Abstract
Aspects of manganese metabolism during normal and acidogenic growth of Aspergillus niger were explored. Arginase from this fungus was a Mn[II]-enzyme. The contribution of the arginase protein towards A. niger manganese metabolism was investigated using arginase knockout (D-42) and arginase over-expressing (ΔXCA-29) strains of A. niger NCIM 565. The Mn[II] contents of various mycelial fractions were found in the order: D-42 strain < parent strain < ΔXCA-29 strain. While the soluble fraction forms 60 % of the total mycelial Mn[II] content, arginase accounted for a significant fraction of this soluble Mn[II] pool. Changes in the arginase levels affected the absolute mycelial Mn[II] content but not its distribution in the various mycelial fractions. The A. niger mycelia harvested from acidogenic growth media contain substantially less Mn[II] as compared to those from normal growth media. Nevertheless, acidogenic mycelia harbor considerable Mn[II] levels and a functional arginase. Altered levels of mycelial arginase protein did not significantly influence citric acid production. The relevance of arginase to cellular Mn[II] pool and homeostasis was evaluated and the results suggest that arginase regulation could occur via manganese availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese is an essential micronutrient found in almost all biological systems where it functions as a co-factor for many enzymes (Crowley et al. 2000). Cellular Mn[II] ions also interact with various metabolites to form low molecular weight complexes; some of these function in protection against oxidative stress (McNaughton et al. 2010). On the other hand, excessive Mn[II] can be toxic and inhibits growth (Kosman 1994). Aspects of manganese metabolism with respect to growth, development and secondary metabolite production have been studied in a few fungi (Auling 1994). A lipid phosphatase (Cdc1p) located in the yeast endoplasmic reticulum is implicated in Mn[II] metabolism but does not affect cellular Mn[II] levels (Paidhungat and Garrett 1998). The cdc1 homolog of Neurospora crassa (frost) is involved in manganese sensitivity and hyphal branching (Sone and Griffiths 1999). Various mushrooms have been analyzed for their Mn[II] contents because of their role in bioaccumulation of metal ions (Tuzen 2003). Our current understanding of manganese metabolism is largely confined to bacteria (Foster et al. 2014) and among fungi Saccharomyces cerevisiae is well represented (Reddi et al. 2009). Mn[II] transporters from the yeast and homologs from N. crassa and Aspergillus fumigatus have also been studied (Bowman et al. 2012; Pinchai et al. 2010).
A large number of enzymes require divalent metal ions for their activity, but very few of them are absolutely specific for Mn[II] (Crowley et al. 2000). Table 1 summarizes the available literature on enzymes from Aspergilli that can use Mn[II] ions. With very few exceptions, arginase is a strict Mn[II] enzyme across organisms (Ash 2004; Mendz et al. 1998). The Aspergillis niger arginase may therefore find a place in Table 1 as a prominent member with tightly bound Mn[II] ions. Arginase hydrolyzes arginine to ornithine and urea. Despite its catabolic role, significant basal levels of arginase are found in fungal mycelia grown on minimal media (Dave et al. 2012; Davis 1986). For these reasons, Aspergillus niger arginase could represent a significant cellular pool of Mn[II] and its levels could potentially influence fungal manganese metabolism. While the global effects of manipulating Mn[II] transport are obvious, the impact of interfering with cellular Mn[II] binding proteins on manganese homeostasis is not.
In the present study, the contribution of A. niger arginase towards manganese metabolism was investigated using arginase knockout (D-42) and arginase over-expressing (ΔXCA-29) strains of A. niger. The cellular arginase protein content influenced the mycelial Mn[II] levels. This report for the first time demonstrates the involvement of arginase in the manganese homeostasis of any organism.
Materials and methods
Strains and growth conditions
The three different fungal strains used in this study were A. niger NCIM 565 (the parent strain), D-42 (an arginase knockout) and ΔXCA-29 (a strain that constitutively expresses arginase) (Dave et al. 2012). Preparation of spore inoculum and media (normal minimal medium—MM and acidogenic medium—AM) was as earlier (Punekar et al. 1985); all the media were prepared in de-ionized water. The Mn[II] concentration measured by inductively coupled plasma atomic emission spectrometry (ICP-AES; 9.2 ± 0.7 μM, n = 4) in typical MM preparations was close to the value calculated from the MM composition (10.8 μM). While no explicit Mn[II] additions were made, the traces contributed by other media components to AM amounted to 0.1 ± 0.1 μM, n = 5 (inductively coupled plasma mass spectrometry, ICP-MS). Mycelial growth was carried out in shake flasks (220 rpm) at 30 °C, either for 4 days (on MM) or for 8 days (on AM). For experiments involving pure arginase protein, Escherichia coli BL21 (DE3) (expressing A. niger arginase cDNA from pETNat) was grown in Luria–Bertani broth (with ampicillin at 0.1 mg/ml) at 37 °C (Jayashri et al. 2009).
