Abstract
Effects of invasive European earthworms in North America have been well documented, but less is known about ecological consequences of exotic Asian earthworm invasion, in particular Asian jumping worms (Amynthas) that are increasingly reported. Most earthworm invasion research has focused on forests; some Amynthas spp. are native to Asian grasslands and may thrive in prairies with unknown effects. We conducted an earthworm-addition mesocosm experiment with before–after control-impact (BACI) design and a complementary field study in southern Wisconsin, USA, in 2014 to investigate effects of a newly discovered invasion of two Asian jumping worms (Amynthas agrestis and Amynthas tokioensis) on forest and prairie litter and soil nutrient pools. In both studies, A. agrestis and A. tokioensis substantially reduced surface litter (84–95 % decline in foliage litter mass) and increased total carbon, total nitrogen, and available phosphorus in the upper 0–5 cm of soils over the 4-month period from July through October. Soil inorganic nitrogen (ammonium– and nitrate–N) concentration increased across soil depths of 0–25 cm, with greater effects on nitrate–N. Dissolved organic carbon concentration also increased, e.g., 71–108 % increase in the mesocosm experiment. Effects were observed in both forest and prairie soils, with stronger effects in forests. Effects were most pronounced late in the growing season when earthworm biomass likely peaked. Depletion of the litter layer and rapid mineralization of nutrients by non-native Asian jumping worms may make ecosystems more susceptible to nutrient losses, and effects may cascade to understory herbs and other soil biota.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasion of non-native earthworms profoundly alters biodiversity and ecosystem function (Bohlen et al. 2004c; Lavelle et al. 2006; Hendrix et al. 2008; Blouin et al. 2013). Such invasions are particularly problematic in glaciated regions of North America that were historically devoid of native earthworms prior to European settlement (James 1995). Prior studies have revealed that earthworm invasions can accelerate litter loss and reduce soil horizon thickness, alter nutrient pools and dynamics (e.g., carbon, nitrogen and phosphorus), deplete soil cations (e.g., calcium, magnesium and potassium), increase soil microbial biomass and activities (e.g., in forest soils), shift understory plant community composition and diversity, and decrease other soil invertebrates (Bohlen et al. 2004c; Groffman et al. 2004; McLean et al. 2006; Blouin et al. 2013; Resner et al. 2015). However, such effects have largely been based on research on European earthworms (family Lumbricidae), and earthworms originating in other regions are less well studied. Of particular concern are non-native Asian earthworms in the genus Amynthas (family Megascolecidae), which have successfully invaded North America and other tropical/subtropical regions (Gates 1982; Burtelow et al. 1998; Greiner et al. 2012). Warming temperatures and longer growing seasons may facilitate northward expansion of some Amynthas species that currently have a more southerly distribution (Bohlen et al. 2004a). Moreover, most earthworm invasion research has focused on forests, but some Asian earthworms native to sedge and grassland communities (Masamichi et al. 2011; Ishizuka and Minagoshi 2014) may colonize other ecosystems besides forests with unknown effects. Hence, understanding the extent to which Amynthas spp. can alter soils and ecosystems is increasingly urgent.
Asian jumping worms (also known as ‘Asian crazy worms’ or ‘Alabama jumpers’) normally refer to a subgroup of epigeic (i.e., litter and surface dwellers) Amynthas spp. such as Amynthas agrestis and Amynthas tokioensis (Ishizuka 2001; Ikeda et al. 2015), that are noted for their rapid, snake-like movements and jumping behavior when disturbed. Their dispersal mechanisms are not well understood, but these earthworms can be unknowingly transported in mulch and nursery plants as adults or cocoons (Belliturk et al. 2015). Unlike many invasive European earthworms in temperate North America that grow more slowly and can live for multiple years, these jumping worms have an annual life cycle. Juveniles emerge in spring, grow rapidly to sexual maturity (within 60–90 days of hatching), then reproduce and die in the fall (Burtelow et al. 1998; Callaham et al. 2003; Greiner et al. 2012; Ikeda et al. 2015). The population persists overwinter via cocoons from which earthworms hatch the following spring. In addition, compared to many other epigeic European species that live in low densities, the Asian jumping worms can live at much higher densities (~10 times of European invaders) (Hale 2007), have notably larger body size (Greiner et al. 2010), exhibit greater dietary flexibility, and can outcompete other soil organisms such as millipedes (Snyder et al. 2011, 2013). Further, some Asian jumping worms are native to grasslands (Masamichi et al. 2011; Ishizuka and Minagoshi 2014) and may thrive in U.S. prairies. These trait differences suggest that Asian jumping worms may have stronger or different effects than European earthworms depending on the ecological context.
