Abstract
The invasion of jumping worms, a small group of pheretimoid earthworm species from Asia, has increasingly become an ecological, environmental and conservation issue in forest ecosystems and urban-suburban landscapes around the world. Their presence is often noticed due to their high abundance, distinctive “jumping” behavior, and prominent granular casts on the soil surface. Although they are known to affect soil carbon dynamics and nutrient availability, no single paper has summarized their profound impacts on soil biodiversity, plant community, and animals of all trophic groups that rely on soil and the leaf litter layer for habitat, food, and shelter. In this study, we summarize the biology, invasion, and ecological impacts of invasive jumping worms across North America. We highlight potential impacts of this second wave of earthworm invasion, contrast them with the preceding European earthworm invasion in temperate forests in North America, and identify annual life cycle, reproductive and cocoon survival strategies, casting behavior and co-invasion dynamics as the key factors that contribute to their successful invasion and distinct ecological impacts. We then suggest potential management and control strategies for practitioners and policy makers, underscore the importance of coordinated community science projects in tracking the spread, and identify knowledge gaps that need to be addressed to understand and control the invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
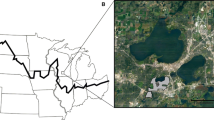
Pheretimoids are earthworms belonging to 12 genera in the family Megascolecidae (Chang et al. 2009a). With more than 1000 described species worldwide, this diverse, species-rich group is native to East and Southeast Asia and is quite often the dominant group in earthworm communities in forest, grassland, and agricultural ecosystems in their native range. However, 16 of the species have invaded 38 states in the USA and one Canadian province (Chang et al. 2016a; Reynolds 2018) (Fig. 1). Their invasion in ecosystems throughout the eastern USA as well as in a few locations in southern Ontario, Canada has increasingly raised concerns among ecologists, conservationists, land managers, horticultural professionals, and the general public (Chang et al. 2018; Moore et al. 2018; McCay et al. 2020).
Some common invasive pheretimoid (a–f) and lumbricid (g–i) earthworms in temperate North America. Amynthas tokioensis (a), Amynthas agrestis (b), Metaphire hilgendorfi (c), Amynthas corticis (d), and Amynthas gracilis (e) are often referred to as jumping worms, whereas Amynthas hupeiensis (f) does not jump. Instead, as seen in the photo, it coils. All of these five jumping worm species are epi-endogeic. In contrast, invasive lumbricid earthworm communities are often composed of three functional groups, including epigeic or epi-endogeic (e.g. Lumbricus rubellus, g), anecic (primarily Lumbricus terrestris, h), and endogeic (e.g. Aporrectodea caliginosa, i) species
In North America, there are several regional or common names for invasive pheretimoid species, all of which make reference to some behavioral characteristics of these organisms. In Kansas they are known as disco worms (S. James, pers. obs.), while in southern states they are known as jumping worms, frequently with a geographic modifier, such as Alabama or Georgia jumpers. In the northeastern states, they may be called Jersey wrigglers, crazy worms, snake worms, or even crazy snake worms. They are called snake worms because they use a serpentine motion on the soil surface while trying to escape. In this paper, we adopt the term jumping worms (Fig. 1). It should be noted that not all pheretimoids “jump,” and that jumping is a consequence of a violent thrashing motion of the body, rather than an effort to propel themselves upward. Another common name is wood eel as some pheretimoids are sometimes found in decaying logs. The majority of the invasive pheretimoids found in North America are active at or near the soil-litter interface, and these are the most likely to show the jumping behavior. However, a few species are active deeper in the mineral soil (Fig. 1); these do not thrash, instead, when exposed, they coil up and may secrete body fluid.
Of the 16 pheretimoid earthworm species currently known in North America, three species, Amynthas agrestis (Goto and Hatai, 1899), Amynthas tokioensis (Beddard, 1892) and Metaphire hilgendorfi (Michaelsen, 1892), appear to be the most invasive and damaging to forested ecosystems (Fig. 1). They look very similar and frequently occur together in the same habitat (Chang et al. 2018). It should be noted that recent changes in taxonomy have affected the names of several jumping worm species, including the three species that this paper highlights (Table 1). Where they co-occur, they are often mistakenly identified as one species, usually A. agrestis (Chang et al. 2016a, 2018). Besides these three species, some information on other species is given in this review when discussing distributions, common ecological traits, or control agents and strategies.
Jumping worms are highly invasive in hardwood forests and found in high abundance in urban parks, residential yards, greenhouses and compost piles. As a result, they are listed as ‘species of concern’, ‘invasive species,’ or ‘pests’ in several USA states, including Wisconsin, New York, Vermont, and California (e.g., New York Department of Environmental Conservation, 2014 [http://www.dec.ny.gov/docs/lands_forests_pdf/islist.pdf]; Wisconsin Department of Natural Resources, 2009 [http://docs.legis.wisconsin.gov/code/admin_code/nr/001/40.pdf]). However, in other states, concerns for natural resources, specifically for forest ecosystems, have not translated into regulatory action. Some of the difficulties in shaping policies and management plans to control jumping worms stem from four factors: (1) lack of management options and recommendations due to incomplete knowledge of their ecology and life history; (2) lack of assessments of economic damage to forest or horticultural production; (3) perceptions that earthworms in general enhance soil quality; and (4) lack of visibility because they are soil fauna. Information and research on jumping worms is inherently interdisciplinary, integrating a wide range of topics, scientific disciplines, published and unpublished data, and anecdotal observations. The objectives of this paper are (1) to increase awareness of this invasion by summarizing the current state of knowledge on jumping worm species for ecologists, natural resource managers, and horticultural professionals, (2) to highlight tools that are currently available for stakeholders, and (3) to provide a conceptual framework and indicate knowledge gaps for future research.
Origin and distribution worldwide and in North America
Overview of pheretimoid earthworms worldwide
Pheretimoid earthworms have been spreading across the world for hundreds of years, aided by human activities and enhanced by the exchange of plant and soil material between Asia and Europe, Africa, and the Americas (Brown 1878; Nelson 1917; Houchins 1995). When trade routes between the continents were based on sailing ships, introductions were likely restricted to port cities and areas receiving materials containing soil from Asia. As shipping routes expanded, the rate of spread of non-native species greatly increased. One proxy to estimate this rate is the record of the materials that end up in quarantine samples and custom agent blockages at international borders. For instance, Gates (several publications, but synthesized in Gates 1972) reported arrivals of pheretimoid earthworms at several entry points in the United States, in shipments coming from around the world, including countries other than their centers of origin. In fact, several pheretimoid species were originally described from specimens collected outside their native range. For instance, Amynthas gracilis (Kinberg, 1867), a species widespread in Latin America (Fragoso and Brown 2007), was described from specimens collected in the Botanic Gardens of Rio de Janeiro (Brazil), and Polypheretima elongata (Perrier, 1872), native to Asia, was described from specimens collected in Peru (Perrier 1872).
Among the 12 pheretimoid earthworm genera, Pithemera, Pheretima, and Polypheretima generally have a tropical origin in Southeast Asia, whereas Amynthas and Metaphire are widespread throughout temperate and tropical East Asia and Mainland Southeast Asia. Species in each of these five genera have been introduced outside their native range. However, with a few exceptions, such as A. agrestis, A. tokioensis, and M. hilgendorfi, the origin and native range of these introduced species are unknown. For instance, the native range of Amynthas corticis (Kinberg, 1867), one of the most common and widely distributed earthworm species throughout both tropical and temperate regions in the world, is believed to be somewhere in China (Gates 1972). There has been no evidence indicating where in China it might have originated, and we also do not know how much of its range in neighboring countries, such as Korea, Japan, Taiwan, and Thailand, was acquired through their active dispersal.
In Latin America, a total of 12 species of pheretimoid earthworms in the genera Amynthas, Metaphire, and Pheretima have been reported from over 35 countries/territories, including every country in mainland Central and South America (Fragoso and Brown 2007). For instance, throughout Brazil they have been found not only in fragments of forests and lawns in urban parks, but also in native forests, crop fields, integrated crop-livestock fields, pastures, and perennial plantations (Brown et al. 2006; Bartz et al. 2009, 2014a, b; Maschio et al. 2014; Ferreira et al. 2018; Demetrio et al. 2020). Interestingly, pheretimoids such as A. corticis and A. agrestis have also colonized oceanic volcanic islands, such as the Galapagos, where they occupy crop fields, pastures (Nakamura 1997); and protected natural forests (J. Ortiz-Pachar, pers. obs.).
In Europe, Darwin (1881) published sketches of relatively large tower-like castings of pheretimoid earthworms in Nice, France. However, Bouché’s (1972) comprehensive volume on earthworms in France showed only a few records of pheretimoid species. Apparently, these earthworms are generally restricted to the warmer, Mediterranean climatic regions (Portugal, Spain, Southern France, and Italy), rather than the colder continental temperate climate of most Central and Northern European countries. In these regions, they have been found in greenhouses in England, Denmark, and Hungary, among others (Drilobase http://taxo.drilobase.org/; Sims and Gerard 1999; Sherlock and Carpenter 2009; Csuzdi et al. 2008). Mechanisms restricting the spread of pheretimoids in these regions remain unclear. In Africa, a total of 16 pheretimoid species have been reported from 11 nations (Drilobase http://taxo.drilobase.org/). In Australia, pheretimoids have been recorded since the late 1800’s (Blakemore 2000); to date a total of 15 species in the genera Amynthas, Metaphire, and Pheretima are known. In New Zealand, three Amynthas species have been documented (Blakemore 2005). In India, eight species in the genera Amynthas, Metaphire, and Pheretima are known (Blakemore 2005).
Historical records and distributions in North America
In the USA and Canada, 172 species of earthworms in 11 families and 43 genera have been documented (Reynolds 2018), about a third of which are non-native (Snyder and Hendrix 2008) and 16 are pheretimoids (Chang et al. 2016a). In Mexico, 102 species in 8 families and 42 genera have been recorded, of which 51 are non-native and 10 are pheretimoids (Fragoso and Rojas 2014). The pheretimoids thus make up a little less than 10% of the earthworm fauna known in North America.
The first pheretimoid earthworm recorded in North America, Metaphire californica (Kinberg, 1867), was collected from San Francisco, California in 1866 and described for the first time in 1867 (Chang et al. 2016a). The new species was named after its type locality, California, hence the specific epithet (Chang et al. 2016a). Although this record predates that of the first European earthworm, Lumbricus terrestris Linnaeus, 1758 in New York in 1871 (Reynolds 2018), it is most likely the first lumbricid earthworms were transported from Europe hundreds of years ago, while pheretimoids are indeed more recent arrivals.
The three earthworm species that are at the focus of this paper were not officially reported in North America until the late 1930s: A. agrestis was first collected in 1939 in Maryland; A. tokioensis, 1947 in New York City; M. hilgendorfi, 1948 in Albany, New York (Chang et al. 2016a). Notably, scientific first records are unlikely to match the first introduction of a non-native species. The three jumping worms may have arrived with the cherry trees donated by Japan to Washington DC and nearby Bethesda in Maryland in 1912 and/or subsequent years (C.-H. Chang, pers. obs.). A couple of decades later, jumping worms were seen in 1939 at the Homewood campus of the Johns Hopkins University in Baltimore, Maryland, less than 60 km northeast from Bethesda. Presumably, as often is the case with accidental introductions, jumping worms arrived and were introduced at multiple locations and multiple times. Regardless of the points of entry, these three species have since been reported in 29 states in the USA and one province in Canada (Reynolds 2018) (Fig. 2). The latitudes at which they occur in North America match those in the source area in Japan and Korea. They are now widespread and common in the Eastern United States but have yet to be documented in the states west of the Rocky Mountains.
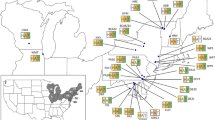
The distribution of Amynthas agrestis (a and d), Metaphire hilgendorfi (b and e) and Amynthas tokioensis (c and f) in Japan and part of South Korea (a–c) and in the south-eastern North America (d–f). Shaded areas in a–c are Japan and South Korea; those in d–f represent the US states and Canadian provinces from which data are available. See “Online Appendix 1” for data details
Identification and parthenogenetic degradation
Most jumping worms are relatively easy to distinguish from other earthworms in North America by their snake-like movement, thrashing behavior (in some species), and the shape and position of the clitellum. Occupying only three segments (from the 14th to the 16th), the clitellum (a swollen region on the body wall that appears only on the adults and functions in cocoon formation) of jumping worms is shorter and closer to the anterior end of the body as compared to the Lumbricidae family (Fig. 1). The clitellum also goes all the way around the body, unlike the saddle-shaped clitellum in most other species. With the use of a hand lens or stereo microscope, pheretimoids are also distinguished by having numerous short setae (bristles) on each segment, which are arranged in a continuous ring. In the Lumbricidae family, only eight setae are present per segment.
In general, earthworms are identified using both external and internal characters, most of which are related to reproduction. This means that immature specimens cannot be identified to species level by morphological characters. In pheretimoids, even adult identification can be difficult because the morphological characters used for species identification, particularly male reproductive organs, can sometimes become degraded as a result of parthenogenesis (Gates 1956; Shen et al. 2011), rendering useful features no longer visible. Multiple morphs that vary in the amount of reduction in the male sexual system can exist within a single population, posing additional challenges.
Despite the difficulties recognizing morphological characters, a key is now available for identifying the 16 pheretimoid species in the USA and Canada (Chang et al. 2016a). This key provides identification based on a small number of external and internal characters, color visuals, and a detailed description of all 16 species. It is intended for a broad range of users, including researchers, practitioners, and students—essentially anybody who would like to know which of these earthworms are present in backyards, nurseries, parks, forests, or any other ecosystems they are studying or managing. With some training and practice, the most common species are relatively easy to key out.
Molecular methods of identification are also available and reliable for a number of pheretimoid earthworm species. One of the most important contributions to ecological investigations is that molecular markers can identify adults (even those with degraded or absent reproductive organs), juveniles, and even cocoons to species. The mitochondrial cytochrome c oxidase subunit I (COI) gene is the standard barcoding region used for animals, and this approach has been proven quite effective and accurate for species-level identification in earthworms since its first use in 2005 (Chang and Chen 2005; Perez-Losada et al. 2005; Chang et al. 2009b, Chang and James 2011; Rougerie et al. 2009; Porco et al. 2013). Identification through barcoding requires a reliable reference database, which can now be found in GenBank and the barcode of life data system (BOLD). Barcoding using COI sequences as a tool may be expensive as the procedure requires DNA sequencing. However, a multiplex method has been developed to distinguish between the species of concern by simple gel electrophoresis of amplified products (Keller et al. 2017; Nouri-Aiin et al. 2021) at a fraction of the cost of sequencing. This method is cheaper than barcoding with COI and has been used to distinguish between species in cocoons and juveniles (Keller et al. 2017; Görres et al. 2018; Nouri-Aiin et al. 2021). To date, this protocol is available for all three jumping worm species this review focuses on (Nouri-Aiin et al. 2021) and has the potential to be further developed to include more species.