A. niger arginase purification
The E. coli BL21 (DE3) harboring pETNat was induced with 0.4 mM IPTG for 3 h at 37 °C (Jayashri et al. 2009). Purification of expressed arginase was performed in three steps: ammonium sulfate precipitation, ion-exchange chromatography using DEAE Sephacel and gel filtration chromatography. All steps except gel filtration were performed at 4 °C. The cell lysates were prepared in Buffer A (200 mM imidazole·HCl, 12 mM MnSO4, 2 mM 2-mercaptoethanol, 1 mM PMSF, pH 7.5). The crude extract was subjected to 0–30 % and further 30–60 % ammonium sulfate fractionation. The pellet obtained was dissolved in Buffer B (25 mM imidazole·HCl, 1.2 mM MnSO4, 2 mM 2-mercaptoethanol, pH 7.5) and loaded onto DEAE Sephacel (Pharmacia LKB, Uppasala, Sweden) column. Elution was performed with a linear gradient of zero to 0.8 M KCl and arginase activity eluted around 0.4 M KCl. The peak activity fractions were pooled, ammonium sulfate precipitated and loaded onto Superdex 200 (HiLoad 16/60 prep grade, GE Healthcare, United Kingdom) gel filtration column, using 25 mM HEPES·NaOH at pH 7.5. The Mn[II] ions were not included in this final step of purification. This ensured that purified arginase protein containing only tightly bound metal ions was isolated. Suitably chosen active fractions from this column when pooled, provided the purified arginase protein. The purified arginase protein was a hexamer with a subunit molecular weight of about 40 kDa.
Preparation of arginase apo-enzyme
De-ionized water was used throughout for metal ion stoichiometry and Mn[II] interaction studies. Whenever the purified protein was needed for Mn[II] interaction/specificity studies MnSO4 was omitted from the gel filtration buffer (as above). The apoenzyme form was prepared using a reported protocol (Lopez et al. 2005) with some modifications. Pure arginase protein was incubated with 25 mM EDTA and 3.0 M guanidinium hydrochloride in 10 mM Tris HCl (pH 7.5) for 1.0 h at 25 °C. The sample was immediately desalted using HiPrep 26/10 desalting column (GE Healthcare) into 25 mM HEPES NaOH buffer (pH 7.5) at 4 °C. The protein thus obtained had negligible arginase activity in absence of MnSO4 and was used as the apoenzyme.
Mn[II] estimation and calculations
All glassware required during mycelial growth and other steps of Mn[II] estimation were washed with 20 % HNO3 and rinsed with de-ionized water. The mycelia after filtration on muslin cloth were washed (five times with de-ionized water), blot dried and stored at −20 °C. Corresponding samples were also dried in a hot air oven to constant weight and stored at −20 °C. Crude extracts were prepared in Buffer A (but without MnSO4), from wet weight samples by crushing frozen mycelia in a pre-chilled mortar and pestle, using acid washed sea sand. For obtaining the soluble fraction devoid of macromolecule ligands, crude extracts were ultra-filtered using a 3 kDa cut off filtration device (Pall Corporation, USA). The strategy for fractionation and the actual fractions analyzed are shown Fig. 1.
For Mn[II] analysis, the samples were digested in a microwave digester. The samples (about 1.0 g of mycelia or 1.0 ml of protein/extract + 6 ml HNO3 + 0.4 ml H2O2 + 6 ml H2O) were digested at 800 W (20 min), 1600 W (20 min), 0 W (20 min). The digested sample was analyzed by ICP-AES or ICP-MS.
The following conversion factors were used to compute physiological Mn[II] concentrations. (i) For mycelia grown on MM, 1.0 g dry weight = 2.54 ml intracellular volume (Slayman and Tatum 1964), (ii) for mycelia grown on AM, 1.0 g wet weight = 0.175 ml intracellular volume (Legisa and Kidric 1989). The following average wet and dry weight inter-conversions were arrived at experimentally. (i) For mycelia grown on MM, 1.0 g dry weight = 5.0 g wet weight, (ii) for mycelia grown on AM, 1.0 g dry weight = 2.9 g wet weight. The proportion of Mn[II] in various fractions was calculated using the following formulae:
-
(1)
\({\rm Soluble\,fraction} = \frac{{\rm Total\,soluble\,Mn[II]\,in}\,\upmu {\rm M}}{{\rm Total\,mycelial\,Mn[II]\,in}\, \upmu {\rm M}}\)
-
(2)
\({\text{`Soluble}} . {\text{unbound' fraction}} = \frac{{{{\text{ng Unbound Mn[II]}} \mathord{\left/ {\vphantom {{\text{ng Unbound Mn[II]}} {\text{mg protein}}}} \right. \kern-0pt} {\text{mg protein}}} \, }}{{{{\text{ng Total soluble Mn[II]}} \mathord{\left/ {\vphantom {{\text{ng Total soluble Mn[II]}} {\text{mg protein}}}} \right. \kern-0pt} {\text{mg protein}}}}}\, \times {\text{Soluble fraction}}\)
-
(3)
\({\text{`Soluble}} . {\text{bound' fraction}} = {\text{Soluble fraction }} - {\text{`Soluble}} . {\text{unbound' fraction}}\)
Arginase assay
Arginase activity was monitored by measuring urea according to Archibald’s method (Dave et al. 2012). The standard assay conducted at 37 °C involved a pre-incubation step with 0.8 mM MnSO4 for 10 min, followed by addition of buffered arginine (150 mM) and incubation for 15 min. Unless stated otherwise, arginase assays were performed at pH 7.5 and in presence of 1 mg/ml BSA. One unit (U) of arginase is the amount of enzyme that liberates 1.0 μmol of urea per min under the respective assay conditions.