A population of Asian jumping worms was discovered at the University of Wisconsin–Madison Arboretum in October 2013. This was the first recorded presence of Amynthas spp. in Wisconsin, which had listed these earthworms as restricted species under Wisconsin’s Invasive Species Rule (Wisconsin Administrative Code Chapter NR 40). Genetic analyses conducted at Colgate University and Johns Hopkins University in 2015 determined that A. agrestis and A. tokioensis were both present (Brad Herrick, University of Wisconsin Arboretum, personal communication). While these two species vary somewhat in body length (A. agrestis: ~8 to 20 cm; A. tokioensis: ~6 to 15 cm), both are epigeic and similar in life history, feeding strategy, reproduction, and movement behavior (Uchida 2004; Uchida et al. 2004; Ishizuka and Minagoshi 2014). Despite their wide occurrence (e.g., A. agrestis has invaded at least 17 states across the eastern and southeastern U.S. since the first reported case in 1939; Gates 1982; Reynolds and Wetzel 2012; Ikeda et al. 2015), studies of these species are relatively infrequent. Most research to date has addressed their physiological tolerance (Richardson et al. 2009), dietary flexibility (Zhang et al. 2010), management and control strategies (Ikeda et al. 2015), and showed declining litter horizon and intensifying food resource competition due to their invasions (Snyder et al. 2013), yet their ecological consequences are not fully known.
We conducted an earthworm-addition mesocosm experiment and a complementary field study in 2014 to investigate effects of the incipient invasion of these two co-occurring Asian jumping worms. We asked: (1) What are the effects of A. agrestis and A. tokioensis invasion on litter quantity and soil nutrient pools (including organic matter, total carbon and nitrogen, available phosphorus, inorganic nitrogen, and dissolved organic carbon)? (2) Do effects on soil nutrients differ with depth (i.e., 0–5, 5–10, and 10–25 cm from the surface)? (3) How do effects on litter and soil nutrient pools differ between forest and prairie ecosystems? We hypothesized that the invasion of A. agrestis and A. tokioensis would increase foliage litter loss because of their high population density and associated feeding activities, and further lead to a transient enrichment of total nutrient pools (e.g., organic matter, total C and N) in the upper soil layers through mixing and incorporation of litter nutrients into soils (Table 1). We also hypothesized that invasion of A. agrestis and A. tokioensis would increase soil inorganic nitrogen and dissolved organic carbon through enhanced mineralization in earthworm guts, secretion of nutrients in the form of earthworm casts and mucus, and changes in soil physical structure that facilitate nutrients mobilization across soil profiles. Because A. agrestis and A. tokioensis are epigeic, we expected stronger effects on nutrient pools in the O horizon and topsoils. We also expected greater effects on forest litter than prairie, as deciduous leaf litter is normally lower in C:N ratio, easier to decompose (decomposed leaf litter is preferred food source for A. agrestis; Snyder et al. 2013), and likely more digestible relative to prairie graminoid litter with its higher C:N ratio and lignin content. However, we still expected to find significant effects in prairies because A. agrestis were found to stimulate ligninolytic enzyme activities (Belliturk et al. 2015), which could promote the consumption and decomposition of prairie graminoid litter.