Ecology, phenology and life history
Life history and phenology
Our knowledge of the life history of jumping worms is largely based on laboratory incubations, field observations, and long-term field monitoring. The most distinguishing life history characteristic of the three invasive jumping worm species A. agrestis, A. tokioensis, and M. hilgendorfi, is their annual life cycle (Fig. 3), both in the invaded range in North America and in their native range in Japan (Uchida and Kaneko 2004). The annual life cycle means that these organisms emerge, grow, reproduce and die within one growing season, with a lifespan of roughly six months after emergence. In contrast, similarly sized European invaders like Lumbricus rubellus Hoffmeister, 1843 (red worm) and L. terrestris (nightcrawler), live for several years and take more time (4–5 months at 15 °C) to reach maturity (Butt 2011).
In a sugar maple stand at the University of Vermont’s Horticultural Research Center in Vermont, USA, where both A. agrestis and A. tokioensis are present (Chang et al. 2018), pheretimoids have been monitored for several years (Görres et al. 2016). The first hatchlings are generally observed in April, shortly after snowmelt. Cocoon hatching typically occurs when temperatures have increased above 10 °C, an experimentally determined hatching threshold (Blackmon et al. 2019), and remained greater than 5 °C, the lowest temperature at which jumping worms survived in laboratory cultures (Richardson et al. 2009). With increasing temperatures in May, the hatching rate and abundance quickly increase. The density of jumping worms peaks at 150–200 individuals m−2 in June, at a point when they are still juveniles. When the first adults appear, which is about 90–120 days after the first hatchlings are observed, abundance is reduced to about half of the peak. This decreases to even fewer individuals during summer droughts (Fig. 4). A similar pattern of rapid growth of the juvenile populations followed by a drop in abundance was observed at high elevations for A. agrestis in Georgia, with a later phenology than in Vermont (Callaham et al. 2003).
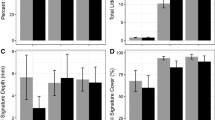
Temporal dynamics of the abundance of annual jumping worm species. a Data collected in a sugar maple stand in the Champlain Valley in Vermont, USA in 2011 shown as the sum of clitellate (adults) and unclitellate (juveniles). Graph redrawn from data in Görres et al. (2016). b Total pheretimoid abundance at the Horticultural Research Center of the University of Vermont, Vermont, USA from 2011 to 2015. Error bars are standard deviation
At some of the sites co-invaded by two or three jumping worm species, A. tokioensis reach maturity earlier than larger syntopic species (Johnston and Herrick 2019), perhaps because smaller organisms generally need less energy and resources. Contrary to these expectations, at Vermont sites, the first adults observed tend to be the larger A. agrestis and M. hilgendorfi. In Japan, A. tokioensis and A. agrestis are similar in size (Ishizuka and Minagoshi 2014; Y. Minamiya, pers. obs.), suggesting that some conditions may limit the growth of A. tokioensis in hardwood forests in Eastern USA.
In Vermont, all individuals were mature by September when first frosts likely reduce total abundance (Görres et al. 2016). Similar observations about the timing of the autumn population reduction were documented at high elevations in northern Georgia (Callaham et al. 2003), where, on average, frost dates are later than in Vermont. At both sites, no jumping worms were observed after late November (Callaham et al. 2003; Görres et al. 2016). Globally, A. agrestis follows this annual pattern with small changes in phenology based on local climate (CABI 2018).
Several independent morphological and ecological studies strongly suggest that North American A. agrestis, A. tokioensis, and M. hilgendorfi are generally parthenogenetic. As mentioned above ("Identification and parthenogenetic degradation" section), their male reproductive organs are often degraded or absent, and an H morph (i.e., specimens with a full suite of reproductive organs) has never been observed for any of the three species in North America. However, A. tokioensis (reported as A. levis) populations in Japan may include about 1% H morph individuals with the full suite of reproductive organs (Gates 1956). Thus, possible sexual reproduction in this species cannot be fully ruled out.
Cocoon production and development
Estimates of cocoon production in A. tokioensis and A. agrestis vary from 0.15 and 0.08 cocoons d−1 adult−1, respectively, in laboratory cultures (Johnston and Herrick 2019) to 0.7 and 0.5 cocoons d−1 adult−1, respectively, from field investigation (Nouri-Aiin and Görres 2019). At the Vermont site, by October the reproductively mature jumping worms are estimated to have produced about 1500 cocoons m−2 (Nouri-Aiin and Görres 2019). These numbers are equivalent to roughly 10–50 cocoons per individual per year, and is on par with cocoon production in L. terrestris (Butt 1991). In contrast, another common peregrine pheretimoid, A. corticis, produced more cocoons under tropical conditions in a laboratory study, reaching 55–282 cocoons per individual per year (García and Fragoso 2002). Nevertheless, it should be noted that adult jumping worms have only up to three months to reproduce before they die. Thus, this cocoon production rate is still substantial, especially when compared to European lumbricids.
In some earthworm species, cocoons are essential survival structures under environmental stress. For instance, cocoons of a common lumbricid, Dendrobaena octaedra (Savigny, 1826), survive freezing temperatures by dehydration, which reduces water content in the cocoon and prevents ice crystals from forming. This in turn prevents cellular damage in the embryos (Holmstrup and Westh 1994). For jumping worms, dehydration in cocoons is easily observed by the deformation of the nearly spherical cocoons into shapes that resemble deflated soccer balls (Fig. 5). Amynthas agrestis and A. tokioensis cocoons remain viable over a wide temperature range. Cocoons in the soil can survive an air temperature of at least − 24 °C in the field (Görres et al. 2016). At the other end of the scale, 75–100% embryos remained viable in the lab at temperatures between 20 and 26 °C, but temperatures greater than 38.4 °C were fatal (Johnston and Herrick 2019).
The sizes of jumping worm cocoons are smaller than those of the family Lumbricidae of comparable body sizes, and thus are harder to see in the soil. At the Vermont monitoring sites, jumping worm cocoons vary in size between 2 and 4.5 mm, depending on species (Nouri-Aiin and Görres 2019). Amynthas tokioensis cocoons average 1–1.5 mm smaller than those of A. agrestis. Metaphire hilgendorfi probably has the largest cocoons of the three species (M. Johnston, pers. obs.).
Based on experimental data (Blackmon et al. 2019), A. agrestis embryos require about 600-degree days to develop and hatch. As a result of cocoon production stretching over several summer and autumn months, a variety of embryonic developmental stages can be found at the same time, and “ready-to-hatch” cocoons exist in the soil all year round. Thus the cocoons may form a “cocoon bank” that stays viable for at least two years (Nouri-Aiin and Görres 2019). Some cocoons produced by the very first adults may hatch in the same year if other conditions such as soil moisture are also optimal (Nouri-Aiin and Görres 2019). This may have occurred at the Vermont monitoring site (Horticultural Research Center) in 2011 when juveniles of A. tokioensis and/or A. agrestis were observed, unexpectedly, as late as early October.
In A. agrestis, A. tokioensis and, presumably, M. hilgendorfi, a portion of embryos is always ready to hatch even during winter warming events (Görres et al. 2018; Nouri-Aiin and Görres 2019). Even though the likelihood of survival for these early hatchlings is low, early hatchlings that survive have more time for development and reproduction. This strategy, which is unique in earthworms, is especially advantageous at the northern edge of their distribution range in the Northern Hemisphere, and may have contributed to their northerly expansion as the frost-free period has increased over the last decades (McMahon et al. 2010).
Horizontal and vertical distribution and abundance
The three focal jumping worm species are categorized as epi-endogeic, living in the litter-soil interface and burrowing slightly into the surface soil (Chang et al. 2016a). European lumbricids that live at or near the soil surface tend to be relatively small. For example, the average mass of adult Dendrobaena octaedra (epigeic; dry weight: less than 0.1 g individual−1) and L. rubellus (epi-endogeic; dry weight: 0.1–0.2 g individual−1), are smaller than adult A. agrestis (dry weight: 0.4–0.5 g individual−1) and M. hilgendorfi (Richardson et al. 2015). With high abundance, e.g. 90–150 individuals per m2 (Callaham et al. 2003; Görres et al. 2016), and high individual biomass, the total biomass of A. agrestis per unit area can be much greater than that of lumbricids (Richardson et al. 2015). Similarly, M. hilgendorfi might attain an estimated biomass as high as about 194 g per m2 (based on a mean fresh biomass of 2.16 g individual−1 reported in Chang et al. (2016c) and a density of 90 individuals per m2). Consequently, forests invaded by pheretimoids are characterized by greater concentration of earthworm biomass and activity in the litter layer as compared to native North American or European earthworm assemblages.
Moisture is one of the factors that drives horizontal and vertical migration. When the leaf litter is wet, jumping worms are mainly found in the litter. If it is dry, they prefer the top-most mineral soil. During drought, they also aggregate around large tree trunks (C.-H. Chang and J. Görres, pers. obs.) or moister areas in the riparian zones (Snyder et al. 2011).
Ecological and environmental impacts
The impacts of invasive earthworms on forest floor litter, soil properties, C dynamics, nutrient cycles, ecosystem functions, and soil communities have received considerable attention over the last two decades in North America. Detailed reviews and meta-analyses can be found in various publications (Bohlen et al. 2004b; Hendrix et al. 2008; Lubbers et al. 2013; Craven et al. 2017; Ferlian et al. 2018, 2020; Frelich et al. 2019). In general, invasive earthworms reduce the leaf litter layer (organic layer) through direct consumption and some of the carbon stored in the litter is released into the atmosphere as CO2, leading to reduced carbon storage in the forest floor (Fig. 6). Their burrowing behavior increases soil mixing and redistributes litter-derived carbon, nitrogen and other nutrients into the soil, creating a soil habitat that has become enriched in various forms of these elements. In addition, their feeding and burrowing behaviors also lead to changes in basic soil properties, such as increased soil pH and bulk density. These dramatic changes in the litter-soil habitat have not only substantial impacts on ecosystem functions, but also cascading effects on organisms living in it, from trees, herbaceous plants, birds and salamanders to millipedes, springtails, mites, nematodes, fungi and bacteria (Fig. 6).
Principal ecological effects of pheretimoid earthworm invasions. Effects are manifested as chemical, physical, and/or biological changes to the aboveground (green box) or belowground (brown box) components of the system. These are produced at the nexus of earthworm behavior and the structure and composition of the forest floor. The intensity of effects and interactions will be dependent upon many factors including earthworm abundance, earthworm species present, individual size, inter- and intra-annual climatic conditions, and time since a particular site was invaded. Not all pathways indicated by arrows will be operating at all times, and for clarity not all possible connections are shown
Most previous efforts on earthworm invasion impacts focused on communities of European species that include a combination of three functional groups: epigeic, endogeic and anecic species. Conceptually, epigeic species are uniformly pigmented litter feeders that live in the litter layer; endogeic species are unpigmented soil feeders that live predominantly in the soil; and anecic species are anteriorly and/or dorsally pigmented litter feeders that feed on the soil surface but live in permanent vertical burrows (Bouché 1977). Epigeic and anecic earthworms tend to produce casts on the soil surface, while endogeic earthworms can produce casts on the soil surface and/or within the soil, depending on the species. Due to these distinct ecological characteristics in burrowing, feeding and casting behaviors, species of different functional groups are expected to affect the soil ecosystem differently. For instance, anecic earthworms can pull organic material deep into the mineral layers, whereas endogeic earthworms can redistribute mineral soil throughout multiple layers.
Compared to the effects of European earthworms, the effects of jumping worms on the soil ecosystem have been less studied and are poorly understood. Most of the work was published recently (Snyder et al. 2009, 2011, 2013; Zhang et al. 2010; Greiner et al. 2012; Bellitürk et al. 2015; Ziemba et al. 2015, 2016; Chang et al. 2016b, c, 2017; Richardson et al. 2016, Richardson 2019; Qiu and Turner 2017; Gao et al. 2017; Laushman et al. 2018; Ziter and Turner 2019; Bethke and Midgley 2020; Price-Christenson et al. 2020; O’Keefe and McCulloh 2021). In general, jumping worms increase litter decomposition (Greiner et al. 2012) and soil aggregation (Snyder et al. 2009, 2011; Greiner et al. 2012), mainly by casting on the soil surface, leading to a shallower Oe/Oa horizon sitting on top of a casting layer. Pheretimoid castings, with their distinctive loose, granular morphology (Fig. 7), are strikingly different from the surface castings of invasive European lumbricids. The extensive layer of granular castings changes the proportion of macropores and the thermal properties of soil, reducing temporal variation in surface temperatures (Görres et al. 2019). This more stable condition potentially provides thermal refuges for pheretimoids. These fundamental changes in the soil habitat may have cascading effects on soil physical and chemical properties, and on the diverse organisms relying on or living in the soil (Frelich et al. 2019). These are reviewed in the following sections.
Photos of soil surface covered with jumping worm casts taken from heavily infested forest patches in eastern USA. a A jumping worm is shown surrounded with its granular casts. b Casts of various ages next to a piece of decomposing wood. A small pile of wet, freshly deposited casts can be seen on the left. Casts may then become drier (lighter in color, upper right) and eventually congregate into aggregates covering soil surface, as seen in the background of the photo
Soil carbon
The feeding, casting and burrowing activities of European lumbricids and Asian pheretimoids profoundly affect soil carbon cycling, especially in forest ecosystems (Bohlen et al. 2004a; b; Fahey et al. 2013). Loss of the organic horizon and the fast disappearance of leaf litter indicate accelerated carbon mineralization. However, not all surface detritus (leaf litter and dead wood) mineralizes; some portion is incorporated into soil organic matter fractions, subsequently going through several transformations. These processes are microbially mediated, but detritivores, such as earthworms can modify location (especially depth), rates, and trajectories of these transformations. The biogeochemical pathways may significantly differ depending on earthworm functional group composition and type of detritus input. For example, in Mid-Atlantic young successional forests with abundant soil feeding (endogeic) lumbricid earthworms, decaying wood was incorporated deeper into soils than in mature forests dominated by epigeic earthworms, presumably due to more intensive mixing in the former (Ma et al. 2014). Young forests exhibited incorporation of fresher lignin into both particulate organic matter and silt and clay fractions, the latter providing greater long-term carbon protection. Detailed biogeochemical studies of earthworm effects on carbon transformations are few even for lumbricid earthworms (Bohlen et al. 2004a; Speratti and Whalen 2008; Kernecker et al. 2014) and have yet to be conducted with pheretimoids. Due to their surface casting and presumably lower mixing activity, jumping worms are assumed to contribute more to carbon mineralization and thus overall carbon loss rather than carbon protection in forest soils, however, this hypothesis needs to be rigorously tested.