Estimations
Citric acid in the spent medium was estimated by an enzymatic (citrate lyase) method as earlier (Dave et al. 2012). Protein estimation was done with the Bradford method (Bradford 1976) using bovine serum albumin as a standard. For metal stoichiometry analysis, arginase protein concentration was additionally measured using the extinction co-efficient (280 nm) of 25,440 M−1 (obtained from ExPASy ProtParam software).
Western blot
Arginase protein from A. niger crude extracts was detected after the samples were run using native polyacrylamide gel electrophoresis. An enriched anti-arginase from rabbit serum served as the primary antibody. The anti-rabbit IgG tagged with alkaline phosphatase was used and detected using Nitro-blue tetrazolium/5-bromo-4-chloro-3′-indolylphosphate (Roche Diagnostics, Mannheim, Germany). The band intensities were quantified using ImageJ software.
Results and discussion
A. niger arginase is a Mn[II] activated enzyme
The A. niger arginase was expressed in E. coli BL21 (DE3) and purified in a buffer without Mn[II] ions. This enzyme showed significant activity even without Mn[II] added in the standard assays (see Materials and methods). The activity was enhanced further (about 5-fold) by including 0.8 mM of Mn[II] ions in the assay (Fig. 2a). Analysis of such an enzyme preparation by ICP-MS indicated that Mn[II] was the major metal ion tightly bound to the enzyme (Table 2) and a Mn[II] stoichiometry of about 3 Mn[II] ions per hexamer could be calculated. This stoichiometry is lower than that observed for mammalian arginases (one Mn[II] per subunit) (Orellana et al. 2002), whereas a much lower value is reported for the N. crassa arginase (Davis 1986).
The A. niger enzyme preparation (containing only the tightly bound Mn[II]) was also assayed by addition of different metal ions; the activity with Mn[II] was the highest followed by Ni[II] and Co[II], while Zn[II] showed a slight inhibitory effect (Fig. 2a). However, the apo-enzyme form generated by stripping off the tightly bound Mn[II] ions, was virtually inactive in the absence of added metal ions. The apo-enzyme was maximally activated by Mn[II] followed by Ni[II] and Co[II] (Fig. 2b). Clearly, the A. niger arginase prefers Mn[II] for optimal activity. The protein does retain tightly bound Mn[II] and is best activated by pre-incubation with Mn[II] ions; the pre-incubation may allow the weaker Mn[II]-binding sites to be occupied. Removal of the tightly bound metal ion appears to affect arginase irreversibly since the activity of the apoenzyme could not be fully reconstituted by pre-incubation with Mn[II] ions (Fig. 2). The metal ion preference is similar to that for well-studied arginases (Ash 2004), whereas Helicobacter pylori and Bacillus anthracis arginases are exceptions and prefer Co[II] and Ni[II], respectively (Mendz et al. 1998; Viator et al. 2008).
Arginase and mycelial Mn[II] levels in A. niger
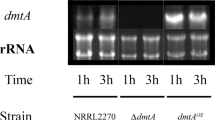
The A. niger arginase is a Mn[II] enzyme and displays Mn[II] specificity in vitro. It was of interest to see if this translates into an effect on Mn[II] metabolism of the fungus. The contribution of the arginase protein towards A. niger manganese metabolism was investigated using arginase knockout (D-42), arginase over-expressing (ΔXCA-29) strains and the parent A. niger NCIM 565. Both the arginase activity (Dave et al. 2012) as well as the arginase protein (Fig. 3) in the three strains grown on MM, followed the pattern, D-42 (none) < NCIM 565 < ∆XCA-29. Since these strains differ only in their arginase protein contents, any change in Mn[II] levels could be attributed either directly or indirectly to arginase itself. The total Mn[II] content of D-42 strain was 10 % lower while that of ΔXCA-29 strain was 15 % higher than in the parent strain (Fig. 4a).
