Abstract
A thermotolerant fungus identified as Aspergillus niveus was isolated from decomposing materials and it has produced excellent levels of hydrolytic enzymes that degrade plant cell walls. A. niveus germinated faster at 40 °C, presenting protein levels almost twofold higher than at 25 °C. The crude extract of the A. niveus culture was purified by diethylaminoethyl (DEAE)-cellulose, followed by Biogel P-100 column. Polygalacturonase (PG) is a glycoprotein with 37.7 % carbohydrate, molecular mass of 102.6 kDa, and isoelectric point of 5.4. The optimum temperature and pH were 50 °C and 4.0–6.5, respectively. The enzyme was stable at pH 3.0 to 9.0 for 24 h. The DEAE-cellulose derivative was about sixfold more stable at 60 °C than the free enzyme. Moreover, the monoaminoethyl-N-aminoethyl-agarose derivative was tenfold more stable than the free enzyme. PG was 232 % activated by Mn2+. The hydrolysis product of sodium polypectate corresponded at monogalacturonic acid, which classifies the enzyme as an exo-PG. The K m, V max, K cat, and K cat/K m values were 6.7 mg/ml, 230 U/mg, 393.3/s, and 58.7 mg/ml/s, respectively. The N-terminal amino acid sequence presented 80 % identity with PglB1, PglA2, and PglA3 putative exo-PG of Aspergillus fumigatus and an exo-PG Neosartorya fischeri.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pectins are complex colloidal acidic polysaccharides constituted basically by galacturonic acid linked by α-1,4-glycosidic bonds. This complex shows three backbones: the unsubstituted homogalacturonan, the (1 → 3)-β-d-xylose-substituted xylogalacturonan, and rhamnogalacturonan II, which is extremely complex, with sugars not yet fully characterized. Also, the backbone can show the repeating disaccharide (1 → 2)-α-l-Rha-(1 → 4)-β-d-GalA, named rhamnogalacturonan I (Round et al. 2010).
Pectic enzymes are enzymes that degrade pectin and they can be divided into de-esterifying and depolymerizing groups based on the attack mechanism to the pectin backbone. The depolymerizing enzymes are classified into polygalacturonase (PG) (EC 3.2.1), which cleaves the α-1,4-glycosidic bonds between galacturonic acid residues, and pectin lyase (EC 4.2.2) or pectate lyase, which catalyzes the β-elimination reaction between methylated residues. Pectinesterase (EC 3.1.1) is classified as a de-esterifying enzyme, which demethoxylates the methylated pectin, producing methanol and pectin (Zeni et al. 2011). Moreover, these enzymes can also be classified as alkaline or acid pectinases. Acid pectinase is used in fruit juice industries and winemaking and often comes from fungal sources (Kashyap et al. 2001).
Fungal species have the potential to produce several kinds of enzyme-degrading compounds of the cellular wall. A novel strain of Aspergillus niveus RP05 was isolated from Brazilian decomposing plant material (Peixoto-Nogueira et al. 2009) and it produced high amylase (da Silva et al. 2009), xylanase (Betini et al. 2009), and pectinase (Maller et al. 2011) levels. A. niveus, as Ascomycetes, may have ascus, the reproductive structure that forms the sexual spores. Then, this may facilitate the laboratory manipulation. Furthermore, this fungus is thermotolerant and its enzymatic activities have shown high thermostability (da Silva et al. 2009). The aim of this study was to purify and to perform the characterization of physicochemical properties of PG produced by A. niveus.
Material and methods
Microorganism and culture conditions
A. niveus RP05 was identified and deposited in the culture collection of Pernambuco Federal University, Recife, Brazil. The fungus was maintained on a slant of potato dextrose agar (PDA) medium at 40 °C for 15 days and stored at 4 °C. Czapek medium (25 ml; in percent: NaNO3, 0.3; KH2PO4, 0.1; MgSO4∙7H2O, 0.05; KCl, 0.05; Fe2(SO4)3∙7H2O, 0.001; yeast extract, 0.1) (Wiseman 1975), with 1.0 % citrus pectin (Sigma-Aldrich) added, was inoculated with 5 × 106 conidia and incubated at 40 °C without agitation for 3 days (Maller et al. 2011). After that, the mycelial pads were removed in a Buchner funnel and the filtrate was saved as the source of crude PG. Fungal growth (0–24 h) was estimated according to Rizzatti et al. (2004), and the total protein of this assay was estimated according to Bradford (1976).