Pheretimoid invasion may also affect soil carbon pools and fluxes in indirect ways, primarily by causing dietary shifts in other detritivores. Using 13C and 15 N labeled leaf litter, Chang et al. (2016c) have shown that in the presence of M. hilgendorfi, L. rubellus, a litter feeding lumbricid species, feeds less on leaf litter and more on soil. Zhang et al. (2010) reported a shift in the opposite direction, and argued that it is the dietary flexibility of the Asian jumping worms that contributes to their invasion success. Additionally, pheretimoids have the potential to change microbial community composition (Zhang et al. 2010; Chang et al. 2016b, c, 2017), further altering rates and pathways of organic carbon transformations.
The ways pheretimoids alter soil carbon cycling will likely depend on the conditions at the time of invasion. Specifically, effects are expected to be different at sites with no earthworms, with established native communities, established European lumbricids, or the mixture of the two. To date only a few studies have explored pheretimoid impacts on carbon cycling, and even fewer have focused on the underlying mechanisms.
Nutrient cycles
Earthworms directly and indirectly affect nutrient cycles, potentially leading to ecosystem-level changes both in natural and managed systems. Earthworms may directly impact the availability of nutrients by converting organic to inorganic forms (e.g., Waqar et al. 2019) or by increasing their mobility in the soil (e.g., Sizmur and Hodson 2009). Another mechanism is alteration of nutrient abundance or storage in organic and mineral soil horizons (e.g., Dobson et al. 2017). Alternatively, Sizmur et al. (2011) postulated that earthworms may not drive nutrients directly but may instead indirectly amplify prevailing processes by making them operate at faster rates or greater magnitude. Due to their fast growth rate and high biomass (Greiner et al. 2012; Richardson et al. 2015), jumping worms have a great potential to further accelerate biochemical transformations that change the availability of soil nutrients.
Jumping worms have been shown to alter N and inorganic nutrient cycling in agricultural and forest ecosystems (Steinberg et al. 1997; Burtelow et al. 1998; Greiner et al. 2012; Qiu and Turner 2017; Bethke and Midgley 2020). Qiu and Turner (2017) demonstrated that jumping worms (A. agrestis and A. tokioensis together) increased the concentration of inorganic nitrogen (NO3− and NH4+) in forest soils, both in the field and in laboratory mesocosms, in Wisconsin. Similar increases were also observed for M. hilgendorfi in a laboratory experiment using soils collected from a riparian forest in Michigan (Greiner et al. 2012) and for A. gracilis in a forest in New York (Burtelow et al. 1998). Jumping worm invasion also was associated with increased potential net N-mineralization and nitrification rates (Steinberg et al. 1997), though this is yet to be confirmed in the field. Additionally, the effect of jumping worms (A. agrestis and A. tokioensis together) on soil nitrogen and phosphorus availability may be tree species-dependent, increasing in white oak forest soils and decreasing in sugar maple forest soils (Bethke and Midgley 2020).
It is still unclear how these changes in N dynamics in the soil may affect other processes within the N cycle, particularly plant N uptake. Increased concentration of inorganic N in the soil may increase N uptake by plants (e.g., Waqar et al. 2019). However, the increased concentration of nutrients in general as a result of earthworm activity may also lead to accelerated leaching and downward movement of nutrients (Resner et al. 2015), and subsequently decrease inorganic nutrient uptake by, for example, understory plants in forests (Dobson et al. 2017). Moreover, the enhanced nutrient availability when earthworms are active may not result in enhanced plant uptake due to misaligned timing or increased leaching rates of nutrients. Nutrient additions may thus be considered “disturbances” when they are misaligned with plant uptake and could result in enhanced leaching. This scenario may very well be the case under jumping worm invasion. In Wisconsin, Qiu and Turner (2017) demonstrated that during the period between July (summer) and October (fall), soil inorganic nitrogen concentration peaked in October in forest plots invaded by jumping worms (A. agrestis and A. tokioensis), whereas the concentration is at an all-time low in forest plots with no earthworms. This dramatic difference in the seasonal dynamics of inorganic nitrogen in the soil between forest sites with and without jumping worms and apparent misalignment between soil nutrient concentration and the timing of plant growth raise concerns regarding potential nutrient loss.
Toxic and essential elements
Earthworms interact with toxic metals in the soil through bioaccumulation, mobilization, and redistribution in the soil profile (Sizmur and Richardson 2020). To date, less than a handful of datasets examined the impact and mechanisms of pheretimoid-metal interactions. Pheretimoid earthworms can enhance uptake of essential nutrients in some mineral soil rooted-plants but suppress nutrient acquisition in forest floor-rooted plants (Dobson et al. 2017). In South China, bioaccumulation of toxic metals in the pheretimoid earthworms M. californica and A. corticis (reported as A. heterochaetus) reached concentrations potentially toxic to predators (Wang et al. 2018). In the USA, Richardson et al. (2015), Richardson (2019) found similar patterns of bioaccumulation of As, Cd, Co, Pb, Hg, and V in A. agrestis and M. hilgendorfi in forests of the northeastern United States. However, the fate of these metals remains unclear. If consumed by a predator, these metals may be directly assimilated into tissues and organs and cause toxicity (Richardson et al. 2015). If the earthworm completes its life cycle, the metals may be retained and sequestered in soil. For instance, after 60 days leaching, > 95% of Pb from decomposing A. agrestis tissues was sequestered within soils (Richardson et al. 2016). Even less is known about how pheretimoids interact with organic contaminants. In a laboratory study, Metaphire guillelmi (Michaelsen, 1895) has been shown to rapidly take up, bioaccumulate, but also detoxify TBBPA, a halogenated phenolic compound commonly used as flame retardant (Gu et al. 2020).
Soil organisms
Concerns over pheretimoid earthworm invasions have emerged in the wake of profound changes exerted on the soil habitats they colonize. The most visible effect of pheretimoid activity is the disappearance of the leaf litter layer and the creation of a casting layer on the soil surface. These alterations in the soil habitats can have major impacts on other organisms living in or on the soil.
Plant-earthworm interactions
Identifying pheretimoid-specific impacts on plant communities in field studies is often confounded by the presence of existing earthworm assemblages at a field site and a comprehensive, mechanistic study of pheretimoid-specific impacts on plants has yet to be conducted. However, in temperate North America, field observations from pheretimoid-invaded sites and studies looking into earthworm invasion in general suggest potential widespread negative impacts on native, horticultural, and agricultural plants (Nuzzo et al. 2009; Dobson and Blossey 2015; Dobson et al. 2017). In contrast, some highly invasive grass species, plants with high concentrations of defensive chemicals, and generalist ferns may benefit from pheretimoid invasion (Greiner et al. 2012; Melnichuk 2016; Bowe et al. 2020). Furthermore, in subtropical agroecosystems (Southern Brazil) under no-tillage practices, invasion by pheretimoids (Amynthas spp.) enhanced macronutrient availability, soil macroaggregation and water infiltration into the soil, leading to increased grain production over several cropping seasons (Peixoto and Marochi 1996). In field macrocosms also in Southern Brazil, pheretimoid inoculation (30, 60, or 90 A. gracilis per m2) increased the growth of early succession tree species (Mimosa sp. seedlings) over a five-month period (Kobiyama et al. 1995).
In North America, earthworm invasion often consists of a diverse mix of co-occurring pheretimoid and lumbricid species, and is associated with reduced plant diversity and changes in plant species composition (Holdsworth et al. 2007; Craven et al. 2017). For instance, invasive earthworms are associated with increased sedge and graminoid abundance (Loss et al. 2012) and with presence of invasive plants, such as garlic mustard (Alliaria petiolata) and Japanese barberry (Berberis thunbergii) (Nuzzo et al. 2009). Conversely, sugar maple (Acer saccharum) productivity (Bal et al. 2018) and survival of long-lived understory herbaceous plants (Dobson and Blossey 2015) tend to decline in earthworm invaded soils. However, the effects of jumping worms (A. agrestis and A. tokioensis together) on tree seedling growth have been shown to be species-dependent, increasing growth in sugar maple and common buckthorn (Rhamnus cathartica) while decreasing growth in white oak (Quercus alba) (Bethke and Midgley 2020). Earthworms drive these changes in plant communities through direct consumption of seeds and seedlings (Griffith et al. 2013), changes in seedbank dynamics (Nuzzo et al. 2015), and effects on mycorrhizal-plant associations (Paudel et al. 2016). Furthermore, co-occurring factors, including soil characteristics, resident earthworm communities, invasive plants, white-tailed deer herbivory, forest age and land use history, are likely to mediate pheretimoid impacts (Dávalos et al. 2015; Laushman et al. 2018; Szlavecz et al. 2018; Cope and Burns 2019) (Fig. 6). Thus, evaluating pheretimoid impacts on plant communities requires long-term analysis of these interacting factors.
Earthworm-earthworm interactions
Pheretimoids in North America colonize not only soils with native earthworms (Callaham et al. 2003) or no earthworms (Görres and Melnichuk 2012; Görres et al. 2014, 2016), but also soils that have already gone through significant changes by European lumbricid species, primarily of the genera Lumbricus, Aporrectodea, and Octolasion (Dávalos et al. 2015; Laushman et al. 2018; Szlavecz et al. 2018). Given that certain lumbricids and pheretimoids utilize the same resources, they are expected to compete when they co-occur, and this may affect litter mass loss and decomposition. In a laboratory mesocosm experiment using the stable isotope technique, Chang et al. (2016c) demonstrated that the jumping worm M. hilgendorfi is a superior competitor for leaf litter against the common European earthworm L. rubellus, and detected a trophic niche shift in two non-native lumbricids (L. rubellus and O. lacteum) in the presence of the jumping worm, but not the native lumbricid Eisenoides lonnbergi (Michaelsen, 1894). However, in two separate laboratory mesocosm experiments, likely due to the short-term nature of these incubations, no apparent changes in biomass or survival were detected between jumping worms and the European lumbricids (Greiner et al. 2012; Chang et al. 2016c).
In the field, only limited, snapshot-type data exist on the trophic niche of pheretimoids and coexisting lumbricids. Consistent with laboratory mesocosm results, these field data provided clear evidence of niche overlap between jumping worms and one of the most common non-native lumbricids, L. rubellus (Chang et al. 2016c). In the American Midwest, a two-year survey detected substantial spreading of A. tokioensis and A. agrestis, and simultaneous reduction in the abundance of European lumbricids (L. rubellus and L. terrestris), suggesting possible interspecific competition and replacement (Laushman et al. 2018). Clearly, long term field monitoring is essential to understand the population dynamics of the invading and resident earthworm groups, and to properly evaluate the effects of invasion on earthworm communities.
Earthworm-microbe interactions
Soil microbes are major drivers of biogeochemical processes, and an integral component of the soil food web. They are also the primary contributors of soil respiration, and play a crucial role in the carbon cycle (Bardgett et al. 2008). The effects of earthworms on soil microbes, including bacteria and fungi, have been extensively reviewed recently (Medina-Sauza et al. 2019). 16S and ITS metagenomics have transformed our understanding of soil microbial community structures, and generated large quantities of data and new questions. However, few studies have taken into account seasonal changes throughout different phases of a growing season, and how different stages in the life history of the investigated earthworm species corresponds to these phases differing in temperature, precipitation and plant growth. These considerations are important when studying the ecological impacts of annual species.
Only a few studies have investigated how jumping worms may affect soil microbial communities, and only one included mycorrhizal fungi (Azevedo 2010). In the field, jumping worm invasion has been shown to be either associated with increased microbial biomass in both O and A horizons in plots invaded by A. gracilis (Burtelow et al. 1998), or not correlated with soil microbial biomass in plots with A. agrestis (Snyder et al. 2011). However, in a Wisconsin field study, plots that had been invaded by A. agrestis and A. tokioensis for longer than a year had different soil bacterial and fungal communities compared to newly invaded plots. Additionally, bacterial communities in worm guts and casts were species-specific (Price-Christenson et al. 2020). These inconsistent results highlight the complexity of the systems, and the need to take different species and their life histories into consideration.
Experimental approaches using laboratory mesocosms have long been used as an effective method to study earthworm impacts. In laboratory mesocosms, both A. agrestis and M. hilgendorfi have been shown to reduce the biomass of both Gram-positive and Gram-negative bacteria (Zhang et al. 2010; Chang et al. 2016b). However, in a two-year field manipulation, A. agrestis increased, not decreased, microbial biomass (Chang et al. 2017). These contrasting results strongly indicate that the short-term laboratory experiments may represent the recovery phase after the initial disturbance of mesocosm construction, rather than what might happen in natural soils. They also highlight the urgent need for observational and experimental field data, especially since it is unclear how the annual jumping worm species affect soil microbes at short and long time scales. Regular and frequent sampling is needed to reveal whether the increase in microbial biomass observed in Chang et al. (2017) is a general phenomenon through the growing season, or just a short-term spike resulting from increased soil N in September–October, related to massive mortality of the earthworms (Qiu and Turner 2017).
Earthworm-invertebrate interactions
Invasive earthworms are generally known to decrease soil biodiversity (Ferlian et al. 2018). Earthworms can interact with other animals in the soil directly by serving as prey or through competition and indirectly through modifying soil physical or chemical properties. There exists a large body of work concerning the direct and indirect effects of European lumbricids on invertebrates (Eisenhauer 2010; McCay and Scull 2019), but little is known about the impacts of pheretimoids and how they differ from those of lumbricids. In a microcosm study, springtail (Collembola) abundance declined in the presence of A. agrestis (Gao et al. 2017) likely due to decreased quantity or quality of food or changes in habitat quality. In the Great Smoky Mountains National Park, in Georgia, USA, presence of A. agrestis in the field was associated with lower richness and abundance of millipedes (Snyder et al. 2011). In another microcosm study, the millipede Pseudopolydesmus erasus assimilated less litter derived C when kept together with A. corticis (Snyder et al. 2009). While this reduction in litter consumption did not affect millipede growth or survival in the short term, over a longer-term experiment, A. agrestis was found to negatively affect survival of another millipede species, Sigmoria ainsliei (Snyder et al. 2013). Collectively, these studies presented clear evidence of competition between the invasive jumping worm species A. agrestis and native millipedes, documented species-specific differences in interspecific interactions, and highlighted impacts that have never been observed with lumbricid earthworm invasion in North American forests.