Methods
Mesocosm experimental design
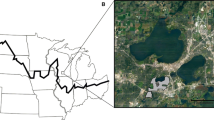
We conducted a paired and replicated mesocosm experiment with before-after control-impact (BACI) design in an open field facility at the University of Wisconsin-Madison Arboretum from July to October 2014. We used a BACI design because it can detect treatment effects while controlling for differences in initial conditions among sites (Underwood 1994). Intact soil cores (N = 99) with in situ leaf litter were collected from six representative uninvaded sites across southern Wisconsin (Fig. 1a). At each site, one forest and one adjoining prairie with the same soil type were selected, except for one site where prairie soils were too compacted to excavate (see online Appendix A for study area and site description). Within each forest and prairie, a 50-m transect was laid out in a random direction. At 0, 25, and 50-m marker along transect, a set of three intact soil cores (20-cm diameter × 25-cm depth) was collected within a 1-m radius.
a Non-invaded sites across southern Wisconsin where intact soil cores were collected for the mesocosm experiment. At each site, 20-cm diameter × 25-cm depth soil cores were excavated from a pair of adjoining forest and prairie ecosystems with similar soil type, except for the site of Goose Pond where only forest soil cores were collected. b Setup of the mesocosm experiment with random blocks in the field facility at the University of Wisconsin–Madison Arboretum (Photo credit: J. Qiu), and a close-up of a block with forest and prairie soil cores. Each earthworm treatment core was paired with a control. Letter “F” (also annotated ‘UW-Arboretum’) in A where the complementary field study was conducted
All soil cores were returned to the Arboretum field facility for incubation (Fig. 1b). Prior to adding earthworms for the mesocosm experiment, one soil core from each set of three was randomly chosen and destructively harvested to measure initial litter and soil conditions (Table 2). The remaining two were paired—one for introducing earthworms and the other as the control—and were placed into completely randomized blocks that accounted for microclimatic variation across the field facility (Fig. 1b). We installed shade cloth above the mesocosm and piled soils around all PVC cores to mimic field conditions. We then introduced six adult earthworms into a randomly selected core of each pair (Fig. 1b); this number of earthworms approximated the maximum density of Asian jumping worms (167/m2) measured in the adjacent invaded forest using mustard pours (in 30-cm × 30-cm quadrats, n = 15) and is consistent with other studies of the similar species (e.g., Greiner et al. 2012; Richardson et al. 2016). Because of dry conditions during summer 2014 and concern that death of the earthworms would cause the experiment to fail, we added two more earthworms in August. All earthworms were collected from the adjacent forest where the invasion was first discovered and earthworms had been collected for identification (Brad Herrick, University of Wisconsin Arboretum, personal communication). Density of Amynthas spp. at the end of the experiment averaged five per core (159/m2), indicating that the densities achieved in the experiment were consistent with the desired densities. At the end of the experiment, we only found three of the 66 cores with non-Amynthas earthworms (two in controls and one in earthworm treatment); each of those cores had a single one Lumbricus terrestris.
The top of each core was covered with a mesh layer to prevent earthworms from escaping, and the bottom was covered with a permeable fabric layer to allow vertical flow of water. Mesocosms (i.e., soil cores) were incubated under ambient conditions from July through October 2014. Throughout the experiment, mesocosms were monitored weekly for soil temperature and moisture, and deionized water was added consistently to all cores (with and without earthworms) as needed to maintain soil moisture, because earthworms in the cores could not move to more favorable sites and we wanted to minimize the likelihood that earthworms would desiccate and die. Cores were also inspected weekly for indications of earthworm activity and changes in surface soil and litter conditions. At the end of experiment, soil and litter samples were collected to measure the same properties as for the initial conditions (Table 1); all soil cores were then harvested to tally the earthworms remaining in each core.
Field study design
The mesocosm experiment tested for effects under controlled conditions but could not detect changes over time in situ. Hence, we also sampled litter and soil monthly in invaded and nearby uninvaded forests with similar soil conditions and stand structure at the University of Wisconsin-Madison Arboretum (Fig. 1). Both stands were dominated by sugar maple (Acer saccharum), interspersed with red maple (Acer rubrum), red oak (Quercus rubra), and American beech (Fagus grandifolia). At each invaded and uninvaded site, three 25-m2 plots were established at random locations separated by >50 m. Monthly sampling occurred the same day of each month from July through October 2014. For each sampling effort, litter and soil samples were collected at each plot from three randomly placed 30-cm × 30-cm quadrats, and the same properties were measured as for the mesocosm experiment (Table 1). Detailed sampling protocols are described below.