PG activity
PG activity was determined by measuring the production of reducing sugar from sodium polypectate from Sigma-Aldrich using 3,5-dinitrosalicylic acid (Miller 1959). One unit of PG activity was defined as the amount of enzyme that produced 1 μmol reducing sugar/min/ml, under the assay conditions. The total activity and total protein were determined by multiplying units per milliliter and milligrams per milliliter, respectively, by the total volume of the filtrate.
Enzyme purification
All the steps were carried out at 4 °C. First, 230 ml of crude PG activity was dialyzed by 18 h in 10 mmol/L Tris–HCl buffer, pH 7.5. After that, the crude extract was applied to a diethylaminoethyl (DEAE)-cellulose column (100 × 20 mm) equilibrated with the same buffer and eluted with a linear concentration gradient (0–1 mol NaCl, 200 ml). The fractions were collected and monitored at OD280 nm and for PG activity. A pool of the PG fractions was dialyzed against 100 mmol/L sodium acetate, pH 6.0, lyophilized, and suspended in 1 ml of the same buffer. This sample was applied to a Biogel P-100 column (1,100 × 20 mm) equilibrated and eluted with acetate buffer. Fractions with PG activity were pooled, dialyzed against same buffer, and utilized for biochemical characterization.
Determination of molecular mass, electrophoresis, and enzymatic activities in gel
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) 10 % (Laemmli 1970) and ladders of 10 to 220 kDa from BenchMark™ (GibcoBRL™) were used to determine the purification degree and molecular mass of the enzyme. The protein was silver stained, as described by Blum et al. (1987). To confirm the PG molecular mass, the sample was applied to Fast Protein Liquid Chromatography BioRad™ Model 2800 Solvent Delivery System, using Bio-Sil SEC 400 column, eluted with 10 mmol/L sodium phosphate buffer, 15 mmol/L NaCl, and 10 mmol/L sodium azide, pH 6.8.
Native PAGE (10 %) was used to identify the PG activity in gel (Davis 1964). The activity was revealed when the gel polymerized with 1 % (w/v) sodium polypectate was incubated in 100 mmol/L sodium acetate, pH 6.0, at 50 °C for 30 min. After that, 0.1 % ruthenium red was used to stain the gel and show the band PG activity. Isoelectric focusing was carried out according to O’Farrell et al. (1977) using Pharmalyte (pH 3.0–10.0). The carbohydrate content was measured according to Dubois et al. (1956).
PG immobilization and thermal inactivation
Anionic exchanger support monoaminoethyl-N-aminoethyl (MANAE)-agarose (Fernandez-Lafuente et al. 1993), polyethyleneimine (PEI)-agarose (Mateo et al. 2000), and DEAE-cellulose were added to 20 ml of purified PG (36 total units) in 5 mmol/L sodium phosphate, pH 7.0, at 25 °C. During adsorptions, samples were withdrawn from the supernatant and the amount of proteins and activity were determined. After that, the matrix containing the adsorbed proteins was washed with 20 ml of 5 mmol/L sodium phosphate buffer, pH 7.0. The thermal stability of free and immobilized PG was determined at 60 °C. Samples were taken at different times intervals, cooled on ice, and the activity determined using sodium polypectate as substrate.
Analysis of hydrolysis products
To analyze the hydrolysis products, the purified PG was incubated with 1 % sodium polypectate in 100 mmol/L sodium acetate solution, pH 6.0, at 50 °C. Samples were removed after 5, 10, 15, 30, 60, and 120 min and 24 h, and the hydrolysis was stopped by heating the samples in boiling water for 5 min. The products were detected by thin layer chromatography (TLC) (DC-Alufolien Kieselgel 60, Merck). The standards used were monogalacturonic acid, digalacturonic acid, and trigalacturonic acid and n-butanol/ethanol/distilled water (5:3:2) as the mobile phase. After the elution, the hydrolysis products were revealed with 0.2 % of orcinol in n-butanol/distilled water/acetic acid (5:3:2) and incubation at 100 °C by 5 min.
Determination of kinetic parameters
The K m, V max, and K cat values for the pure enzyme were determined by incubating the enzyme with 0–30 mg/ml sodium pectate in 100 mmol/L sodium acetate (pH 6.0) at 50 °C. The values were calculated from the plot of Hanes (1932).
N-terminal sequencing of the PG
The N-terminal purified PG was sequenced with the PPSQ/23 sequencer (Shimadzu Corporation™), with a high-performance liquid chromatography isocratic system, using the Edman degrading method. The primary sequence was analyzed using the Basic Local Alignment Search Tool (BLAST) database (http://www.ncbi.nih.gov/BLAST).