Earthworm-vertebrate interactions
Birds and salamanders are two groups of vertebrates affected by earthworm invasion. In general, the disappearance of leaf litter layers caused by invasive earthworms reduces habitat quality for forest floor salamanders and ground-nesting birds (Loss and Blair 2011; Ransom 2012), but earthworms themselves may become food for some species. Thus, the overall influences are likely to be decided by the interaction of these factors.
As generalist predators, salamanders can exert top-down regulation of forest floor invertebrates (Wyman 1998; Walton 2013), and at high densities even reduce the rate of litter decomposition (Hickerson et al. 2017). Forest floor salamanders are typically regarded as the most abundant vertebrate predators in the northeastern United States (Burton and Likens 1975). Most salamander species consume earthworms as prey (Petranka 1998; Ransom 2012; Pinder 2013), and some use anecic earthworm (Lumbricus terrestris) burrows as shelters (Ransom 2012). Pheremitoids have been shown to disrupt cover objects used by red-backed salamanders (Plethodon cinereus) (Ziemba et al. 2015), and when they were present, red-backed salamanders were less likely to be found under cover objects (Ziemba et al. 2016). Also, red-backed salamanders used lower quality habitats and foraged less successfully when housed with pheretimoid earthworms (Ziemba et al. 2016). In addition, while earthworms are part of salamanders’ diets, seal salamanders (Desmognathus monticola) were less likely to successfully consume A. agrestis compared to lumbricid earthworms (Gorsuch and Owen 2014).
Research examining the impacts of pheretimoid earthworms on other North American vertebrates is lacking. The presence of European lumbricids in a previously earthworm-free forest in Wisconsin lowered nest survival of Ovenbirds (Seiurus aurocapilla) by decreasing leaf litter and increasing sedge cover (Loss and Blair 2011). Additionally, the abundance of both Ovenbirds and Hermit Thrushes, another ground-nesting species, decreased in European earthworm-invaded areas. In contrast, invasive lumbricids may have a positive impact on generalist vertebrate consumers, such as American Robins (Cameron and Bayne 2012). Presumably, pheretimoid earthworms may have effects similar to those of epi-endogeic invasive European species, especially in regions of North America lacking a native earthworm fauna. However, more research on potential impacts is needed, especially because, unlike their European counterparts, several pheretimoid species exhibit a “boom and bust” annual life cycle, with large accumulations of biomass over a short period of time (Greiner et al. 2012; Görres et al. 2016).
Invasion processes
Jumping worms in their native range
In their native range in Japan, the three focal jumping worm species of this paper, M. hilgendorfi, A. tokioensis and A. agrestis, are widespread (Ishizuka and Minagoshi 2014; http://japanese-mimizu.jimdo.com/). They are more abundant in human-dominated environments, such as parks in urban areas and university campuses, than in natural habitats (Ishizuka and Minagoshi 2014; Uchida and Kaneko 2004; Minamiya et al. 2009; http://japanese-mimizu.jimdo.com/). Human-mediated dispersal might have occurred in such areas, but to date no studies have focused on this topic. In the Bonin Islands, an archipelago of oceanic islands south of Tokyo, a member of the three species, identified as “Amynthas hilgendorfi species complex” (Nakamura 1994) and A. tokioensis (Hasegawa et al. 2009), has been reported. It is unclear how this species arrived on the offshore islands, and whether it could be considered an invasive species there. Furthermore, these three species have also been found on an artificial island in Tokyo Bay (Y. Minamiya, pers. obs.), presumably brought there with soil and plants.
Unlike in North America, in their native range in Japan the three jumping worms are generally not the dominant species in earthworm assemblages. (Ikeda et al. 2012, 2018; Uchida and Kaneko 2004; Minamiya et al. 2010, 2013, 2015). Although a large number of specimens can usually be found along roads and in roadside ditches in secondary forests and plantation forests, the density is much lower than that in the US (Y. Minamiya, J.Görres, and D. McHugh, pers. obs.). Additionally, other species in the families Megascolecidae and Lumbricidae are abundant in Japanese forests. In general, none of the three jumping worm species or the other widespread cosmopolitan species in the families Megascolecidae and Lumbricidae are dominant in forest habitats. Such dramatic differences between Japan and the US are intriguing. In Japan, there are many native earthworm species (Ikeda et al. 2012, 2018; Uchida and Kaneko 2004; Minamiya et al. 2009; Ishizuka 2001) that may co-occur with the three jumping worms. These native earthworms, together with other decomposers such as isopods and millipedes (Kaneko 2018), are potential competitors for habitats and food resources. There are also many predators, such as birds, moles, and insects, adapted to feeding on pheretimoid earthworms (Ueno 1999; Okuzaki and Sota 2018; Imaizumi 1979, 1983). Thus, we suspect that the absence of effective competitors and predators may have contributed to the successful invasion and rapid spread of the three jumping worm species in the USA.
Phylogeography, population genetics and adaptation
Molecular data allow analyses of phylogeography and population genetics of invasive species. They can also be used to elucidate cryptic diversity of invasive taxa, and to assess whether and how populations of invasive species adapt to their new environment. To date, molecular studies of the pheretimoid earthworms have included analysis of mitochondrial and/or nuclear gene sequences (Chang and Chen 2005; Chang et al. 2007, 2008, 2009b; Minamiya et al. 2009, 2011; Novo et al. 2015; Schult et al. 2016; Aspe and James 2018), whole genome (Cunha et al. 2017), mitogenome (Zhang et al. 2016), Random Amplified Polymorphic DNA (RAPD) (Keller et al. 2017; Nguyen et al. 2018), and microsatellites (Cunha et al. 2017). However, only a few of these studies focused on invasive species of Amynthas or Metaphire.
Studies looking at populations of pheretimoids in their invaded ranges have generally indicated potential cases of multiple introductions and widespread co-occurrence of several species. For example, Schult et al. (2016) used two mitochondrial markers (COI and 16S rRNA) to examine the phylogeography and genetic divergence of Amynthas populations in the eastern United States. Their results supported the co-occurrence of three “cryptic lineages” at several sites sampled in New York, Wisconsin, and Alabama. The three lineages were later confirmed to correspond to A. agrestis, A. tokioensis, and M. hilgendorfi by the examination of internal reproductive systems and external morphology (Schult et al. 2016; Chang et al. 2018). Additionally, within each of the three species, populations across broad geographic distances exhibit shared haplotypes, indicating multiple occasions of introductions (Schult et al. 2016).
Also drawing on COI and 16S rRNA markers, Novo et al. (2015) highlighted that the invasion patterns of A. corticis and A. gracilis in the Azores, Portugal were shaped by environmental variables and that abundances of different genetic lineages were influenced by soil metal concentrations, topographical elevation and degree of human influence. The inclusion of samples from putative source populations revealed a complex invasion history with multiple introductions of these species, with different mitochondrial lineages present in the Azores and shared across other native and non-native ranges (Novo et al. 2015). Hence, phylogeographic patterns of pheretimoid populations in their invaded ranges are mainly shaped by human introductions together with their capability to adapt to different environments (Novo et al. 2015).
Molecular mechanisms by which pheretimoid earthworms may adapt to diverse environments are mostly unknown but will be important in understanding the role of adaptation in invasion success (Cunha et al. 2011; Novo et al. 2015). In response to these conditions, A. gracilis can change its gene expression patterns and its epigenome (Rimmington 2018), suggesting that genetic mechanisms can confer a great plasticity for adaptation of these earthworms to new environments.
Population genetics studies are scarce for pheretimoid earthworms, both in their native and invasive ranges. By applying RAPD markers to samples of three populations of A. agrestis and two populations of A. tokioensis in Vermont, USA, Keller et al. (2017) reported substantial genetic variation within both species. However, they also found no difference in genetic structure among populations, and concluded that the invasion into the three sites came from a single source population. In addition, clones, individuals with identical genotypes, represented 50% of the samples at one site for A. agrestis and 64% at another site for A. tokioensis, confirming pervasive parthenogenetic reproduction in these species.
Microsatellites provide consistent, reliable results and have recently been used in population genetics analyses of invasive pheretimoid species. Specifically, Cunha et al. (2017) provided a large set of microsatellite markers for A. corticis, designed from a low coverage genome. Microsatellites tested on Amynthas populations in the Azores showed similar results to those yielded by the mitochondrial markers, albeit at a finer scale (Cunha et al. 2017). Microsatellite libraries have recently been developed for A. agrestis, A. tokioensis, and M. hilgendorfi (D. McHugh, J. Görres, and M. Nouri-Aiin, unpub. data). Preliminary work indicates that populations of these three species are mostly triploid, which will need to be accounted for in future genetic analyses.
While some efforts are underway to understand the invasion patterns of pheretimoid earthworms, it is obvious that our current knowledge is still sparse. Populations from invasive and native ranges need to be extensively analyzed to reconstruct invasion histories using haplotypes from mitochondrial and nuclear markers. The study of genetic diversity and distribution of distinct mitochondrial lineages in relation to environmental variables has been proven useful for understanding invasion patterns (Novo et al. 2015); thus, this approach should be the focus of future studies and can be used for facilitating management measures. Relationships of recovered lineages with environmental variables will help understand whether ecological preferences exist and, if so, could motivate further examination of adaptation capacities within invasive pheretimoid populations. At a finer scale, microsatellite markers will provide valuable information on dispersal capacity, population admixture, and reproductive strategies. Next-generation sequencing techniques should also be considered for the analyses of single nucleotide polymorphisms (SNPs) in the study of population genomics.
Genomic approaches will be helpful in identifying positive selection that may be linked to local environmental conditions or climatic variables at a broader scale. Transcriptomic approaches can also be used to assess selection and adaptation (e.g., Laricchia et al. 2018). Furthermore, analyses of gene expression patterns across transcriptomes for individual worms of similar genotype can reveal any plasticity that might exist in response to different environmental variables in invaded habitats. This information will be important in assessing the potential for rapid adaptation of invasive pheretimoid species to new and different environments. For all of these molecular approaches, comparison between populations from native and invasive ranges of each species will be essential, and thorough sampling across those ranges is an urgent and important first step.
Co-invasion dynamics
Widespread co-invasion of the three jumping worm species, A. agrestis, A. tokioensis, and M. hilgendorfi, in northeastern USA was recently reported after examining DNA and morphological data (Schult et al. 2016; Chang et al. 2018). Their co-invasion most likely has been repeatedly taking place for a long time, and thus the recent discovery is a result of the lack of taxonomic expertise, rather than evidence of a new phenomenon. In fact, the earliest record on co-occurrence can be traced to samples collected in 2002–2003 (Chang et al. 2018). Therefore, we know for sure that co-invasion has been happening for almost 20 years. This leaves us with many unanswered questions: Is co-invasion evidence of any form of interspecific interaction? Is there niche differentiation among the species? Could the presence of one species facilitate the invasion or survival of another one? Is there competition between any co-invading species? Are there non-additive effects regarding ecological processes and ecosystem functions?
In areas of North America that were earthworm-free after the last glaciation, Hale et al. (2005) found that non-native epigeic and epi-endogeic earthworms invaded earlier and appeared to facilitate the invasion of other European earthworm species. With the limited knowledge we have about pheretimoid ecology, we can only speculate that facilitation among the three co-invading jumping worms is possible. M. hilgendorfi is larger than A. agrestis, which is in turn larger than A. tokioensis. This size difference among the co-invading species may indicate potential food or habitat resource partitioning. Stable isotope studies have shown that the lumbricid Aporrectodea longa shifts its diet as it grows (Schmidt 1999). This diet shift could be a result of changing nutrient requirements during development, or simply body size differences. Additionally, different earthworm species from the same functional group may prefer leaf litter at different stages of decomposition (Zicsi et al. 2011). Jumping worms are known to increase extracellular enzyme activities and facilitate decomposition (Bellitürk et al. 2015). Presumably, one species that prefers leaf litter at an early stage of decomposition may make more food available for another species that relies on leaf litter at a slightly later decomposition stage. Coprophagy is also present in some pheretimoids (Kaplan et al. 1980), although, to our knowledge, nothing is known about the potential use of castings of one pheretimoid species by another.
The second wave of earthworm invasion and shifting baseline
It has been proposed that the success of earthworm invasion is influenced by physical or chemical characteristics of sites more so than interactions with resident native earthworms (Hendrix et al. 2006). Additionally, invasion history and environmental characteristics may play an important role in structuring observed earthworm communities (Szlavecz et al. 2018; Pinder and Robinson 2019). However, when invading pheretimoid species meet resident communities, interspecific interaction is destined to play an important role in this second wave of earthworm invasion.
A central question in ecology is how systems may change under different global change stressors, such as different assemblages of invasive species. Different invasion histories or invasive species may lead to alternative stable states. Before European settlement, forests in temperate North America were either earthworm-free as a result of the last glaciation, or had only native species, such as Diplocardia spp., Bimastos spp., and Eisenoides spp. Many of these forests were later invaded by European lumbricid earthworms, resulting in two new states, in which a forest has either only the invasive European species or both native and European species. The second wave of earthworm invasion, i.e., the invasion of jumping worms, further complicates the scenarios, adding four more states that are theoretically possible. Do these states, which have different combinations of invasive earthworm groups (i.e., lumbricids-only, pheretimoids-only, or lumbricids and pheretimoids), differ qualitatively and quantitatively from one another? Specifically, is pheretimoid invasion causing a shift of the baseline in a system? Addressing this question requires researchers to identify the specific ecological structures or processes in question, and the answers may be context-dependent. However, given the dramatic life history and behavior differences between lumbricid and pheretimoid earthworms, and the reported competition between these two groups (Chang et al. 2016c; Laushman et al. 2018), we expect to see a baseline shift in C and N biogeochemistry and nutrient cycles under pheretimoid invasion, regardless of the pre-invasion status of earthworm community.