Sample collection and laboratory analyses
Protocols for litter and soil sampling and subsequent laboratory analyses were identical for the mesocosm experiment and field study. In brief, for each soil core (mesocosm experiment) or quadrat (field study), litter depth was recorded at three locations and averaged, and percent litter cover was visually estimated. All fresh litter was removed from each core or quadrat, and separated into foliage (e.g., dead leaves) and woody litter (e.g., twigs, fragments). Percent cover of earthworm castings in each mesocosm was visually estimated as an indication of earthworm activity. Litter was oven-dried at 70 °C for 48 h or until constant mass.
Ten soil subsamples were collected evenly across each mesocosm or quadrat using a 2.5-cm diameter soil probe sampler; soil samples were divided at incremental depths of 0–5, 5–10 and 10–25 cm, and pooled by depth. Soils were then homogenized and sieved (2-mm mesh) to remove fine roots, coarse fragments or gravel, and placed in a plastic bag and kept cool for transport to the laboratory.
For laboratory analyses, gravimetric soil moisture was first determined by wet and dry weights (oven-drying at 105 °C for 48 h). Subsamples were then oven-dried (70 °C for 48 h) and ground with a ball mill to pass through a 100 mesh (0.15 mm) sieve to determine total soil C and N. Total C and N were analyzed by high-temperature catalytic combustion using a Carlo-Erba Model NA 1500 C and N analyzer (CE Instruments, Milan, Italy). Other subsamples were air dried and sent to the Soils and Plant Analysis Lab at the University of Wisconsin-Madison for analyzing soil organic matter and available phosphorus. Soil organic matter was determined by dry combustion of a 5-g subsample using Tekmar-Dohrman 183 TOC Boat Sampler DC-190 (Tekmar-Dohrman, Mason OH), and available phosphorus was analyzed using Bray P1 extraction procedure (Bray and Kurtz 1945).
Inorganic N (\({\text{NH}}_{4}^{ + }\)-N and \({\text{NO}}_{3}^{ - }\)-N) was extracted within 24 h of soil sampling. We placed 8 ± 0.1 g of sieved (2-mm mesh) fresh subsamples of soil into centrifuge tubes with 40 ml of 2 M KCl. After capping and shaking the tubes for 1 h on an orbit shaker at 200 rpm, the samples were centrifuged (3000 rpm) for 10 min (Wiltshire and Laubscher 1989; Robertson et al. 1999). Supernatants were then extracted and filtered through 0.7 um Whatman GF/F glass microfiber filters, and frozen (−18 °C) until analysis. Concentrations of \({\text{NH}}_{4}^{ + }\)-N and \({\text{NO}}_{3}^{ - }\)-N were analyzed on a Technicon segmented flow autoanalyzer (Tarrytown, New York, USA). Dissolved organic carbon (DOC) was extracted following the same protocol as extractions for inorganic N, except for using 0.5 M K2SO4 solution (Balser and Firestone 2005). Extracts were frozen pending analysis. DOC concentrations were analyzed using a Shimadzu TOC-V carbon analyzer.
Statistical analyses
For the mesocosm experiment, we calculated changes between end and initial measurement of soil and litter properties. Effects of earthworms were tested using a general linear-mixed effect model in which the end measurement was the response variable and the initial condition was a covariate (Vickers 2001). Linear-mixed effect models were used to account for unbalanced design and inherent variation from random effects. Random effects included site (six locations where mesocosm soil cores were collected) and block (a factor nested within site that allows for comparison between the earthworm treatment and control in each pair). Earthworm presence, ecosystem type, soil depth, and their interactions were considered as fixed effects.
To evaluate earthworm effects from the field study, we used linear-mixed effect models to handle repeated measures and subsampling. To avoid pseudoreplication, samples taken within each plot were considered as subsamples, and monthly sampling efforts were treated as repeated measurements. Random effects included subsampling (nested within plot), and plot (as repeated statement), and fixed effects included earthworm presence, month, soil depth and their interactions. We interpreted a significant interaction (P < 0.05) between earthworm presence and month (a test for the rate of change) for a given soil depth as evidence of earthworm effects.