Statistical analysis
Statistical determinations of the difference between means of assays were performed using one-way analysis of variance. Differences of p < 0.05 were considered to be statistically significant. All data are the mean of at least three independent experiments showing consistent results.
Results and discussion
Influence of temperature in the germination and growth of A. niveus
A. niveus RP05 had high spore production in PDA medium and it had thermotolerance when incubated at 40 and 45 °C (data not shown). Figure 1a shows the temperature effect in fungal germination. The spores germinated faster at 40 °C after incubation of 9 h, and the growth was higher than at 25 °C up to 24 h (Fig. 1b). A similar result was found by Rizzatti et al. (2004) incubating Aspergillus phoenicis at 25 and 42 °C. Analogous to this, A. niveus showed high production at 40 °C, with 52.4 U/mg (Table 1) when incubated in Czapek medium with 1.0 % citrus pectin (Sigma-Aldrich) for 96 h (Maller et al. 2011). So, incubation in this temperature was used to induce PG production with thermostable characteristics, which was confirmed in the thermal influence assays.
Purification and physicochemical properties of PG from A. niveus
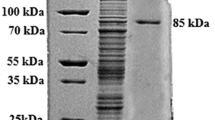
The purification protocol using DEAE-cellulose followed by Biogel P-100 column resulted in PG purified approximately 4.4-fold, with 17.4 % recovery (Table 1). This enzyme migrated as a single band between 100 and 120 kDa in SDS-PAGE (Fig. 2). The activity was confirmed in native PAGE and showed sodium polypectate degradation band with migration close to the protein band. The molecular mass of PG was 102.6 kDa with 37.7 % carbohydrate, classifying it as a glycoprotein. Several works describe PGs with small molecular mass, as 32 kDa (Fahmy et al. 2008) and 60–70 kDa (Pedrolli and Carmona 2010), or with similar molecular mass of 92 kDa (Gomes et al. 2009). The isoelectric point (pI) of A. niveus PG was 5.4. This result corroborated with the reports of Paecilomyces variotii (Damásio et al. 2010) and Penicillium expansum (Jurick et al. 2010) PGs.
PG immobilization and thermal effect
The PG optimum temperature was evaluated by measuring the activity at 40–70 °C in 100 mmol/L sodium acetate solution, pH 6.0. A. niveus PG was found to have an optimum temperature at 50 °C (Fig. 3a), showing 94 and 67 % of relative activity at 55 and 60 °C, respectively. The literature relates same or small optimum temperatures as the Aspergillus giganteus PG (Pedrolli and Carmona 2010) and Mucor circinelloides (Thakur et al. 2010), with 55–60 and 42 °C, respectively.
The thermal stability was determined by free and immobilized PG in the absence of substrate at 60 °C by several times, following enzyme assay. The activity remaining after incubation with supports was 75 % for PEI-agarose, 100 % for DEAE-cellulose, and 90 % for MANAE-agarose (Table 2). The thermal inactivation profiles of PG showed that, except with PEI-agarose, the derivatives DEAE-cellulose and MANAE-agarose presented improved stability profiles at 60 °C, when compared to soluble enzyme (Fig. 3b). The DEAE-cellulose derivative was about more stable than free enzyme at 60 °C; moreover, the MANAE-agarose derivative was 10.0 times more stable than the free enzyme.
Effect of pH on PG activity and stability
The enzyme presented a range of optimum pH 4.0–6.5 (Fig. 3c), similar to the literature that report pH 4.5 for P. expansum PG (Jurick et al. 2010) and pH 5.5 for Thermoascus aurantiacus (Martins et al. 2007), M. circinelloides (Thakur et al. 2010), and Curvularia inaequalis PGs (Gomes et al. 2001). At pH 7.0, the enzyme maintained 80 % of activity, and at pH 3.0 and 8.0, the enzyme showed about 60 % of activity.
The effect of pH was investigated by incubating the enzyme at low temperature and different pH values for 24 h. After that, the residual activity was measured with 200 mmol/L sodium acetate solution, pH 6.0. The enzyme was stable at all pH values tested, mainly at pH 6.5, retaining more than 70 % of activity at pH 3.0 and 85 % at pH 8.0 (Fig. 3d). The literature reports that P. variotii PG was more stable at the pH range of 3.0–6.0 (Damásio et al. 2010) and A. giganteus PG at the pH range of 6.5–10.0 (Pedrolli and Carmona 2010).