Transport mechanisms
European earthworm invasions have been associated with historical and present-day human activities, including land use change, expansion of road systems, landscaping practices, and distribution of fishing bait (Cameron and Bayne 2009; Sackett et al. 2012; Shartell et al. 2013; Beausejour et al. 2015; Yesilonis et al. 2016; Szlavecz et al. 2018). Similarly, human-mediated dispersal of pheretimoid earthworms and cocoons is thought to be the main mechanism for spread. Pheretimoids are often associated with horticultural materials and nursery infrastructure, such as potting mixes, nursery stocks, wood mulches, and compost from infested nurseries, gardens, ornamental beds, and greenhouses (Gates 1958; Görres and Melnichuk 2012; Bellitürk et al. 2015; Moore et al. 2018). As with many species of earthworms, pheretimoids are also used for soil improvement and as fishing bait (Gates 1982; Callaham et al. 2003; Görres and Melnichuk 2012; Gorsuch and Owen 2014). Therefore, disposal of unused fishing bait and the transport and use of natural landscaping materials containing either earthworms or cocoons are important vectors for their dispersal. Moreover, cocoons of pheretimoid earthworms are small, and can be easily and accidentally transported by anything “contaminated” with soil, including shoes and tires of vehicles.
Once introduced into an ecosystem, it is unclear what exactly facilitates their spread beyond movement over the soil surface (estimated at 12 m yr−1 for the invasion front; Snyder et al. 2011). Naturalized populations often exhibit patchy distributions. Pheretimoid earthworms have been observed along riparian habitats (Szlavecz et al. 2014), sometimes as a result of runoff from upland areas. Schwert and Dance (1979) demonstrated that viable cocoons of European earthworms were able to drift downstream, furthering their spread. Additionally, overland flow can move pheretimoids as well as their cocoons (Görres et al. 2014). Studies determining the extent streams and rivers facilitate transport of pheretimoid earthworms and cocoons are urgently needed in order to better predict their spread in undisturbed ecosystems and develop management plans for conservation areas.
Understanding and modeling distribution and dispersal
As understanding of life history, physiology, and ecology of pheretimoids increases, models can be developed to predict the rate and extent of jumping worm colonization in North America. The spread of exotic earthworms into northern forests can have particularly substantial impacts due to changes to the large amount of carbon stored in the thick forest floors there (Lubbers et al. 2017; Angst et al. 2019). Because of their annual life cycle, the northern extent of pheretimoid spread may be limited by a minimum number of frost-free days, which were estimated at approximately 90 days for Vermont forests (Görres and Melnichuk 2012; Görres et al. 2016). Using the same parameter, Moore et al. (2018) predicted that much of southeastern Canada is or will soon be invasible for the pheretimoids. Soil moisture (Snyder et al. 2011) and soil pH (Bernard et al. 2009; Moore et al. 2013) also can constrain suitable habitats for pheretimoids. Future work in this area should consider climate projections and soil types to develop robust predicted distributions.
Understanding and predicting the distributions of pheretimoids at finer scales will require more detailed models of invasion spread. Although only limited data are available on rates of spread, approximate Bayesian computation may be used to infer these rates from distributional data, similar to a model focusing on the spread of European earthworms in Canadian forests (Chkrebtii et al. 2015). Other approaches, so far used for European lumbricid colonizations, are individual based models (Armstrong et al. 2017) and Leslie-matrix models (Pelosi et al. 2008). These models are parameterized using information on population dynamics, such as mortality rates and dispersal. Additional data on distributions and population dynamics will be needed for these small-scale spread models. Work has already begun to examine changes in pheretimoid distributions over time (e.g., Laushman et al. 2018; Szlavecz et al. 2018), and such studies will be important for both development and testing of models of pheretimoid spread.
Control and policy
Human-mediated dispersal
Conversion of land to urban-suburban uses favors both establishment and spread of pheretimoids. First, cities as centers of international trade and transport are often the entry points of species introductions. Second, humans create favorable habitats that overcome environmental limitations, allowing exotic species to survive. In North America, landscaping practices in urban and suburban areas, such as irrigation, composting, mulching, and transportation of these substrates, are some of the ways humans may facilitate the spread of pheretimoids and alter their phenology to faster development and protection from drought. Gardeners are well aware of their presence in planting beds; in many regions, the gardening community was the first to raise concern about their presence. While urban and suburban landscapes are highly modified and jumping worms are just one of many non-native organisms, their ability to quickly spread into wildlands nearby and their dramatic effects on soils and soil biota have resulted in their listing as species of concern in the states of Wisconsin and New York.
The practice of raking or blowing leaves from residential or commercial lawns could mobilize earthworms and cocoons and concentrate their abundance in leaf piles. In turn, many municipalities collect this material and redistribute it in the form of inexpensive leaf mulch or compost for use in landscaped parks, rights-of-way, or other public spaces. Some municipalities give away this collected material to residents for personal use. These practices serve as another potential vector of pheretimoid earthworms. Environmental and horticultural non-profit groups (e.g., garden clubs, plant societies, ‘friends’ organizations) often hold plant sales to raise money for their operations. In many cases, plants are dug from residential properties, potted, and sold. If the source soil or litter harbors pheretimoid earthworms or cocoons, this practice could also lead to new infestations. Recently, many garden clubs in Wisconsin, New York, and other states have either cancelled their sales, only sold plants with bare roots, or purchased wholesale from nurseries that are thought to be free of pheretimoid earthworms.
Management and control
The most effective means of controlling the invasion of non-native earthworms into new habitats is prevention of introduction (Hendrix and Bohlen 2002; Callaham et al. 2006). Most of the considerations and recommendations in these papers are also applicable to pheretimoid earthworm species. However, when introductions have already occurred, it is necessary to develop management approaches to eliminate or control populations. Management and control of invasive pheretimoid earthworms has scarcely been applied at any operational scale, and data are available mainly from golf courses, where these worms have been controlled predominantly through pesticides (Redmond et al. 2016). However, a few studies have attempted to develop management guidelines to address this problem (McCay et al. 2020). Managing any kind of pest requires detailed knowledge of the life cycle of the species in question, as well as information about the life stages most likely to be susceptible to management intervention. This kind of information is currently being collected for pheretimoid earthworms. We consider that management efforts aimed at eliminating/controlling pheretimoid earthworms fall into two general categories: (1) integrated pest management involving introduction or promotion of natural enemies of pheretimoid earthworms, and (2) environmental modification to render the invaded habitat inhospitable for completion of the pheretimoid life cycle.
Integrated pest management approaches
To date, we are unaware of any coordinated efforts to develop integrated pest management for pheretimoid earthworms, but there are a few potential avenues for such an approach. For example, there are earthworm predatory turbellarian flatworms (Bipalium spp.), themselves non-native to North America (Stokes et al. 2014), which may have utility in controlling pheretimoid populations, but these flatworms have been shown to more effectively capture lumbricid earthworms than pheretimoids (Gorsuch and Owen 2014). This apparent preference for lumbricids gives rise to concerns about non-target effects of planarian biocontrol, particularly in soils with native earthworm species present. Furthermore, in Europe some introduced planarians have themselves become invasive species causing problems for the local soil (mainly earthworm) fauna (Sluys 2016). Likewise, centipedes (in this case, using species native to North America), show some promise for being effective predators of pheretimoid earthworms (Gorsuch and Owen 2014; Gao et al. 2017), but these have not yet been applied in an operational context.
Habitat modification approaches
Controlling pheretimoid earthworm populations through habitat modifications could be accomplished by physical or chemical means. One example of a physical habitat modification would be the use of prescribed fire to remove food resources (leaf litter in a forest floor), and/or apply heat to earthworm propagules (either juveniles or cocoons). The applicability of fire as a method to control invasive earthworms has been tested for lumbricid species (Blackmon 2009), and for pheretimoid species (Ikeda et al. 2015). Ikeda et al. (2015) conducted small scale, controlled burns in test beds containing adults of A. agrestis, and observed a significant decline in cocoon viability in burned plots, but not in the number of adults compared to control plots, as adult earthworms can temporarily escape by burrowing into the soil, whereas cocoons mixed in the casting layer on the soil surface are more susceptible to fire. In the laboratory, Johnston and Herrick (2019) found that cocoons of A. agrestis and A. tokioensis were not viable when subjected to temperatures of 40 °C or above for at least three days. This study mirrored many USA states’ standards for commercial compost facilities. Compost piles are required to be heated to at least 55 °C for 3 or 15 days depending on the type of pile. This temperature will render pheretimoid cocoons nonviable, which suggests that unless finished compost is infested after treatment, commercially prepared material should be free of pheretimoid earthworms and cocoons.
Policy
The global to regional commercial movement of plants, soil materials, and earthworms as bait has undoubtedly led to the spread of these non-native species. States such as California, Wisconsin and New York have legally restricted the movement of pheretimoid earthworms within and across state boundaries (New York Department of Environmental Conservation, 2014 [http://www.dec.ny.gov/docs/lands_forests_pdf/islist.pdf]; Wisconsin Department of Natural Resources, 2009 [http://docs.legis.wisconsin.gov/code/admin_code/nr/001/40.pdf]). In these States, it is illegal to knowingly sell, trade, purchase, import, or transport pheretimoid earthworms or infested materials. In Wisconsin, state officials, academics, non-profit groups, and the green industry developed a list of Best Management Practices (BMP’s) to help homeowners, gardeners, landscapers, horticulturalists, land managers, and others to limit the spread of pheretimoid earthworms (https://dnr.wi.gov/topic/invasives/fact/jumpingworm/index.html#manage).
Community science
One of the challenges of managing and controlling invasive earthworms is that, as organisms living at or under the soil surface, their presence may often go unnoticed until populations are firmly established. While a number of researchers are currently examining the impacts of invasive pheretimoids in North America, we do not yet know the true scope of pheretimoid invasion at the continental scale, as thorough documentation often only occurs in regions with established research or education programs (Moore et al. 2018). With the ubiquity of smartphones and data collection applications such as iNaturalist, eBird, and Project BudBurst, many scientists have turned towards the public to enhance their ability to monitor biodiversity over large areas by enabling ordinary people to collect data for them. With community science, the general public can contribute to research by collecting data, allowing scientists to monitor biodiversity, including invasive species, over large areas relatively easily and often fairly accurately. For instance, in a comparison of techniques for monitoring butterflies in urban meadows, iNaturalist observers recorded 73% of the butterflies present and significantly more butterfly species than Malaise trapping (Prudic et. al. 2018). The Earthworm Society of Britain, in collaboration with the staff at London Natural History Museum, regularly organizes earthworm collecting and identification training sessions, and oversees earthworm survey campaigns. Records collected by community scientists go through a verification process, and then become part of several datasets, including the National Biodiversity Network Atlas.
Community science projects have been used successfully to detect and monitor invasive species in both marine and terrestrial systems. For example, citizen-submitted observations documented lionfish invasion 1–2 years earlier than a traditional monitoring program (Scyphers et al. 2015). Great Lakes Worm Watch (www.greatlakeswormwatch.org) was an early adopter of the collection of data by local non-scientists to detect non-native European earthworms in the Great Lakes region of the United States. Earthworms Across Kansas, which operated in the early 2010’s, recruited school classes to collect earthworms, resulting in many new records, including Amynthas spp. (B. Snyder, pers. obs.). Recently, citizen scientists, students, and scientists conducted a one-day earthworm survey of urban habitats in Madison, WI. This participatory field campaign resulted in the first confirmed record of Metaphire hilgendorfi in Wisconsin and showed that the presence and abundance of pheritimoid earthworms differed among urban green spaces (Ziter et al. 2021). Today, the Cornell University Cooperative Extension and NY iMapinvasives have tools in place to detect and map pheretimoid sightings throughout the United States, with jumping worms listed as a “species of interest” (www.nyimapinvasives.org/species-of-interest), and they have been the focal invasive species in the their annual Invasive Species Mapping Challenge for several years. Likewise, the genus Amynthas—identified as “Snake Earthworm”—is listed on iNaturalist (www.inaturalist.org), enabling citizens to submit sightings and location information.
In addition, community science programs offer potential benefits both to scientists, by gathering data that may otherwise have been difficult or impossible to collect, and to community participants, by providing opportunities to be involved in and learn about science (e.g., Lucky et al. 2014). To support learning, these programs often include curricular material that allow them to be used effectively in an educational context, and actually enhance the educational experience (Hardy and Hardy 2018). For example, the Ecological Research as Education Network (EREN; http://erenweb.org/), which is a network of collaborative research projects involving undergraduate students, includes a project on earthworm distributions in the United States. For middle and high school grades, Earthworms Across Kansas provided lesson plans to match the life science curriculum in Kansas. A key next step for programs such as these would be to test their effectiveness in meeting educational objectives and enhancing student learning.
Community science programs can allow the large amounts of data needed to effectively track invasive species to be collected, while simultaneously informing the public about species invasions (Jordan et al. 2011). Consequently, community science and education-based programs are particularly promising for studying both local and regional jumping worm invasion dynamics in North America. This is especially important in light of the uncertainty of climate change and continued anthropogenic impacts.
Knowledge gaps
We identified seven critical knowledge gaps regarding jumping worm invasion centering around six fundamental questions (Fig. 8).
1. Differences in the ecological impacts, especially on C and N cycles and microbial communities, between jumping worms and European lumbricids
Given the distinct, granular casts and annual life cycle of the three focal jumping worm species, we believe that their impacts on C and N cycles and soil microbial communities are not equivalent to those of the invasive European earthworms. The granular casts deposited on the soil surface, which can form a casting layer as thick as 5 cm, might increase soil erosion. Casts that are not eroded can change soil bulk density, which is generally assumed to increase under the influence of invasive earthworms. However, quantitative data on soil bulk density in habitats with jumping worms are still lacking. All these changes will impact soil C and N biogeochemistry. The three annual species of jumping worms can cause dramatic increase in soil N towards the end of the growing season (Qiu and Turner 2017), a striking phenomenon not observed under European earthworm invasion. However, the fate of the N is still unknown. Chang et al. (2017) documented that A. agrestis increased soil microbial biomass in soil samples taken in early fall. Although this increase in microbial biomass coincided with the increase in total nitrogen and total inorganic nitrogen in the soil, we cannot just assume a causal relationship between the two. There is an urgent need to take the jumping worm’s annual life cycle into account and carefully follow the soil microbial community and soil nitrogen throughout the growing season to understand the true dynamics of these interconnected factors.
2. Impacts of leaf litter loss and the casting layer on other soil fauna
Habitat loss caused by the disappearance of leaf litter is a major change for other soil invertebrates. The impact of leaf litter loss on soil fauna might superficially be similar to that under European earthworm invasion. However, this inference does not take into account the casting layer. The casting layer is something unique to the annual jumping worms. This layer of granular, organic material-rich structure might become a hotspot for soil mesofauna, such as mites, springtails, and nematodes. However, data on jumping worms’ impact on soil mesofauna are still lacking. A casting layer can also form by the invasive earthworm Pontoscolex corethrurus (Müller, 1857), a species of the Rhinodrilidae family that has spread throughout the tropics and subtropics, including Florida (Gates 1973; Taheri et. al. 2018). In contrast to the loose, granular casting layer of the annual jumping worms, the casting layer of P. corethrurus is a compacted, water-impermeable layer that can be harmful to plants and other soil organisms (Barros et al. 2004). This dramatic difference in the morphology and physical property of the casting layer highlights the possibility of different impacts between different groups of invasive earthworms.