Mn[II] contents in various fractions of A. niger mycelia. a Total mycelial Mn[II] normalized with mycelial dry weight (d.w.), *P < 0.01, n = 13, **P < 0.05, n = 8, b total soluble Mn[II] normalized with protein content in the crude extract, *P < 0.1, n = 6; **P = 0.05, n = 6, c ‘soluble.unbound’ Mn[II] normalized with protein content in the corresponding crude extract, *P < 0.1, n = 4. Results are presented as average ± SEM. P values are from paired t tests performed with corresponding Mn[II] values of NCIM 565 within the same experiment. Samples for a and b were analyzed by ICP-AES and c by ICP-MS
A systematic analysis of the Mn[II] content of various A. niger mycelial fractions (Fig. 1) was undertaken. The total mycelial Mn[II] was broadly divided into particulate and soluble components. The soluble component consists of Mn[II] ions: (a) bound to various macromolecules (‘soluble.bound’) and (b) free in solution consisting of all Mn[II] species either unbound or complexed with small cellular metabolite chelators (‘soluble.unbound’). This ill-defined Mn[II] buffer consists of small molecules such as amino acids, glutathione, organic acids and inorganic ligands (Foster et al. 2014). The arginase content of A. niger mycelia significantly affected the total soluble as well as the ‘soluble.unbound’ Mn[II] levels (Fig. 4). This was anticipated since arginase is a cytosolic protein. Accordingly, the total soluble Mn[II] in D-42 strain was 30 % lower than in the parent strain; and it was 55 % higher in the ΔXCA-29 strain (Fig. 4b). The ‘soluble.unbound’ Mn[II] levels in these three strains also followed a similar pattern (Fig. 4c). The results indicate that arginase protein levels influence mycelial Mn[II] levels. It is therefore likely that arginase may well function as a Mn[II] reservoir in A. niger. Fungal cytosolic arginine concentration during growth on MM (as was done here) is too low for significant catabolism by arginase (Davis 2010). Therefore, the effect of arginase on manganese metabolism appears to be independent of its catalytic function. This is the first study of arginase involvement in manganese metabolism and the effects are significant. The only other example which considered the effect of a Mn[II]-enzyme on Mn[II] metabolism is manganese superoxide dismutase (in yeast, E. coli and Deinococcus radiodurans); but there appears to be no agreement on the extent of superoxide dismutase contribution to cellular Mn[II] pools (Sharma et al. 2013; Tabares and Un 2013).
Interestingly, changes in the arginase content affected the absolute Mn[II] levels but not its distribution in the three fractions (Fig. 5). About 60 % of the total Mn[II] was in the soluble fraction while the remaining 40 % as the insoluble (particulate) form. About 20 % of the total Mn[II] was unbound to any macromolecular ligand (‘soluble.unbound’). This distribution of Mn[II] ions in the soluble versus the insoluble fractions is similar to that of Saccharomyces carlsbergensis (Okorokov et al. 1977). A higher level of control(s) seems to operate in sub-cellular manganese distribution in fungi.
The distribution of mycelial Mn[II] in A. niger: The proportions of Mn[II] in the ‘soluble.bound’ (gray), ‘soluble.unbound’ (white) and insoluble (black) fractions have been shown. The total soluble component includes the ‘soluble.bound’ and the ‘soluble.unbound’ fractions. The proportions were derived from data in Fig. 4, see “Materials and methods” section for details of calculations
Arginase kinetics under physiological Mn[II] concentrations
From the Mn[II] measurements reported above, the physiological concentrations of Mn[II] in A. niger NCIM 565 could be computed, albeit with certain assumptions about intracellular volume (see Materials and methods). The total mycelial Mn[II], soluble Mn[II] and ‘soluble.unbound’ Mn[II] concentrations of 100, 60 and 20 µM, respectively were estimated. The total A. niger mycelial Mn[II] concentration is comparable to that in S. cerevisiae (Luk and Culotta 2001). Much higher concentrations were found in D. radiodurans (radiation resistance; Sun et al. 2010) and mushrooms (metal bioaccumulation; Tuzen 2003).
Near physiological concentrations of arginine and Mn[II] when used, are known to significantly influence the observed kinetic parameters in some arginases (Carvajal et al. 1982; Davis et al. 1978; Maggini et al. 1992). A knowledge of mycelial Mn[II] distribution permitted the kinetic evaluation of A. niger arginase under near-physiological conditions. The enzyme would experience around 20 μM of Mn[II] ions in the cytosol. Therefore, arginine saturation was performed at this near in vivo Mn[II] concentration; the saturation was hyperbolic with a KM for arginine of 20 mM and a Vmax of around 200 U/mg protein (Fig. 6a). The near-physiological Mn[II] concentrations appear adequate for efficient functioning of arginase over a wide range of arginine concentrations. At 150 mM arginine, pure arginase displayed a hyperbolic activity pattern over an initial range of Mn[II] concentrations; a Mn[II] dissociation constant (KMn[II]) of 15 μM was obtained (Fig. 6b). Higher concentrations of Mn[II] were inhibitory with a KI of 250 μM. Since the pure protein contains tightly bound Mn[II] as well (see earlier section), the KMn[II] determined by Mn[II] saturation represents the affinity of arginase to loosely bound Mn[II]. Interestingly, the KMn[II] closely mirrors the ‘soluble.unbound’ Mn[II] concentration in A. niger. Yet another key enzyme from this fungus namely, glutamine synthetase also responds to Mn[II] concentration in the lower μM range (Punekar et al. 1985). That arginase activity in vivo can be modulated by changes in Mn[II] availability appears feasible. This may hold particular significance when the fungus experiences diverse Mn[II] environments.