Kinetic studies
The kinetic parameters of A. niveus PG for polygalacturonic acid hydrolysis were carried out at 50 °C and pH 6.0 and analyzed by the plots of Hanes (1932). The K m, V max, K cat, and K cat/K m values were 6.7 mg/ml, 230 U/mg, 393.3/s, and 58.7 mg/ml/s, respectively. The literature reports smaller values (Pedrolli and Carmona 2010; Damásio et al. 2010)
Effect of metal ions on enzyme activity
The PG activity produced by fungus can be inhibited or activated by several ions (Damásio et al. 2010). Thus, the effect of several ions and ethylenediaminetetraacetic acid (EDTA) on the catalytic activity of A. niveus PG was studied. Before the enzymatic assay, the purified enzyme was dialyzed against 10 mmol/L EDTA for 12 h followed by other dialysis in 100 mmol/L sodium acetate solution, pH 6.0, for 12 h. After that, the enzyme was incubated with 1.0 and 10 mmol/L ions or EDTA and 1 % sodium polypectate in 100 mmol/L sodium acetate solution, pH 6.0, at 50 °C. The control for the enzymatic assay was carried out in the absence of ions or EDTA.
The enzyme was activated by several ions tested, mainly Mn2+ (232 %), at 10 mmol/L concentration, and F− (17 %), K+ (17 %), and Br− (10 %) (Table 3). The divalent ion Mn2+ increases the activity of A. giganteus PG (Pedrolli and Carmona 2010). Divalent ions can indirectly activate the PG activity because they act directly on the pectin molecule, stabilizing the negatively charged carboxyl groups (Gomes et al. 2009). The enzyme was strongly inhibited by Ba2+ and Pb2+, like A. giganteus (Pedrolli and Carmona 2010) and P. variotii (Damásio et al. 2010). Other ions and EDTA tested had low influence on A. niveus PG activity.
Thin layer chromatography of hydrolysis products
The classification of A. niveus PG was based on the analysis of the hydrolysis products of sodium polypectate using TLC. After short or long incubation times, only galacturonic acid was detected (Fig. 4) and no oligogalacturonate acids were produced. This shows that the enzyme specifically hydrolyzed the end-glycosidic bond of the polymer and can be classified as exo-PG.
Partial amino acid sequence of the PG
The amino terminal residues analysis showed six PG amino acids from A. niveus, SPPA(T)VI. Because A. niveus genomic DNA was sequenced and showed high similarity with Aspergillus fumigatus Af293 DNA (unpublished data), this microorganism was adopted as target sequence. It identified seven putative exo-PG-encoding genes (namely, PgxA, PgxB, and PgxC) in A. fumigatus genomic DNA (Fig. 5). A protein BLAST with amino terminal residues sequence obtained in this work showed 80 % of identity with PglB1, PglA2, and PglA3 putative exo-PGs by A. fumigatus. Furthermore, this segment showed 80 % identity with exo-PG Neosartorya fischeri. These results corroborate that the purified enzyme is an exo-PG.
References
Betini JHA, Michelin M, Peixoto-Nogueira SC, Jorge JA, Terenzi HF, Polizeli MLTM (2009) Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess Biosyst Eng 32:819–824. doi:10.1007/s00449-009-0308-y
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant-proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
da Silva TM, Maller A, Damásio AR, Michelin M, Ward RJ, Hirata IY, Jorge JA, Terenzi HF, de Polizeli ML (2009) Properties of a purified thermostable glucoamylase from Aspergillus niveus. J Ind Microbiol Biotechnol 36:1439–1446. doi:10.1007/s10295-009-0630-z
Damásio ARD, da Silva TM, Maller A, Jorge JA, Terenzi HF, Polizeli MDTD (2010) Purification and partial characterization of an exo-polygalacturonase from Paecilomyces variotii liquid cultures. Appl Biochem Biotechnol 160:1496–1507. doi:10.1007/s12010-009-8682-0
Davis BJ (1964) Disc electrophoresis: method and application to human serum proteins. Ann N Y Acad Sci 121:404–427
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fahmy AS, El-beih FM, Mohamed SA, Abdel-Gany SS, Abd-Elbaky EA (2008) Characterization of an exopolygalacturonase from Aspergillus niger. Appl Biochem Biotecnol 149:205–217. doi:10.1007/s12010-007-8107-x