3. Impacts on wildlife and plants
Currently, there is nearly no data on the impacts of jumping worms on organisms of conservation importance, such as vertebrates and plants. Moreover, as a result of their behaviors and annual life cycle, their impacts may be different from those of the European earthworms. For instance, earthworm communities dominated by European species usually include L. terrestris, which is often the only anecic earthworm in those communities. The burrows of this species can be used by the red-backed salamander as shelters (Ransom 2012). In some areas previously occupied by European earthworms, L. terrestris is now being replaced by A. agrestis and A. tokioensis (Laushman et al. 2018). As these two epi-endogeic species do not form permanent vertical burrows, and their “jumping” behavior could make them harder to catch by salamanders compared to European earthworms (Gorsuch and Owen 2014), the consequence of their invasion on the salamanders may not be comparable. Additionally, jumping worm impacts are likely mediated through complex interactions with multiple, co-occurring stressors, such as invasive plants and white-tailed deer herbivory. Analysis based on long-term monitoring of these interacting factors would help disentangle whether jumping worms are driving ecological impacts or responding to other co-occurring stressors, which has important implications for conservation and management.
4. Habitat invasibility and the factors leading to the boom-and-bust dynamics
A central question in invasion biology is what drives the successful invasion of a species in some places, but not in others. For jumping worms, it is unclear why they in general have not spread and become invasive in European countries the same way as in the USA. In their native range in Japan, their densities appear to be low and for A. tokioensis, there are apparent differences in body size between Japan and USA populations. The answers to these questions may help us better understand jumping worm invasion in the USA, as well as locate other parts of the world with a high risk of becoming invaded. Multi-year long-term data are also needed to investigate the temporal dynamics of the three annual species, to monitor if their high density can be sustained throughout multiple years, and to uncover factors leading to the boom-and-bust dynamics that have been observed by several researchers. This can be done with significant involvement of community scientists.
5. Facilitation and competition of the three co-invading species
Another knowledge gap regarding jumping worm invasion is their co-invasion dynamics. For European earthworms, it has been documented that the epigeic species may invade first, and facilitate invasion of anecic and endogeic species that come afterwards (Hale et al. 2005; Holdsworth et al. 2007). Thus, co-invasion of European earthworms involves different functional groups and possible facilitation among them. However, this is not the case in the annual jumping worms. The three jumping worm species that co-invade are all epi-endogeic. We have few data on the ecological differences among the three species that seem similar ecologically, and no knowledge regarding their interspecific interactions, e.g., competition or facilitation, during the co-invasion process.
6. Different trajectories of invasion progression in habitats with and without previous histories of European earthworm invasion
Earthworm invasion can lead to irreversible changes to forest soils. Ma et al. (2014) and Yesilonis et al. (2016) highlighted that forests that have gone through different land use and earthworm invasion histories are on different trajectories of soil evolution. Here, we further propose that under the second wave of earthworm invasion, the differences in initial conditions, i.e., not invaded, lumbricid dominant, or native earthworms dominant, may determine pheretimoid effects on soil properties and biogeochemical processes. Thus, the topics we discussed above can lead to different outcomes and conclusions, depending on the initial conditions. In addition to forests, as the three annual jumping worms are known to be abundant in residential areas and riparian zones in cities, they could also have profound impacts on urban ecosystems.
7. Field experiments to complement laboratory mesocosm and field observational studies
While many laboratory studies have provided valuable data on jumping worms, particularly their life histories and potential mechanistic explanations on their ecological impacts, there are few field experimental studies that provide the critical missing link between controlled laboratory microcosm/mesocosm experiments and real-world conditions (Greiner et al. 2012; Chang et al. 2017). In particular, some results based on short-term laboratory experiments either cannot be replicated in field conditions (e.g., Greiner et al. 2012) or were qualitatively contradictory to field experimental results (e.g. Zhang et al. 2010 vs. Chang et al. 2017), let alone reasonably extrapolating these results to estimate ecosystem responses.
Conclusions
The second wave of earthworm invasion by jumping worms in temperate deciduous forests and urban and suburban landscapes is a relatively new phenomenon. This group of highly invasive species has increasingly raised concerns primarily due to its high abundance, “jumping” behavior and visible impacts on the appearance of the leaf litter layer and surface soil. Jumping worms have an annual life cycle; they hatch from cocoons in spring, mature and reproduce in summer, and die by the end of fall. Their annual life cycle, reproductive and cocoon survival strategies, unique casting behavior, and co-invasion dynamics may contribute to their successful invasion and distinct ecological impacts. These species may compete with and even replace the resident European earthworms, leading to potential baseline shift and altered ecosystem functions, such as C and N biogeochemistry and nutrient cycles. Particularly, a misaligned timing between increased soil inorganic nitrogen concentration and plant nitrogen uptake in a forest may lead to increased leaching and promote microbial growth. However, in sharp contrast with the ample studies focusing on European earthworms, the limited number of studies examining jumping worms severely hinders our understanding on potential ecological consequences, such as how trees and other animals may be affected.
Jumping worms are most often transported as juveniles or cocoons through horticultural products, especially mulches, potting mixes, and compost. The practice of raking or blowing leaves can mobilize and concentrate earthworms and cocoons, creating centers of high jumping worm abundance in leaf piles and yard waste bags, which further facilitate their spreading. Prevention of introduction or spreading is the most effective way of controlling the invasion of jumping worms into new habitats. In the USA, some states have legally restricted the movement of jumping worms within and across state boundaries. For practitioners and land managers, changing horticultural and landscaping practice may be the most effective way to control jumping worms in their properties. Long-term monitoring and field experimental studies are urgently needed to be coupled with well-designed laboratory experiments to address the ecological questions and concerns raised by jumping worm invasion. Scientists as well as the general public can benefit from community science projects that help detect the occurrence of jumping worms and monitor their spread and long-term dynamics.
References
Angst G, Mueller CW, Prater I et al (2019) Earthworms act as biochemical reactors to convert labile plant compounds into stabilized soil microbial necromass. Commun Biol 2:441
Armstrong GW, Mahmood A, Nugent A et al (2017) WORMSPREAD: an individual-based model of invasive earthworm population dynamics. Comput Ecol Softw 7:109–122
Aspe NM, James SW (2018) Molecular phylogeny and biogeographic distribution of pheretimoid earthworms (clitellata: Megascolecidae) of the Philippine archipelago. Eur J Soil Biol 85:89–97
Bal TL, Storer AJ, Jurgensen MF (2018) Evidence of damage from exotic invasive earthworm activity was highly correlated to sugar maple dieback in the Upper Great Lakes region. Biol Invasions 20:151–164
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2:805–814
Barros E, Grimaldi M, Sarrazin M et al (2004) Soil physical degradation and changes in macrofaunal communities in Central Amazon. Appl Soil Ecol 26:157–168
Bartz MLC, Brown GG, da Rosa MG et al (2014a) Earthworm richness in land-use systems in Santa Catarina, Brazil. Appl Soil Ecol 83:59–70
Bartz MLC, Brown GG, Pasini A et al (2009) Earthworm communities in organic and conventional coffee cultivation. Pesqui Agropecu Bras 44:928–933
Bartz MLC, Pasini A, Brown GG (2014b) Earthworm richness, abundance and biomass in different land use systems in northern Paraná, Brazil (Oligochaeta). In: Pavlíček T, Cardet P, Almeida MT, Pascoal C, Cássio F (eds) Advances in earthworm taxonomy VI (Annelida: Oligochaeta). Kasparek Verlag, Heidelberg, pp 59–73
Beausejour R, Handa IT, Lechowicz MJ et al (2015) Historical anthropogenic disturbances influence patterns of non-native earthworm and plant invasions in a temperate primary forest. Biol Invasions 17:1267–1281
Bellitürk K, Görres JH, Kunkle J et al (2015) Can commercial mulches be reservoirs of invasive earthworms? Promotion of ligninolytic enzyme activity and survival of Amynthas agrestis (Goto and Hatai, 1899). Appl Soil Ecol 87:27–31
Bernard MJ, Neatrour MA, McCay TS (2009) Influence of soil buffering capacity on earthworm growth, survival, and community composition in the Western Adirondacks and Central New York. Northeast Nat 16:269–284
Bethke PG, Midgley MG (2020) Amynthas spp. impacts on seedlings and forest soils are tree species-dependent. Biol Invasions 22:3145–3162
Blackmon JH (2009) The use of fire in the control of invasive, epigeic earthworms species in the Southeastern United States. (MS Thesis) University of Georgia, Athens, GA, USA.
Blackmon JH, Taylor MK, Carrera-Martinez R et al (2019) Temperature affects hatching success of cocoons in the invasive Asian earthworm Amynthas agrestis from the Southern Appalachians. Southeast Nat 18:270–280
Blakemore RJ (2000) Cosmopolitan earthworms- an eco-taxonomic guide to the peregrine species of the world. VermEcology, pp. 426
Blakemore RJ (2005) A series of searchable texts on earthworm biodiversity, ecology and systematics from various regions of the world. Yokohama National University, Yokohama
Bohlen PJ, Pelletier DM, Groffman PM et al (2004a) Influence of earthworm invasion on redistribution and retention of soil carbon and nitrogen in northern temperate forests. Ecosystems 7:13–27
Bohlen PJ, Scheu S, Hale CM et al (2004b) Non-native invasive earthworms as agents of change in northern temperate forests. Front Ecol Environ 2:427–435
Bouché MB (1972) Lombriciens de France. Écologie et Systématique. Institut National de la Recherche Agronomique, Dijon, France
Bouché MB (1977) Strategies lombriciennes. In: Lohm U and Persson T (eds) Soil organisms as components of ecosystems. Ecological Bulletins Stockholm, pp 122–132
Bowe A, Dobson A, Blossey B (2020) Impacts of invasive earthworms and deer on native ferns in forests of northeastern North America. Biol Invasions 22:1431–1445
Brown A (1878) Plants introduced with ballast and on made land. Bull Torrey Bot Club 6(45):255–258
Brown GG, James SW, Pasini A et al (2006) Exotic, peregrine, and invasive earthworms in Brazil: diversity, distribution, and effects on soils and plants. Carib J Sci 42:339–358
Burtelow AE, Bohlen PJ, Groffman PM (1998) Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Appl Soil Ecol 9:197–202
Burton TM, Likens GE (1975) Energy flow and nutrient cycling in salamander populations in Hubbard Brook Experimental Forest, New Hampshire. Ecology 56:1068–1080
Butt KR (1991) The effects of temperature on the intensive production of Lumbricus terrestris (Oligochaeta, Lumbricidae). Pedobiologia 35:257–264
Butt KR (2011) Food quality affects production of Lumbricus terrestris (L.) under controlled environmental conditions. Soil Biol Biochem 43:2169–2175
CABI (2018) Amynthas agrestis. Invasive Species Compendium. CAB International, Wallingford, UK
Callaham MA, Hendrix PF, Phillips RJ (2003) Occurrence of an exotic earthworm (Amynthas agrestis) in undisturbed soils of the southern Appalachian Mountains, USA. Pedobiologia 47:466–470
Callaham MA, Gonzalez G, Hale CM et al (2006) Policy and management responses to earthworm invasions in North America. Biol Invasions 8:1317–1329
Cameron EK, Bayne EM (2009) Road age and its importance in earthworm invasion of northern boreal forests. J Appl Ecol 46:28–36
Cameron EK, Bayne EM (2012) Invasion by a non-native ecosystem engineer alters distribution of a native predator. Divers Distrib 18:1190–1198
Chang CH, Chen JH (2005) Taxonomic status and intraspecific phylogeography of two sibling species of Metaphire (Oligochaeta: Megascolecidae) in Taiwan. Pedobiologia 49:591–600
Chang CH, James S (2011) A critique of earthworm molecular phylogenetics. Pedobiologia 54:S3–S9
Chang CH, Lin YH, Chen IH et al (2007) Taxonomic re-evaluation of the Taiwanese montane earthworm Amynthas wulinensis Tsai, Shen & Tsai, 2001 (Oligochaeta: Megascolecidae): Polytypic species or species complex? Org Divers Evol 7:231–240
Chang CH, Lin SM, Chen JH (2008) Molecular systematics and phylogeography of the gigantic earthworms of the Metaphire formosae species group (Clitellata, Megascolecidae). Mol Phylogenet Evol 49:958–968
Chang CH, Shen HP, Chen JH (2009a) Earthworm fauna of Taiwan. National Taiwan University Press, Taipei
Chang CH, Rougerie R, Chen JH (2009b) Identifying earthworms through DNA barcodes: Pitfalls and promise. Pedobiologia 52:171–180