Arginase kinetic features under simulated physiological conditions. a Arginine saturation of purified arginase with 20 μM Mn[II] in the assay. b Mn[II] saturation kinetics using 150 mM arginine. Circles represent actual data points while the line represents the best fit using SigmaPlot 11.2 Enzyme Kinetics software. Data are representative of three independent experiments, each performed in duplicates
The A. niger arginase is a cytosolic enzyme. Based on the observed values for KMn[II] and [Mn[II]] (15 μM and 20 μM, respectively), the Mn[II] binding equilibrium for arginase (at the loosely Mn[II]-binding site) could be estimated. About one half of the total arginase protein in A. niger (grown on MM) would be in fully Mn[II]-bound state. The rest would contain only the tightly bound Mn[II]. The actual proportion of fully Mn[II]-saturated arginase will depend on the concentration of free Mn[II] in the fungal cytoplasm. The cytosolic manganese availability can vary because of factors such as sequestration by the fungal vacuole (Okorokov et al. 1977; Reddi et al. 2009), buffering by polydisperse metal ion buffers and other environmental cues (Foster et al. 2014). For instance, the Mn[II] levels in a bacterial cytosol can vary; the Mn[II] is elevated in response to oxidants, to correctly metallate manganese superoxide dismutase (Imlay 2014).
Acidogenesis—an altered Mn[II] metabolism?
Acidogenesis (citric acid production) is a well known example of Mn[II]-deficient growth of A. niger. This process is highly sensitive to the presence of Mn[II] ions in the growth medium; presence of ≥50 ppb of Mn[II] reduce citric acid yields significantly in various strains of A. niger (Karaffa and Kubicek 2003). However, the primary roles of Mn[II] deficiency in citric acid accumulation remain unclear even today. We therefore analyzed A. niger NCIM 565 mycelia, harvested during maximal growth phase on AM (48 h) and MM (24 h), for their Mn[II] content. The mycelia committed to acidogenic growth contained about 8-fold lower Mn[II] content (in both the total and the soluble fractions) when compared to normal mycelia (Table 3). The proportion of Mn[II] ions in the mycelial soluble fraction was similar when the fungus was grown either on AM or on MM. Nevertheless, this Mn[II] content of acidogenic mycelia is notable as it is found despite the fact that AM is a manganese limited growth medium (see Materials and methods). The growth of A. niger is very sensitive to Mn[II] availability and its ability to cadge this micronutrient was exploited for manganese bioassay (Sulochana and Lakshmanan 1968). Efficient and specific manganese transporter(s) is reported to function under citric acid production conditions (Hockertz et al. 1987). The degradation of Mn[II] transporters may be inhibited in yeast starved of manganese (Bleackley and Macgillivray 2011). Such factors may be at play and thereby resulting in significant mycelial Mn[II] levels during acidogenic growth of A. niger.
Arginase during acidogenic growth
In terms of mechanistic details, citric acid fermentation by A. niger continues to be an enigma. Many morphological, physiological and biochemical studies are reported over the years to explain acidogenesis. These include bulbous, branched mycelia with pellet morphology, metabolic and enzyme regulation at the level of glycolysis, Krebs cycle and sub-cellular compartments, altered membrane phospholipids and β-glucan content of the cell wall etc., (Karaffa and Kubicek 2003). These effects are a cumulative outcome of the fermentative growth of A. niger on AM and not addressed by manganese limitation alone. Despite extensive physiological/biochemical literature the metabolic circuits leading to acidogenesis are ill defined; the contribution from nitrogen metabolism is poorly understood (Kubicek and Rohr 1986; Papagianni et al. 2005; Punekar et al. 1985). A possible involvement of A. niger arginase in the acidogenic metabolism is suggested by the following: (a) acidogenesis is a manganese sensitive process and manganese deficiency leads to elevated amino acid pools (and arginine in particular) during acidogenic growth (Kubicek and Rohr 1986), (b) arginase is a Mn[II] enzyme and its levels influence the fungal Mn[II] pool (this study) and (c) arginase is the sole route for arginine degradation in this fungus (Dave et al. 2012).
Arginase activity could be detected in A. niger NCM 565 mycelia throughout the course of growth on AM. The arginase content (expressed as total U/g wet weight) was lower in acidogenic mycelia when compared to mycelia grown on MM (Fig. 7, top panel). However, the arginase specific activity patterns were comparable on the two growth media (Fig. 7, bottom panel). Growth on AM results in very different mycelia morphology and significantly lowered protein content and intracellular volume per unit biomass (see Materials and methods). Clearly, active arginase protein does exist during acidogenic growth and sufficient Mn[II] is available in these mycelia (see Table 3; Figs. 6a, 7) for arginase to function in vivo. Therefore, it is unlikely that elevated arginine pools observed during acidogenic growth is due a compromised arginase activity because of Mn[II] deficiency.