All statistical analyses were performed using R statistical software (R Development Core Team 2009). Models were fit using restricted maximum likelihood (REML), and the significance of differences in earthworm effects between levels of ‘soil depth’ and ‘ecosystem type’ was tested using Tukey’s multiple comparison with the glht function in the “multcomp” package (Hothorn et al. 2008). Residual plots were visually assessed for assumptions of normality and homogeneity of variance; no violations were detected. Linear-mixed effect models were analyzed using the “lme4” package (Bates et al. 2015), and significance of fixed effects was evaluated using the Satterthwaite’s approximation for degrees of freedom in the “lmerTest” package (Kuznetsova et al. 2014).
Results
Mesocosm experiment
Earthworm activities and leaf litter
Most A. agrestis and A. tokioensis survived in mesocosms for the incubation period, with an average of 5.0 ± 0.48 Asian jumping worms recovered. By the end of the experiment, earthworm casts covered >75 % of the surface area of soil cores that had earthworms introduced but were absent in controls (Fig. 2a).
Effects of A. agrestis and A. tokioensis on: a percent area with earthworm casts (%); b litter cover (%); c litter depth (mm); d foliage litter mass (g/m2) from the mesocosm experiment. Bars represent mean changes as compared to initial conditions for the earthworm treatment (black) and control (white), and error bars are standard errors (SE). Bars with different lowercase letters indicate a significant difference (α = 0.05) between the earthworm treatment and control from Tukey’s multiple comparison test
As expected, A. agrestis and A. tokioensis substantially reduced the litter layer in forest and prairie mesocosms (Fig. 2). Litter cover, litter depth and foliage litter mass declined more in earthworm treatments than in controls (all P < 0.001). While declines in litter cover and depth were comparable across ecosystems (Fig. 2b, c), declines in foliage litter mass were much greater in forest than prairie mesocosms (95 vs. 34 % loss respectively, relative to initial litter mass, Fig. 2d; P < 0.05, ANOVA model results in online Appendix B Table B1).
Soil nutrient pools
Soil nutrient pools were enriched in the earthworm treatment for both forest and prairie soils (Figs. 3, 4). Specifically, at 0–5 cm soil depths, percent organic matter, total C and total N content substantially increased in both forest and prairie soils with introduced A. agrestis and A. tokioensis (Fig. 3a, b, c). Changes in C and N content resulted in net increases of 2.2 and 0.8 in the C:N ratio of affected forest and prairie soils, respectively (Fig. 3d). The magnitude of effects on percent organic matter, total C and total N content did not differ between forest and prairie soils (online Appendix B Table B2). For example, soil organic matter increased 25 % relative to initial conditions in the earthworm treatment for 0-5 cm forest soils (vs. 16 % increase for 0–5 cm prairie soils). Available P increased (46 %) at 0–5 cm depth in forest soils, but no effects were found for prairie soils (Fig. 3e). These nutrients were not affected by the earthworms at greater depths (i.e., 5–10, 10–25 cm; online Appendix B Fig. B1).
