A, Henry NL, Loprinzi CL. Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncol Pract. 2020;11:OP2000113. https://doi.org/10.1200/OP.20.00113.
Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, et al. Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol. 2015;33(17):1910–7. https://doi.org/10.1200/JCO.2014.59.5595.
Shen S, Unger JM, Crew KD, Till C, Greenlee H, Gralow J, et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res Treat. 2018;172(3):603–10. https://doi.org/10.1007/s10549-018-4946-0.
Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, et al. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat. 2011;129(1):107–16. https://doi.org/10.1007/s10549-011-1644-6.
Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, Nydegger JL, et al. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res Treat. 2017;166(2):491–500. https://doi.org/10.1007/s10549-017-4429-8.
Shapiro AC, Adlis SA, Robien K, Kirstein MN, Liang S, Richter SA, et al. Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat. 2016;155(3):501–12. https://doi.org/10.1007/s10549-016-3710-6.
• Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, et al. Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol. 2018;36(4):326–32. https://doi.org/10.1200/JCO.2017.74.6651This randomized, double blind, phase 3 study compares duloxetine to placebo for AIMSS in breast cancer patients. By 12 weeks, pain and functioning scores were better for patients receiving duloxetine.
Henry NL, Unger JM, Till C, Schott AF, Crew KD, Lew DL, et al. Association between body mass index and response to duloxetine for aromatase inhibitor-associated musculoskeletal symptoms in SWOG S1202. Cancer. 2019;125(12):2123–9. https://doi.org/10.1002/cncr.32024.
Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat. 2010;120(1):127–34. https://doi.org/10.1007/s10549-009-0692-7.
Colleoni M, Luo W, Karlsson P, Chirgwin J, Aebi S, Jerusalem G, et al. Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(1):127–38. https://doi.org/10.1016/S1470-2045(17)30715-5.
Ramchand SK, Cheung YM, Yeo B, Grossmann M. The effects of adjuvant endocrine therapy on bone health in women with breast cancer. J Endocrinol. 2019;241(3):R111–24. https://doi.org/10.1530/JOE-19-0077.
Tseng OL, Spinelli JJ, Gotay CC, Ho WY, McBride ML, Dawes MG. Aromatase inhibitors are associated with a higher fracture risk than tamoxifen: a systematic review and meta-analysis. Ther Adv Musculoskelet Dis. 2018;10(4):71–90. https://doi.org/10.1177/1759720X18759291.
Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. https://doi.org/10.1016/j.jbo.2017.03.001.
•• Shapiro CL, Van Poznak C, Lacchetti C, Kirshner J, Eastell R, Gagel R, et al. Management of osteoporosis in survivors of adult cancers with nonmetastatic disease: ASCO clinical practice guideline. J Clin Oncol. 2019;37(31):2916–46. https://doi.org/10.1200/JCO.19.01696Although this ASCO guideline provides recommendations for management of osteoporosis in all survivors of adult cancer, many studies reviewed have included breast cancer patients treated with endocrine therapy. Therefore, its recommendations can be applied only for this group of patients.
Coleman R, Hadji P, Body JJ, Santini D, Chow E, Terpos E, et al. Bone health in cancer: ESMO clinical practice guidelines. Ann Oncol. 2020. In press;31:1650–63. https://doi.org/10.1016/j.annonc.2020.07.019.
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333. https://doi.org/10.1002/14651858.CD000333.pub2.
Dalla Via J, Daly RM, Fraser SF. The effect of exercise on bone mineral density in adult cancer survivors: a systematic review and meta-analysis. Osteoporos Int. 2018;29(2):287–303. https://doi.org/10.1007/s00198-017-4237-3.
Fornusek CP, Kilbreath SL. Exercise for improving bone health in women treated for stages I-III breast cancer: a systematic review and meta-analyses. J Cancer Surviv. 2017;11(5):525–41. https://doi.org/10.1007/s11764-017-0622-3.
Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–201. https://doi.org/10.1002/cncr.26313.
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013;24(2):398–405. https://doi.org/10.1093/annonc/mds277.
Mei M, Xiang Z, Yang J, Xiang R. Efficacy of zoledronic acid for prevention of bone loss in early-stage breast cancer patients receiving adjuvant therapy: a meta-analysis of 13 randomized controlled trials. Curr Probl Cancer. 2020;44(2):100507. https://doi.org/10.1016/j.currproblcancer.2019.100507.
Early Breast Cancer. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–61. https://doi.org/10.1016/S0140-6736(15)60908-4.
Sestak I, Blake GM, Patel R, Coleman RE, Cuzick J, Eastell R. Comparison of risedronate versus placebo in preventing anastrozole-induced bone loss in women at high risk of developing breast cancer with osteopenia. Bone. 2019;124:83–8. https://doi.org/10.1016/j.bone.2019.04.016.
Livi L, Scotti V, Desideri I, Saieva C, Cecchini S, Francolini G, et al. Phase 2 placebo-controlled, single-blind trial to evaluate the impact of oral ibandronate on bone mineral density in osteopenic breast cancer patients receiving adjuvant aromatase inhibitors: 5-year results of the single-centre BONADIUV trial. Eur J Cancer. 2019;108:100–10. https://doi.org/10.1016/j.ejca.2018.12.005.
Kyvernitakis I, Kann PH, Thomasius F, Hars O, Hadji P. Prevention of breast cancer treatment-induced bone loss in premenopausal women treated with zoledronic acid: final 5-year results from the randomized, double-blind, placebo-controlled ProBONE II trial. Bone. 2018;114:109–15. https://doi.org/10.1016/j.bone.2018.06.007.
Wagner-Johnston ND, Sloan JA, Liu H, Kearns AE, Hines SL, Puttabasavaiah S, et al. 5-year follow-up of a randomized controlled trial of immediate versus delayed zoledronic acid for the prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen: N03CC (Alliance) trial. Cancer. 2015;121(15):2537–43. https://doi.org/10.1002/cncr.29327.
Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–43. https://doi.org/10.1016/S0140-6736(15)60995-3.
Coleman R, Finkelstein DM, Barrios C, Martin M, Iwata H, Hegg R, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60–72. https://doi.org/10.1016/S1470-2045(19)30687-4.
Johansson H, Gray KP, Pagani O, Regan MM, Viale G, Aristarco V, et al. Impact of CYP19A1 and ESR1 variants on early-onset side effects during combined endocrine therapy in the TEXT trial. Breast Cancer Res. 2016;18(1):110. https://doi.org/10.1186/s13058-016-0771-8.
Ingle JN, Schaid DJ, Goss PE, Liu M, Mushiroda T, Chapman JA, et al. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol. 2010;28(31):4674–82. https://doi.org/10.1200/JCO.2010.28.5064.
US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bethânia Soares dos Santos and Cláudia Bordignon declare no conflict of interest. Daniela Dornelles Rosa has received compensation from Roche, AstraZeneca, Eli Lilly, GlaxoSmithKline, Sanofi, Libbs Farmacêutica, and Novartis for service as a consultant and has received compensation from Roche, Eli Lilly, Novartis, Pfizer and Zodiac for providing expert testimony.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
dos Santos, B.S., Bordignon, C. & Rosa, D.D. Managing Common Estrogen Deprivation Side Effects in HR+ Breast Cancer: an Evidence-Based Review. Curr Oncol Rep 23, 63 (2021). https://doi.org/10.1007/s11912-021-01055-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01055-5