Arginase activity during acidogenic and normal growth of A. niger. Arginase total activity (top panel) and arginase specific activity (bottom panel) in mycelia grown on AM (open circles) and MM (filled circles) were measured. The arginase assays were performed at pH 10.6 using 1 mM MnSO4. Citric acid concentration in the spent medium at the corresponding time points for AM (open triangles) and MM (filled triangles) is shown in the bottom panel. Data are representative of three independent experiments, each performed in duplicates
Comparable citrate yields (between 6 and 10 mM) were obtained for NCIM 565, D-42 and ΔXCA-29 strains of A. niger, suggesting that lack or over-expression of arginase did not affect the ability of the fungus to produce citric acid. The A. niger NCIM 565 studied here produces citric acid but is not an industrial strain. While it may be worthwhile extending this study to other high yielding A. niger strains, that there is a strain-to-strain variation in the manganese effect (for example, see Gupta and Sharma 1995) makes the analysis difficult.
Arginase and Mn[II] toxicity
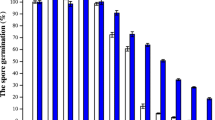
Variations in mycelial Mn[II] pools consequent to altered arginase levels can potentially influence aspects of fungal physiology including Mn[II] toxicity. The effect of a range of Mn[II] concentrations on the growth of A. niger on MM was assessed. Despite their differences in mycelial Mn[II] levels, the three A. niger strains (D-42, NCIM 565 and ΔXCA-29) showed similar growth and Mn[II] toxicity profiles (Fig. 8). Concentrations higher than 5 mM were toxic to A. niger. The intracellular Mn[II] contents are affected in the Mn[II] transport mutants of S. cerevisiae and D. radiodurans and they display significantly altered growth responses to Mn[II] toxicity (Lapinskas et al. 1995; Sun et al. 2010). The mycelial Mn[II] pool changes due to differing arginase levels may not be this drastic and the effects may become apparent only under specific physiological states. Fungal vacuoles play a significant role is sequestering Mn[II] ions (Okorokov et al. 1977) and may account for masking any growth effects in these A. niger strains. Nevertheless, a change in cellular Mn[II] levels may have implications for manganese homeostasis (Reddi et al. 2009) and/or mis-metallation of enzymes (Foster et al. 2014).
Effect of Mn[II] on growth of A. niger strains. Radial growth (after point inoculation with equal number of conidia) was recorded at 60 h as a function of Mn[II] concentration supplemented to MM. High Mn[II] concentrations made the MM turbid and subsequent radial growth was surrounded by zones of clearance possibly due to chelation of Mn[II] ions by secreted organic acids. (0.01 mM of Mn[II] is normally present in MM)
Conclusions
Access to three A. niger strains (D-42, NCIM 565 and ΔXCA-29) which differ in their arginase content served to evaluate the contribution of arginase to manganese metabolism of this fungus. Changes in the cellular arginase protein and the total Mn[II] content were directly correlated. The A. niger arginase is a Mn[II]-enzyme and forms an important fraction of soluble Mn[II] pool of the mycelia. Despite the manganese deficient nature of AM, acidogenic mycelia harbor significant Mn[II] levels and a functional arginase. This study brings out the relevance of arginase in cellular Mn[II] homeostasis and suggests that arginase regulation could occur via manganese availability.
References
Ash DE (2004) Structure and function of arginases. J Nutr 134:2760S–2764S
Auling G (1994) Manganese: function and transport in fungi. In: Winkelmann G, Winge DR (eds) Metal ions in fungi. Marcel Dekker, New York, pp 215–236
Benassi VM, Pasin TM, Facchini FD, Jorge JA, de Moraes Teixeira, MdeL Polizeli (2014) A novel glucoamylase activated by manganese and calcium produced in submerged fermentation by Aspergillus phoenicis. J Basic Microbiol 54:333–339
Bleackley MR, Macgillivray RT (2011) Transition metal homeostasis: from yeast to human disease. Biometals 24:785–809
Bowman BJ, Abreu S, Johl JK, Bowman EJ (2012) The pmr gene, encoding a Ca2+-ATPase, is required for calcium and manganese homeostasis and normal development of hyphae and conidia in Neurospora crassa. Eukaryot Cell 11:1362–1370
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carvajal N, Acoria M, Rodriguez JP, Fernandez M, Martinez J (1982) Evidence for cooperative effects in human liver arginase. Biochim Biophys Acta 1760:146–148
Crowley JD, Traynor DA, Weatherburn DC (2000) Enzymes and proteins containing manganese: An overview. In: Sigel A, Sigel H (eds) Metal ions in biological systems. Marcel Dekker, New York, pp 209–277