Effects of A. agrestis and A. tokioensis on 0–5 cm soil: a organic matter (%); b total C (%); c total N (%); d total C:N ratio; e available P (ppm) from the mesocosm experiment. Bars represent mean changes relative to initial conditions for the earthworm treatment (black) and control (white), and error bars are standard errors (SE). Bars with different lowercase letters indicate a significant difference (α = 0.05) between the earthworm treatment and control from Tukey’s multiple comparison test
Effects of A. agrestis and A. tokioensis on: a nitrate (NO3 −) (mg N kg −1soil ); b ammonium (NH4 +) (mg N kg −1soil ); c total inorganic N (TIN) (mg N kg −1soil ); d total dissolved organic carbon (DOC) (mg C kg −1soil ) from the mesocosm experiment. Bars represent mean changes as compared to initial conditions for the earthworm treatment (black) and control (white), and error bars are standard errors (SE). Bars with different lowercase letters indicate a significant difference (α = 0.05) between the earthworm treatment and control from Tukey’s from Tukey’s multiple comparison test
As predicted, soil inorganic N and DOC concentrations were very responsive to the presence of A. agrestis and A. tokioensis (Fig. 4). Nitrate–N concentrations largely increased across all depths of forest soils in the earthworm treatment, but either declined or showed minimal increases in no-earthworm controls (Fig. 4a). The effects on nitrate–N differed by ecosystem (earthworm × ecosystem interaction, P < 0.001; online Appendix B Table B2), with greater effects in forest than prairie soils, in which significant effects were only observed for 0–5 cm soils (Fig. 4a). Ammonium–N concentration did not differ between earthworm treatments and controls, except for 0–5 cm prairie soils (Fig. 4b). Patterns of total inorganic N mirrored those of nitrate–N concentrations, largely because nitrate dominated the available inorganic forms of N (Fig. 4c). Effects on soil DOC concentrations were consistent and pervasive across all depths of forest and prairie soils. DOC concentrations increased from 71 to 108 % in the earthworm treatment relative to initial conditions (all P < 0.001; Fig. 4d).
Field study
Leaf litter
Presence of A. agrestis and A. tokioensis was associated with accelerated loss of forest leaf litter through time. From July through October, foliage litter mass declined by 84 % in invaded forests (most declines occurred from September to October), compared to 43 % in the adjacent uninvaded forests (P < 0.01, Fig. 5a). Litter depth also decreased more rapidly in invaded forests relative to uninvaded forests (79 vs. 43 %, respectively; Fig. 5b; online Appendix C Table C1).
Temporal changes in a foliage litter mass (g/cm2); b litter depth (mm) in forest invaded by A. agrestis and A. tokioensis (solid circle), and adjacent non-invaded forest (open circle) from the field study. Sampling occurred monthly from July to October 2014. Errors represent standard errors (N = 9), and P values are the significance level of the interaction term between month and absence/presence of earthworms
Soil nutrient pools
Changes in soil nutrient pools closely paralleled loss of leaf litter. In invaded forests, soil organic matter, total C and total N content in 0–5 cm soils did not change noticeably over the first three months, but increased sharply from September to October (Fig. 6a, b, c); in contrast, in uninvaded forests, these soil attributes remained constant or declined slightly. Soil C:N ratio did not change significantly in 0–5 cm soils in invaded forests, but declined from 15.1 to 12.9 in uninvaded forests (Fig. 6d). We also found that available P in the upper 5-cm of soils increased by 128 % in invaded forests from July to October (vs. 18 % in uninvaded forests) (Fig. 6e). Similar to the mesocosm experiment, effects on these nutrient pools were insignificant for soils deeper than 5-cm (online Appendix C Table C2 and Fig. C1).
Temporal changes in 0–5 cm soil: a organic matter (%); b total C (%); c total N (%); d total C:N ratio; e available P (ppm) in forest invaded by A. agrestis and A. tokioensis (solid circle), and adjacent non-invaded forest (open circle) from the field study. Sampling occurred monthly from July to October 2014. Errors represent standard errors (N = 9), and P values are the significance level of the interaction term between month and absence/presence of earthworms
Consistent with the mesocosm experiment, presence of A. agrestis and A. tokioensis substantially altered inorganic N and DOC concentrations in field conditions (Fig. 7). Nitrate–N concentrations increased in 0–5 and 5–10 cm soils over time in invaded forests but remained unchanged or declined in uninvaded areas (Fig. 7a). Effects on ammonium–N were also significant in the upper 5-cm of soils, where ammonium–N initially declined but spiked in the last month (Fig. 7b). Changes in total inorganic N again were akin to those of nitrate–N concentrations, with increases of 54 and 18 % from July through October in 0–5 cm and 5–10 cm invaded soils, respectively (vs. 17 and 5 % declines in respective uninvaded soils; Fig. 7c). DOC concentrations in 0–5 cm soils increased by 91 % in invaded forests, but was unchanged in uninvaded areas (P = 0.05; Fig. 7d). Effects on DOC concentrations at deeper soils (i.e., 10–25 cm) were weaker than those found in the mesocosm experiment.