Fernandez-Lafuente R, Rosell CM, Rodriguez V, Santana C, Soler G, Bastida A, Guisan JM (1993) Preparation of activated supports containing low pK amino groups. A new tool for protein immobilization via the carboxyl coupling method. Enzyme Microb Technol 15:546–550
Gomes E, Iembo T, da Silva R (2001) Production, characterization and properties of polysaccharide depolymerizing enzymes from a strain of Curvularia inaequalis. Folia Microbiol 46:303–308
Gomes E, Leite RS, da Silva R, Silva D (2009) Purification of an exopolygalacturonase from Penicillium viridicatum RFC3 produced in submerged fermentation. Int J Microbiol 2009:631942. doi:10.1155/2009/631942
Hanes CS (1932) Studies on plant amylases. I. The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J 26:1406–1421
Jurick WM, Vico I, Gaskins VL, Garrett WM, Whitaker BD, Janisiewicz WJ, Conway WS (2010) Purification and biochemical characterization of polygalacturonase produced by Penicillium expansum during postharvest decay of ‘Anjou’ pear. Phytopathol 100:42–48. doi:10.1094/Phyto-100-1-0042
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in the commercial sector: a review. Bioresour Technol 77:215–227
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227:680–685
Maller A, Damásio ARL, Silva TM, Jorge JA, Terenzi HF, Polizeli MLTM (2011) Biotechnological potential of agro-industrial wastes as a carbon source to thermostable polygalacturonase production in Aspergillus niveus. Enzyme Res 2011:289206. doi:10.4061/2011/289206
Martins ES, Silva D, Leite RSR, Gomes E (2007) Purification and characterization of polygalacturonase produced by thermophilic Thermoascus aurantiacus CBMAI-756 in submerged fermentation. Anton Leeuw Int J G 91:291–299. doi:10.1007/s10482-006-9114-6
Mateo C, Abian O, Fernandez-Lafuente R, Guisan JM (2000) Reversible enzyme immobilization via a very strong and nondistorting ionic adsorption on support-polyethylenimine composites. Biotechnol Bioeng 68:98–105
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
O'Farrell PZ, Goodman HM, O'Farrell PH (1977) High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12:1133–1141
Pedrolli DB, Carmona EC (2010) Purification and characterization of the exopolygalacturonase produced by Aspergillus giganteus in submerged cultures. J Ind Microbiol Biotechnol 37:567–573
Peixoto-Nogueira SD, Michelin M, Betini JHA, Jorge JA, Terenzi HF, Polizeli MDTD (2009) Production of xylanase by Aspergilli using alternative carbon sources: application of the crude extract on cellulose pulp biobleaching. J Ind Microbiol Biotechnol 36:149–155. doi:10.1007/s10295-008-0482-y
Rizzatti AC, Sandrim VC, Jorge JA, Terenzi HF, Polizeli MLTM (2004) Influence of temperature on the properties of the xylanolytic enzymes of the thermotolerant fungus Aspergillus phoenicis. J Ind Microbiol Biotechnol 31:88–93. doi:10.1007/s10295-004-0120-2
Round AN, Rigby NM, MacDougall AJ, Morris VJ (2010) A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr Res 345:487–497. doi:10.1016/j.carres.2009.12.019
Thakur A, Pahwa R, Singh S, Gupta R (2010) Production, purification, and characterization of polygalacturonase from Mucor circinelloides ITCC 6025. Enzyme Res 2010:170549
Wiseman A (1975) Handbook of enzyme biotechnology. Wiley, London
Zeni J, Cence K, Grando CE, Tiggermann L, Colet R, Lerin LA, Cansian RL, Toniazzo G, de Oliveira D, Valduga E (2011) Screening of pectinase-producing microorganisms with polygalacturonase activity. Appl Biochem Biotecnol 163:383–392. doi:10.1007/s12010-010-9046-5
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho de Desenvolvimento Científico e Tecnológico (CNPq). J.A.J. and M.L.T.M.P. are research fellows of CNPq. A.M., A.R.L.D., and T.M.S. were recipients of a FAPESP Fellowship. We thank Dr. Dalton Amorim and Diego Fachin for the image support and Ricardo Alarcon and Mauricio de Oliveira for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maller, A., da Silva, T.M., Damásio, A.R.d.L. et al. Functional properties of a manganese-activated exo-polygalacturonase produced by a thermotolerant fungus Aspergillus niveus . Folia Microbiol 58, 615–621 (2013). https://doi.org/10.1007/s12223-013-0249-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-013-0249-3