Chang CH, Snyder BA, Szlavecz K (2016a) Asian pheretimoid earthworms in North America north of Mexico: an illustrated key to the genera Amynthas, Metaphire, Pithemera, and Polypheretima (Clitellata: Megascolecidae). Zootaxa 4179:495–529
Chang CH, Szlavecz K, Buyer JS (2016b) Species-specific effects of earthworms on microbial communities and the fate of litter-derived carbon. Soil Biol Biochem 100:129–139
Chang CH, Szlavecz K, Filley T et al (2016c) Belowground competition among invading detritivores. Ecology 97:160–170
Chang CH, Szlavecz K, Buyer JS (2017) Amynthas agrestis invasion increases microbial biomass in Mid-Atlantic deciduous forests. Soil Biol Biochem 114:189–199
Chang CH, Johnston MR, Görres JH et al (2018) Co-invasion of three Asian earthworms, Metaphire hilgendorfi, Amynthas agrestis and Amynthas tokioensis in the USA. Biol Invasions 20:843–848
Chkrebtii OA, Cameron EK, Campbell DA et al (2015) Transdimensional approximate Bayesian computation for inference on invasive species models with latent variables of unknown dimension. Comput Stat Data Anal 86:97–110
Cope CG, Burns JH (2019) Effects of native deer on invasive earthworms depend on earthworm functional feeding group and correlate with earthworm body size. For Ecol Manage 435:180–186
Craven D, Thakur MP, Cameron EK et al (2017) The unseen invaders: introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Glob Change Biol 23:1065–1074
Csuzdi C, Pavlíček T, Nevo E (2008) Is Dichogaster bolaui (Michaelsen, 1891) the first domicole earthworm species? Eur J Soil Biol 44:198–201
Cunha L, Campos I, Montiel R et al (2011) Morphometry of the epidermis of an invasive megascolecid earthworm (Amynthas gracilis, Kinberg 1867) inhabiting actively volcanic soils in the Azores archipelago. Ecotoxicol Environ Saf 74:25–32
Cunha L, Thornber A, Kille P et al (2017) A large set of microsatellites for the highly invasive earthworm Amynthas corticis predicted from low coverage genomes. Appl Soil Ecol 119:152–155
Darwin C (1881) The formation of vegetable mould through the action of worms with some observations on their habits. John Murray, London
Davalos A, Simpson E, Nuzzo V et al (2015) Non-consumptive effects of native deer on introduced earthworm abundance. Ecosystems 18:1029–1042
Demetrio W, Assis O, Niva CC et al (2020) Comparison of soil invertebrate communities in organic and conventional production systems in Southern Brazil. Soil Org 92:143–156
Dobson A, Blossey B (2015) Earthworm invasion, white-tailed deer and seedling establishment in deciduous forests of north-eastern North America. J Ecol 103:153–164
Dobson AM, Blossey B, Richardson JB (2017) Invasive earthworms change nutrient availability and uptake by forest understory plants. Plant Soil 421:175–190
Eisenhauer N (2010) The action of an animal ecosystem engineer: Identification of the main mechanisms of earthworm impacts on soil microarthropods. Pedobiologia 53:343–352
Fahey TJ, Yavitt JB, Sherman RE, Maerz JC, Groffman PM, Fisk MC, Bohlen PJ (2013) Earthworms, litter and soil carbon in a northern hardwood forest. Biogeochemistry 114:269–280
Ferlian O, Eisenhauer N, Aguirrebengoa M et al (2018) Invasive earthworms erode soil biodiversity: a meta-analysis. J Anim Ecol 87:162–172
Ferlian O, Thakur MP, Gonzalez AC et al (2020) Soil chemistry turned upside down: a meta-analysis of invasive earthworm effects on soil chemical properties. Ecology 101:12
Ferreira T, Santos A, Demetrio WC et al (2018) Earthworm species in public parks in Curitiba, Parana, Brazil. Zootaxa 4496:535–547
Fragoso C, Brown GG (2007) Ecología y taxonomía de las lombrices de tierra en Latino-América: el primer Encuentro Latino-Americano de Ecología y Taxonomía de Oligoquetas (ELAETAO1). In: Brown GG and Fragoso C (eds) Minhocas na América Latina: biodiversidade e ecologia. Embrapa Soja, Londrina, pp 33–75
Fragoso C, Rojas P (2014) Biodiversidad de lombrices de tierra (Annelida: Oligochaeta: Crassiclitellata) en México. Rev Mex Biodivers 85:S197–S207
Frelich LE, Blossey B, Cameron EK et al (2019) Side-swiped: ecological cascades emanating from earthworm invasions. Front Ecol Environ 17:502–510
Gao MX, Taylor MK, Callaham MA (2017) Trophic dynamics in a simple experimental ecosystem: Interactions among centipedes, Collembola and introduced earthworms. Soil Biol Biochem 115:66–72
Garcia JA, Fragoso C (2002) Growth, reproduction and activity of earthworms in degraded and amended tropical open mined soils: laboratory assays. Appl Soil Ecol 20:43–56
Gates GE (1956) Reproductive organ polymorphism in earthworms of the oriental megascolecine genus Pheretima Kinberg 1867. Evolution 10:213–227
Gates GE (1958) On some species of the oriental earthworm genus Pheretima Kinberg, 1867, with a key to species reported from the Americas. Am Mus Novit 1888:1–33
Gates GE (1972) Burmese earthworms—introduction to systematics and biology of megadrile oligochaetes with special reference to Southeast Asia. Trans Am Philos Soc 62:5–324
Gates GE (1973) Contributions to North American earthworms (Annelida). No. 6: contributions to a revision of the earthworm family Glossoscolecidae. I. Pontoscolex corethrurus (Müller, 1857). Bull Tall Timbers Res Station 14:1–12
Gates GE (1982) Farewell to North American Megadriles. Megadrilogica 4:12–77
Görres JH, Melnichuk RDS (2012) Asian invasive earthworms of the genus Amynthas Kinberg in Vermont. Northeast Nat 19:313–322
Görres J, Bellitürk K, Keller E (2014) Failure of an Amynthas agrestis (Goto & Hatai 1899) (Oligochaeta: Megascolecidae) population to expand its range within a sugar maple (Acer saccharum) stand. Megadrilogica 17:7–13
Görres JH, Bellitürk K, Melnichuk RDS (2016) Temperature and moisture variables affecting the earthworms of genus Amynthas Kinberg, 1867 (Oligachaeta: Megascolecidae) in a hardwood forest in the Champlain Valley, Vermont, USA. Appl Soil Ecol 104:111–115
Görres JH, Connolly ST, Chang CH et al (2018) Winter hatching in New England populations of invasive pheretimoid earthworms Amynthas agrestis and Amynthas tokioensis: a limit on population growth, or aid in peripheral expansion? Biol Invasions 20:1651–1655
Görres JH, Martin C, Nouri-Aiin M et al (2019) Physical properties of soils altered by invasive pheretimoid earthworms: does their casting layer create thermal refuges? Soil Syst 3:52
Gorsuch JP, Owen PC (2014) Potential edaphic and aquatic predators of a nonindigenous Asian earthworm (Amynthas agrestis) in the Eastern United States. Northeast Nat 21:652–661
Greiner HG, Kashian DR, Tiegs SD (2012) Impacts of invasive Asian (Amynthas hilgendorfi) and European (Lumbricus rubellus) earthworms in a North American temperate deciduous forest. Biol Invasions 14:2017–2027
Griffith B, Turke M, Weisser WW et al (2013) Herbivore behavior in the anecic earthworm species Lumbricus terrestris L.? Eur J Soil Biol 55:62–65
Gu JQ, Chen X, Wang YF, et al. (2020) Bioaccumulation, physiological distribution, and biotransformation of tetrabromobisphenol a (TBBPA) in the geophagous earthworm Metaphire guillelmi - hint for detoxification strategy. J Hazard Mater 388
Hale CM, Frelich LE, Reich PB (2005) Exotic European earthworm invasion dynamics in northern hardwood forests of Minnesota, USA. Ecol Appl 15:848–860
Hardy CR, Hardy NW (2018) Adapting traditional field activities in natural history education to an emerging paradigm in biodiversity informatics. Am Biol Teach 80:501–519
Hasegawa M, Sugiura S, Ito MT et al (2009) Community structures of soil animals and survival of land snails on an island of the Ogasawara Archipelago. Pesqui Agropecuaria Bras 44:896–903
Hendrix PF, Bohlen PJ (2002) Exotic earthworm invasions in North America: ecological and policy implications. Bioscience 52:801–811
Hendrix PF, Baker GH, Callaham MA et al (2006) Invasion of exotic earthworms into ecosystems inhabited by native earthworms. Biol Invasions 8:1287–1300
Hendrix PF, Callaham MA, Drake JM et al (2008) Pandora’s box contained bait: the global problem of introduced earthworms. Annu Rev Ecol Evol Syst 39:593–613
Hickerson CAM, Anthony CD, Walton BM (2017) Eastern red-backed salamanders regulate top-down effects in a temperate forest-floor community. Herpetologica 73:180–189
Holdsworth AR, Frelich LE, Reich PB (2007) Regional extent of an ecosystem engineer: earthworm invasion in northern hardwood forests. Ecol Appl 17:1666–1677
Holmstrup M, Westh P (1994) Dehydration of earthworm cocoons exposed to cold—a novel cold-hardiness mechanism. J Comp Physiol B Biochem Syst Environ Physiol 164:312–315
Houchins CS (1995) Artifacts of diplomacy: smithsonian collections from Commodore Matthew Perry's Japan expedition (1853–1854). Smithsonian Institution Press, Washington D.C.
Ikeda H, Tsuchiya Y, Nagata N et al (2012) Altitudinal life-cycle and body-size variation in ground beetles of the genus Carabus (subgenus Ohomopterus) in relation to the temperature conditions and prey earthworms. Pedobiologia 55:67–73
Ikeda H, Callaham MA, O’Brien JJ et al (2015) Can the invasive earthworm, Amynthas agrestis, be controlled with prescribed fire? Soil Biol Biochem 82:21–27
Ikeda H, Fukumori K, Shoda-Kagaya E et al (2018) Evolution of a key trait greatly affects underground community assembly process through habitat adaptation in earthworms. Ecol Evol 8:1726–1735
Imaizumi Y (1979) Hunting methods in relation to hunting situations in Japanese shrew-mole, Urotrichus talpoides. II: detection of the earthworm “head.” Annot Zool Jpn 52:212–224
Imaizumi Y (1983) Hunting of moles. In: Hidaka T (ed) Meanings of animal behaviors. Toukai Daigaku Shuppankai, Tokyo, Japan, pp 123–147
Ishizuka K (2001) Taxonomic study of the genus Pheretima s. lat. (Oligochaeta, Megascolecidae) from Japan. Bull Seikei Univ 33:1–125
Ishizuka K, Minagoshi Y (2014) Pictorial book of earthworm. Zenkoku Noson Kyoiku Kyokai Co. Ltd., Tokyo
Johnston MR, Herrick BM (2019) Cocoon heat tolerance of pheretimoid earthworms Amynthas tokioensis and Amynthas agrestis. Am Midl Nat 181:299–309
Jordan RC, Gray SA, Howe DV et al (2011) Knowledge gain and behavioral change in citizen-science programs. Conserv Biol 25:1148–1154
Kaneko N (2018) Soil ecology. Asakura Shoten, Tokyo (in Japanese)
Kaplan DL, Hartenstein R, Neuhauser EF (1980) Coprophagic relations among the earthworms Eisenia foetida, Eudrilus eugeniae and Amynthas spp. Pedobiologia 20:74–84
Keller EL, Görres JH, Schall JJ (2017) Genetic structure of two invasive earthworms, Amynthas agrestis and Amynthas tokioensis (Oligochaeta, Megascolecidae), and a molecular method for species identification. Megadrilogica 22:140–148
Kernecker M, Whalen JK, Bradley RL (2014) Litter controls earthworm-mediated carbon and nitrogen transformations in soil from temperate riparian buffers. Appl Environ Soil Sci 2014:329031
Kobiyama M, Barcik C, Santos HR (1995) Influência da minhoca (Amynthas hawayanus) sobre a produção de matéria seca de Bracatinga (Mimosa scabrella Benth). Rev Setor Ciências Agrárias 13:199–203
Laricchia KM, Johnson MG, Ragone D et al (2018) A transcriptome screen for positive selection in domesticated breadfruit and its wild relatives (Artocarpus spp.). Am J Bot 105:915–926
Laushman KM, Hotchkiss SC, Herrick BM (2018) Tracking an invasion: community changes in hardwood forests following the arrival of Amynthas agrestis and Amynthas tokioensis in Wisconsin. Biol Invasions 20:1671–1685
Loss SR, Blair RB (2011) Reduced density and nest survival of ground-nesting songbirds relative to earthworm invasions in northern hardwood forests. Conserv Biol 25:983–992
Loss SR, Niemi GJ, Blair RB (2012) Invasions of non-native earthworms related to population declines of ground-nesting songbirds across a regional extent in northern hardwood forests of North America. Landsc Ecol 27:683–696
Lubbers IM, van Groenigen KJ, Fonte SJ et al (2013) Greenhouse-gas emissions from soils increased by earthworms. Nat Clim Change 3:187–194
Lubbers IM, Pulleman MM, Van Groenigen JW (2017) Can earthworms simultaneously enhance decomposition and stabilization of plant residue carbon? Soil Biol Biochem 105:12–24
Lucky A, Savage AM, Nichols LM et al (2014) Ecologists, educators, and writers collaborate with the public to assess backyard diversity in The School of Ants Project. Ecosphere 5:78
Ma YN, Filley TR, Szlavecz K et al (2014) Controls on wood and leaf litter incorporation into soil fractions in forests at different successional stages. Soil Biol Biochem 69:212–222
Maschio W, Vezzani FM, Brown GG (2014) Earthworm populations in Eucalyptus spp. plantations at Embrapa Forestry, Brazil (Oligochaeta). Proceedings of the 6th International Oligochaeta Taxonomy Meeting (6th IOTM). Heidelberg: Kasparek Verlag, Palmeira de Faro, pp 114–126
McCay TS, Scull P (2019) Invasive lumbricid earthworms in northeastern North American forests and consequences for leaf-litter fauna. Biol Invasions 21:2081–2093
McCay TS, Brown G, Callaham M et al (2020) Tools for monitoring and study of peregrine pheretimoid earthworms (Megascolecidae). Pedobiologia 83:150669. https://doi.org/10.1016/j.pedobi.2020.150669
McMahon SM, Parker GG, Miller DR (2010) Evidence for a recent increase in forest growth. Proc Natl Acad Sci USA 107:3611–3615
Medina-Sauza RM, Alvarez-Jimenez M, Delhal A et al (2019) Earthworms building up soil microbiota, a review. Front Environ Sci 7:81
Melnichuk R (2016) Earthworms in Vermont forest soils: a study of nutrient, carbon, nitrogen and native plant responses. (PhD Thesis) University of Vermont, Vermont, USA
Minamiya Y, Tamura F, Maruyama K et al (2009) On the earthworm fauna of main campus and adjunctive faculties of Nara University of Education, Nara prefecture, central Japan. Bull Center Nat Environ Educ 9:1–4
Minamiya Y, Tamura F, Torii H et al (2010) On the megadrile earthworm fauna of Kinki district, central Japan. Bull Kansai Org Nat Conserv 32:113–125