Dave K, Ahuja M, Jayashri TN, Sirola RB, Punekar NS (2012) A novel selectable marker based on Aspergillus niger arginase expression. Enzyme Microb Technol 51:53–58
Davis RH (1986) Compartmental and regulatory mechanisms in the arginine pathways of Neurospora crassa and Saccharomyces cerevisiae. Microbiol Rev 50:280–313
Davis RH (2010) Amino acids and polyamines: polyfunctional proteins, metabolic cycles and compartmentation. In: Borkovich KA, Ebbole DJ (eds) Cellular and molecular biology of filamentous fungi. ASM Press, Washington, DC, pp 339–358
Davis RH, Weiss RL, Bowman BJ (1978) Intracellular metabolite distribution as a factor in regulation in Neurospora. In: Microenvironments and metabolic compartmentation. Academic Press, New York, pp 197–207
Emiliani E, Bekes P (1964) Enzymatic oxalate decarboxylation in Aspergillus niger. Arch Biochem Biophys 105:488–493
Foster AW, Osman D, Robinson NJ (2014) Metal preferences and metallation. J Biol Chem 289:28095–28103
Gong G, Zheng Z, Liu H, Wang L, Diao J, Wang P, Zhao G (2014) Purification and characterization of a β-glucosidase from Aspergillus niger and its application in the hydrolysis of geniposide to genipin. J Microbiol Biotechnol 24:788–794
Gupta S, Sharma CB (1995) Citric acid fermentation by the mutant strain of Aspergillus niger resistant to manganese ions inhibition. Biotechnol Lett 17:269–274
Hockertz S, Schmid J, Auling G (1987) A specific transport system for manganese in the filamentous fungus Aspergillus niger. J Gen Microbiol 133:3513–3519
Imlay JA (2014) The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121–28128
Jalving R, Bron P, Kester HC, Visser J, Schaap PJ (2002) Cloning of a prolidase gene from Aspergillus nidulans and characterisation of its product. Mol Genet Genomics 267:218–222
Jayashri TN, Anuradha R, Punekar NS (2009) Single-stranded megaprimer splicing through OE-PCR: construction of full-length Aspergillus niger arginase cDNA. Ind J Biochem Biophys 46:266–268
Jernejc K, Legisa M (2002) The influence of metal ions on malic enzyme activity and lipid synthesis in Aspergillus niger. FEMS Microbiol Lett 217:185–190
Kanayama N, Tohru S, Keiichi K (2002) Purification and characterization of an alkaline manganese peroxidase from Aspergillus terreus LD-1. J Biosci Bioeng 93:405–410
Karaffa L, Kubicek CP (2003) Aspergillus niger citric acid accumulation: do we understand this well working black box? Appl Microbiol Biotechnol 61:189–196
Kawasaki L, Farres A, Aguirre J (1995) Aspergillus nidulans mutants affected in acetate metabolism isolated as lipid nonutilizers. Exp Mycol 19:81–85
Komachi Y, Hatakeyama S, Motomatsu H, Futagami T, Kizjakina K, Sobrado P, Ekino K, Takegawa K, Goto M, Nomura Y, Oka T (2013) gfsA encodes a novel galactofuranosyltransferase involved in biosynthesis of galactofuranose antigen of O-glycan in Aspergillus nidulans and Aspergillus fumigatus. Mol Microbiol 90:1054–1073
Kosman DJ (1994) Transition metal ion uptake in yeasts and filamentous fungi. In: Winkelmann G, Winge DR (eds) Metal ions in fungi. Marcel Dekker, New York, pp 1–38
Kubicek CP, Rohr M (1986) Citric acid fermentation. CRC Crit Rev Biotechnol 3:331–373
Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC (1995) Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol 15:1382–1388
Legisa M, Kidric J (1989) Initiation of citric acid accumulation in the early stages of Aspergillus niger growth. Appl Microbiol Biotechnol 31:453–457
Lopez V, Alarcon R, Orellana MS, Enriquez P, Uribe E, Martinez J, Carvajal N (2005) Insights into the interaction of human arginase II with substrate and manganese ions by site-directed mutagenesis and kinetic studies. Alteration of substrate specificity by replacement of Asn149 with Asp. FEBS J 272:4540–4548
Luk EE, Culotta VC (2001) Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J Biol Chem 276:47556–47562
MacKenzie DA, Guillemette T, Al-Sheikh H, Watson AJ, Jeenes DJ, Wongwathanarat P, Dunn-Coleman NS, van Peij N, Archer DB (2005) UPR-independent dithiothreitol stress-induced genes in Aspergillus niger. Mol Genet Genomics 274:410–418
Maggini S, Stoecklin-Tschan FB, Morikofer-Zwez S, Walter P (1992) New kinetic parameters for rat liver arginase measured at near-physiological steady-state concentrations of arginine and Mn2+. Biochem J 283:653–660
Maller A, da Silva TM, Damasio AR, Hirata IY, Jorge JA, Terenzi HF, Polizeli ML (2013) Functional properties of a manganese-activated exo-polygalacturonase produced by a thermotolerant fungus Aspergillus niveus. Folia Microbiol (Praha) 58:615–621
McNaughton RL, Reddi AR, Clement MH, Sharma A, Barnese K, Rosenfeld L, Gralla EB, Valentine JS, Culotta VC, Hoffman BM (2010) Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci USA 107:15335–15339