Minamiya Y, Hayakawa H, Ohga K et al (2011) Variability of sexual organ possession rates and phylogenetic analyses of a parthenogenetic Japanese earthworm, Amynthas vittatus (Oligochaeta: Megascolecidae). Genes Genet Syst 86:27–35
Minamiya Y, Niwa S, Honma K et al (2013) Earthworm fauna of Sado Island compared to those of mainland Niigata Prefecture. Bull Saitama Mus Nat Hist 1:67–78
Minamiya Y, Ikeda H, Kaneko N (2015) Earthworm fauna of Aomori Prefecture, northern Japan. J Aomori Prefect Hist 20:81–90
Moore JD, Ouimet R, Bohlen PJ (2013) Effects of liming on survival and reproduction of two potentially invasive earthworm species in a northern forest Podzol. Soil Biol Biochem 64:174–180
Moore JD, Görres JH, Reynolds JW (2018) Exotic Asian pheretimoid earthworms (Amynthas spp., Metaphire spp.): Potential for colonisation of south-eastern Canada and effects on forest ecosystems. Environ Rev 26:113–120
Nakamura M (1994) Earthworms (Annelida: Oligochaeta) of Ogasawara Archipelagos. Chuo Daigaku Ronshu 15:21–32
Nakamura M (1997) Ecological study of earthworms in Galapagos archipelago. Chuo Daigakku Occas Pap 18:1–12
Nelson JC (1917) The introduction of foreign weeds in ballast as illustrated by ballast-plants at Linnton. Oregon Torreya 17(9):151–160
Nguyen VT, Tran VG, Nguyen TT et al (2018) Genetic diversity of earthworm Amynthas rodericensis (Grube, 1879) (Clitellata: Megascolecidae) in Vietnam by randomly amplified polymorphic DNA analysis. J Chem Biol Phys Sci 8:870–883
Nouri-Aiin M, Görres JH (2019) Earthworm cocoons: the cryptic side of invasive earthworm populations. Appl Soil Ecol 141:54–60
Nouri-Aiin M, Schall JJ, Keough CA, Wen Y, Görres JH (2021) Identifying the unidentifiable: a PCR multiplex protocol for the diagnosis of invasive pheretimoid earthworm species, verified by morphological and barcode identification. Appl Soil Ecol 161:103822. https://doi.org/10.1016/j.apsoil.2020.103822
Novo M, Cunha L, Maceda-Veiga A et al (2015) Multiple introductions and environmental factors affecting the establishment of invasive species on a volcanic island. Soil Biol Biochem 85:89–100
Nuzzo VA, Maerz JC, Blossey B (2009) Earthworm invasion as the driving force behind plant invasion and community change in northeastern North American forests. Conserv Biol 23:966–974
Nuzzo V, Davalos A, Blossey B (2015) Invasive earthworms shape forest seed bank composition. Divers Distrib 21:560–570
O’Keefe K, McCulloh KA (2021) Do invasive jumping worms impact sugar maple (Acer saccharum) water-use dynamics in a Central Hardwoods forest? Biol Invasions 23:129–141
Okuzaki Y, Sota T (2018) Predator size divergence depends on community context. Ecol Lett 21:1097–1107
Paudel S, Longcore T, MacDonald B et al (2016) Belowground interactions with aboveground consequences: invasive earthworms and arbuscular mycorrhizal fungi. Ecology 97:605–614
Peixoto RT, Marochi AI (1996) A influência da minhoca Pheretima sp. nas propriedades de um latossolo vermelho escuro álico e no desenvolvimento de culturas em sistema de plantio direto, em Arapoti-PR. Plantio Direto 35:23–25
Pelosi C, Bertrand M, Makowski D et al (2008) WORMDYN: a model of Lumbricus terrestris population dynamics in agricultural fields. Ecol Model 218:219–234
Perez-Losada M, Eiroa J, Mato S, Dominguez J (2005) Phylogenetic species delimitation of the earthworms Eisenia fetida (Savigny, 1826) and Eisenia andrei Bouche, 1972 (Oligochaeta, Lumbricidae) based on mitochondrial and nuclear DNA sequences. Pedobiologia 49:317–324
Perrier E (1872) Recherches pour server a l’histoire des lombriciens terrestres. Nouvelles Arch Mus D’hist Nat Paris 8:5–198
Petranka JW (1998) Salamanders of the United States and Canada. Smithsonian Institution Press, Washington, DC
Pinder RA (2013) Ecology of earthworms in riparian habitats. University at Albany, New York
Pinder RA, Robinson GR (2019) Niche-structured assemblages of exotic earthworms in headwater streambanks in eastern New York State, USA. Pedobiologia 75:15–23
Porco D, Decaens T, Deharveng L et al (2013) Biological invasions in soil: DNA barcoding as a monitoring tool in a multiple taxa survey targeting European earthworms and springtails in North America. Biol Invasions 15:899–910
Price-Christenson GJ, Johnston MR, Herrick BM et al (2020) Influence of invasive earthworms (Amynthas spp) on Wisconsin forest soil microbial communities and soil chemistry. Soil Biol Biochem 149:107955. https://doi.org/10.1016/j.soilbio.2020.107955
Prudic KL, Oliver JC, Brown BV et al (2018) Comparisons of citizen science data-gathering approaches to evaluate urban butterfly diversity. Insects 9(4):186
Qiu JX, Turner MG (2017) Effects of non-native Asian earthworm invasion on temperate forest and prairie soils in the Midwestern US. Biol Invasions 19:73–88
Ransom TS (2012) Comparison of direct, indirect, and ecosystem engineering effects of an earthworm on the red-backed salamander. Ecology 93:2198–2207
Redmond CT, Saeed A, Potter DA (2016) Seasonal biology of the invasive green stinkworm Amynthas hupeiensis and control of its casts on golf putting greens. Crop Forage Turfgrass MANAG 2:1–9
Resner K, Yoo K, Sebestyen SD et al (2015) Invasive earthworms deplete key soil inorganic nutrients (Ca, Mg, K, and P) in a northern hardwood forest. Ecosystems 18:89–102
Reynolds JW (2018) First earthworm (Annelida: Oligochaeta) species’ collections in Canada and the continental United States. Megadrilogica 23:1–50
Richardson DR, Snyder BA, Hendrix PF (2009) Soil moisture and temperature: tolerances and optima for a non-native earthworm species, Amynthas agrestis (Oligochaeta: Opisthopora: Megascolecidae). Southeast Nat 8:325–334
Richardson JB (2019) Trace elements in surface soils and megascolecidae earthworms in urban forests within four land-uses around Poughkeepsie, New York, USA. Bull Environ Contam Toxicol 103:385–390
Richardson JB, Görres JH, Jackson BP et al (2015) Trace metals and metalloids in forest soils and exotic earthworms in northern New England, USA. Soil Biol Biochem 85:190–198
Richardson JB, Renock DJ, Görres JH et al (2016) Nutrient and pollutant metals within earthworm residues are immobilized in soil during decomposition. Soil Biol Biochem 101:217–225
Rimmington OJ (2018) The mechanisms of evolutionary flexibility in earthworm genomes. (Ph.D. Thesis) Cardiff University, Cardiff, UK.
Rougerie R, Decaens T, Deharveng L et al (2009) DNA barcodes for soil animal taxonomy. Pesqui Agropecuaria Bras 44:789–802
Sackett TE, Smith SM, Basiliko N (2012) Exotic earthworm distribution in a mixed-use northern temperate forest region: influence of disturbance type, development age, and soils. Can J for Res Revue Can Rech for 42:375–381
Schmidt O (1999) Intrapopulation variation in carbon and nitrogen stable isotope ratios in the earthworm Aporrectodea longa. Ecol Res 14:317–328
Schult N, Pittenger K, Davalos S et al (2016) Phylogeographic analysis of invasive Asian earthworms (Amynthas) in the northeast United States. Invertebr Biol 135:314–327
Schwert DP, Dance KW (1979) Earthworm cocoons as a drift component in a southern Ontario stream. Can Field Nat 93:180–183
Scyphers SB, Powers SP, Akins JL et al (2015) The role of citizens in detecting and responding to a rapid marine invasion. Conserv Lett 8:242–250
Shartell LM, Lilleskov EA, Storer AJ (2013) Predicting exotic earthworm distribution in the northern Great Lakes region. Biol Invasions 15:1665–1675
Shen HP, Tsai CF, Fang YP et al (2011) Parthenogenesis, polyploidy and reproductive seasonality in the Taiwanese mountain earthworm Amynthas catenus Tsai et al., 2001 (Oligochaeta, Megascolecidae). Pedobiologia 54:133–139
Sherlock E, Carpenter D (2009) An updated earthworm list for the British Isles and two new “exotic” species to Britain from Kew Gardens. Eur J Soil Biol 45:431–435
Sims RW, Gerard BM (1999) Earthworms: Synopses of the British fauna (new series). Linnean Society of London, London
Sizmur T, Hodson ME (2009) Do earthworms impact metal mobility and availability in soil? A review. Environ Pollut 157:1981–1989
Sizmur T, Richardson J (2020) Earthworms accelerate the biogeochemical cycling of potentially toxic elements: Results of a meta-analysis. Soil Biol Biochem 148:107865. https://doi.org/10.1016/j.soilbio.2020.107865
Sizmur T, Tilston EL, Charnock J et al (2011) Impacts of epigeic, anecic and endogeic earthworms on metal and metalloid mobility and availability. J Environ Monit 13:266–273
Sluys R (2016) Invasion of the flatworms. Am Sci 104:288–295
Snyder BA, Hendrix PF (2008) Current and potential roles of soil macroinvertebrates (earthworms, millipedes, and isopods) in ecological restoration. Restor Ecol 16:629–636
Snyder BA, Boots B, Hendrix PF (2009) Competition between invasive earthworms (Amynthas corticis, Megascolecidae) and native North American millipedes (Pseudopolydesmus erasus, Polydesmidae): effects on carbon cycling and soil structure. Soil Biol Biochem 41:1442–1449
Snyder BA, Callaham MA, Hendrix PF (2011) Spatial variability of an invasive earthworm (Amynthas agrestis) population and potential impacts on soil characteristics and millipedes in the Great Smoky Mountains National Park, USA. Biol Invasions 13:349–358
Snyder BA, Callaham MA, Lowe CN et al (2013) Earthworm invasion in North America: food resource competition affects native millipede survival and invasive earthworm reproduction. Soil Biol Biochem 57:212–216
Speratti AB, Whalen JK (2008) Carbon dioxide and nitrous oxide fluxes from soil as influenced by anecic and endogeic earthworms. Appl Soil Ecol 38:27–33
Steinberg DA, Pouyat RV, Parmelee RW et al (1997) Earthworm abundance and nitrogen mineralization rates along an urban-rural land use gradient. Soil Biol Biochem 29:427–430
Stokes AN, Ducey PK, Neuman-Lee L et al (2014) Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense). PLoS ONE 9:e100718
Szlavecz K, Chang CH, Burgess JL et al (2014) Earthworms (Annelida: Clitellata) of Plummers Island, Maryland, USA, with description of a new species. Proc Biol Soc Wash 126:312–322
Szlavecz K, Chang CH, Bernard MJ et al (2018) Litter quality, dispersal and invasion drive earthworm community dynamics and forest soil development. Oecologia 188:237–250
Taheri S, Pelosi C, Dupont L (2018) Harmful or useful? A case study of the exotic peregrine earthworm morphospecies Pontoscolex corethrurus. Soil Biol Biochem 116:277–289
Uchida T, Kaneko N (2004) Life history of Megascolecidae earthworms in forest soils at Kanagawa, Japan. Edaphologia 74:35–45
Ueno Y (1999) The first breeding record of the fairy pitta Pitta brachyura from Chugoku District, Honshu, Japan. Jpn J Ornithol 47:139–141
Walton BM (2013) Top-down regulation of litter invertebrates by a terrestrial salamander. Herpetologica 69:127–146
Wang K, Qiao YH, Zhang HQ et al (2018) Bioaccumulation of heavy metals in earthworms from field contaminated soil in a subtropical area of China. Ecotoxicol Environ Saf 148:876–883
Waqar A, Shah GM, Bakhat HF et al (2019) The earthworm species Pheretima hawayana influences organic wastes decomposition, nitrogen mineralization and maize N recovery. Eur J Soil Biol 90:1–8
Wyman RL (1998) Experimental assessment of salamanders as predators of detrital food webs: effects on invertebrates, decomposition and the carbon cycle. Biodivers Conserv 7:641–650
Yesilonis I, Szlavecz K, Pouyat R et al (2016) Historical land use and stand age effects on forest soil properties in the Mid-Atlantic US. For Ecol Manag 370:83–92
Zhang LL, Sechi P, Yuan ML et al (2016) Fifteen new earthworm mitogenomes shed new light on phylogeny within the Pheretima complex. Sci Rep 6:20096
Zhang WX, Hendrix PF, Snyder BA et al (2010) Dietary flexibility aids Asian earthworm invasion in North American forests. Ecology 91:2070–2079
Zicsi A, Szlavecz K, Csuzdi C (2011) Leaf litter acceptance and cast deposition by peregrine and endemic European lumbricids (Oligochaeta: Lumbricidae). Pedobiologia 54:S145–S152
Ziemba JL, Cameron AC, Peterson K et al (2015) Invasive Asian earthworms of the genus Amynthas alter microhabitat use by terrestrial salamanders. Can J Zool 93:805–811
Ziemba JL, Hickerson CAM, Anthony CD (2016) Invasive Asian earthworms negatively impact keystone terrestrial salamanders. PLoS ONE 11:e0151591
Ziter C, Turner MG (2019) No evidence of co-facilitation between a non-native Asian earthworm (Amynthas tokioensis) and invasive common buckthorn (Rhamnus cathartica) in experimental mesocosms. Biol Invasions 21:111–122
Ziter C, Herrick BM, Johnston MR et al (2021) Ready, set, go: Community science field campaign reveals habitat preferences of nonnative Asian earthworms in an urban landscape. Bioscience 71:280–291
Acknowledgements
We appreciate two anonymous reviewers for their constructive comments on an earlier version of this article. This review was conceptualized during the symposium “Diversity and Ecology of Invasive Megascolecid Earthworms”' supported by the Picker Interdisciplinary Science Institute at Colgate University, USA.
Funding
C.-H. Chang was supported by the Ministry of Science and Technology (MOST108-2621-B-002-001-MY3) and the Ministry of Education, Taiwan (Yushan Scholar Program); K. Szlavecz, the National Science Foundation (DEB-1855277); G. Brown, the Brazilian National Council for Scientific and Technological Development (310690/2017-0 and 404191/2019-3); J. Görres and M. Nouri-Aiin, a Hatch Grant (SAES—UVM Accession No. 1018366) and the Eppley Foundation for Research; T. McCay and D. McHugh, the Picker Interdisciplinary Science Institute at Colgate University, USA; M. Novo, a Ramón y Cajal Fellowship (RYC2018-024654-I) from the Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, CH., Bartz, M.L.C., Brown, G. et al. The second wave of earthworm invasions in North America: biology, environmental impacts, management and control of invasive jumping worms. Biol Invasions 23, 3291–3322 (2021). https://doi.org/10.1007/s10530-021-02598-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02598-1