Mendz GL, Holmes EM, Ferrero RL (1998) In situ characterization of Helicobacter pylori arginase. Biochim Biophys Acta 1388:465–477
Netik A, Torres NV, Riol JM, Kubicek CP (1997) Uptake and export of citric acid by Aspergillus niger is reciprocally regulated by manganese ions. Biochim Biophys Acta 1326:287–294
Okorokov LA, Lichko LP, Kadomtseva VM, Kholodenko VP, Titovsky VT, Kulaev IS (1977) Energy-dependent transport of manganese into yeast cells and distribution of accumulated ions. Eur J Biochem 75:373–377
Orellana MS, Lopez V, Uribe E, Fuentes M, Salas M, Carvajal N (2002) Insights into the interaction of human liver arginase with tightly and weakly bound manganese ions by chemical modification and site-directed mutagenesis studies. Arch Biochem Biophys 403:155–159
Paidhungat M, Garrett S (1998) Cdc1 and the vacuole coordinately regulate Mn2+ homeostasis in the yeast Saccharomyces cerevisiae. Genetics 148:1787–1798
Papagianni M, Wayman F, Mattey M (2005) Fate and role of ammonium ions during fermentation of citric acid by Aspergillus niger. Appl Environ Microbiol 71:7178–7186
Pinchai N, Juvvadi PR, Fortwendel JR, Perfect BZ, Rogg LE, Asfaw YG, Steinbach WJ (2010) The Aspergillus fumigatus P-type Golgi apparatus Ca2+/Mn2+ ATPase PmrA is involved in cation homeostasis and cell wall integrity but is not essential for pathogenesis. Eukaryot Cell 9:472–476
Promper C, Schneider R, Weiss H (1993) The role of the proton-pumping and alternative respiratory chain NADH:ubiquinone oxidoreductases in overflow catabolism of Aspergillus niger. Eur J Biochem 216:223–230
Punekar NS, Vaidyanathan CS, Rao NA (1985) Role of glutamine synthetase in citric acid fermentation by Aspergillus niger. J Biosci 7:269–287
Reddi AR, Jensen LT, Culotta VC (2009) Manganese homeostasis in Saccharomyces cerevisiae. Chem Rev 109:4722–4732
Ruijter GJ, van de Vondervoort PJ, Visser J (1999) Oxalic acid production by Aspergillus niger: an oxalate-non-producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiology 145:2569–2576
Sharma A, Gaidamakova EK, Matrosova VY, Bennett B, Daly MJ, Hoffman BM (2013) Responses of Mn2+ speciation in Deinococcus radiodurans and Escherichia coli to γ-radiation by advanced paramagnetic resonance methods. Proc Natl Acad Sci USA 110:5945–5950
Siddiqui KS, Azhar MJ, Rashid MH, Ghuri M, Rajoka MI (1997) Purification and the effect of manganese ions on the activity of carboxymethylcellulases from Aspergillus niger and Cellulomonas biazotea. Folia Microbiol (Praha) 42:303–311
Slayman CW, Tatum EL (1964) Potassium transport in Neurospora. I. intracellular sodium and potassium concentrations, and cation requirements for growth. Biochim Biophys Acta 88:578–592
Sone T, Griffiths AJ (1999) The frost gene of Neurospora crassa is a homolog of yeast cdc1 and affects hyphal branching via manganese homeostasis. Fungal Genet Biol 28:227–237
Sulochana CB, Lakshmanan M (1968) Aspergillus niger technique for the bioassay of manganese. J Gen Microbiol 50:285–293
Sun H, Xu G, Zhan H, Chen H, Sun Z, Tian B, Hua Y (2010) Identification and evaluation of the role of the manganese efflux protein in Deinococcus radiodurans. BMC Microbiol 10:319
Tabares LC, Un S (2013) In situ determination of manganese[II] speciation in Deinococcus radiodurans by high magnetic field EPR: detection of high levels of Mn[II] bound to proteins. J Biol Chem 288:5050–5055
Tatsuno K, Yamada-Okabe H, Takagi M, Arisawa M, Sudoh M (1997) Properties of yeast expressed Aspergillus nidulans chitin synthase B which is essential for hyphal growth. FEMS Microbiol Lett 149:279–284
Tuzen M (2003) Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem J 74:289–297
Viator RJ, Rest RF, Hildebrandt E, McGee DJ (2008) Characterization of Bacillus anthracis arginase: effects of pH, temperature and cell viability on metal preferences. BMC Biochem 9:15
Xu DB, Rohr M, Kubicek CP (1989) Aspergillus niger cyclic AMP levels are not influenced by manganese deficiency and do not correlate with citric acid accumulation. Appl Microbiol Biotechnol 32:124–128
Acknowledgments
We acknowledge Sophisticated Analysis and Instrumentation Facility (SAIF), Department of Earth Sciences, and Centre for Environmental Science and Engineering (CESE) at IIT Bombay for support in Mn[II] analyses. Dr. Neetu Singh performed early standardizations of A. niger growth inhibition by Mn[II]. The work was supported by a grant from Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE) and fellowship (to Sarita Keni) by Council of Scientific and Industrial Research (CSIR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Keni, S., Punekar, N.S. Contribution of arginase to manganese metabolism of Aspergillus niger . Biometals 29, 95–106 (2016). https://doi.org/10.1007/s10534-015-9900-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9900-6