Temporal changes in: a nitrate (NO3 −) (mg N kg −1soil ); b ammonium (NH4 +) (mg N kg −1soil ); c total inorganic N (TIN) (mg N kg −1soil ); d total dissolved organic carbon (DOC) (mg C kg −1soil ) in forest invaded by A. agrestis and A. tokioensis (solid circle), and adjacent non-invaded forest (open circle) from the field study. Sampling occurred monthly from July to October 2014. Errors represent standard errors (N = 9), and P values are the significance level of the interaction term between month and absence/presence of earthworms
Discussion
Invasion of A. agrestis and A. tokioensis substantially reduced leaf litter and enriched soil nutrient pools in temperate deciduous forest and prairie soils. The rapid transformation of organic matter into more mobile forms may change nutrient availability for plants and could potentially make ecosystems more susceptible to nutrient losses. Effects of Asian jumping worms were also significant in the prairies (at least in the experimental microcosms, as the worms had not yet been observed in situ in the prairies), which are of great conservation value (Samson and Knopf 1994). Thus, effects of Asian jumping worms might rival those of better-studied European invasive earthworms—the major drivers of ecological changes in northern temperate and boreal forests (Bohlen et al. 2004c). Given the potential northward expansion of Amynthas spp. (Bohlen et al. 2004a), this study provides timely insights into their ecological impacts.
Earthworm effects on leaf litter and soil nutrient pools
Effects of A. agrestis and A. tokioensis on leaf litter were consistent with our hypotheses and other studies that earthworm invasion accelerates leaf litter loss in northern temperate forests (Hale et al. 2008; Fahey et al. 2013). However, the magnitude of effects might exceed those of other earthworm species. In the present study, for example, A. agrestis and A. tokioensis caused a 63 % greater reduction in forest foliage litter mass in earthworm treatment than control in the 4-month experiment, and a 41 % more decline in litter mass in invaded than uninvaded forests under the field conditions. Greiner et al. (2012) reported 23 and 40 % more losses in forest leaf litter mass due to Amynthas hilgendorfi and Lumbricus rubellus, respectively, compared to uninvaded areas. In central New York, USA, Suarez et al. (2006) found 31 % more litter mass loss in hardwood forests invaded by Lumbricus rubellus and Octolasion tyrtaeum than control plots after 540 days of experiment. Great effects of A. agrestis and A. tokioensis on leaf litter corroborate findings of Snyder et al. (2011), and may be due to their high population densities (~10 times of other non-native earthworms) (Ikeda et al. 2015), dietary flexibility and superior feeding on the gram-positive soil bacteria essential to earthworms for litter digestion (Zhang et al. 2010). We also found significant effects on prairie litter, albeit of smaller magnitude than in forests, perhaps because of the lower C:N ratio of the deciduous foliage litter (Snyder et al. 2009).
Our results of nutrient enrichment in the upper 5-cm of soils agree with previous findings on Asian jumping worms (Snyder et al. 2009; Greiner et al. 2012), but contradict research on other earthworm species (Alban and Berry 1994; Suarez et al. 2004; Bohlen et al. 2004b; Eisenhauer et al. 2007; Crumsey et al. 2013). Such differences are possibly due to the relative dominance of two mechanisms that control earthworm effects on nutrient dynamics: (1) earthworm mixing and incorporation of litter nutrients into soils; (2) earthworm-induced nutrient losses through gaseous fluxes (e.g., CO2, N2O) (Speratti and Whalen 2008) and leaching (e.g., dissolved organic matter) (Bohlen et al. 2004b). It is likely that given high densities of Amynthas, the incorporation of litter nutrients into soils exceeds earthworm-induced nutrient losses, leading to a transient enrichment of nutrient pools in topsoils. Differences in effects can also be attributable to earthworms of distinct functional groups: A. agrestis and A. tokioensis are epigeic and exert greater effects on O horizon and topsoils, whereas for anecic species (which feed on surface litter but form deep vertical burrows, e.g., L. terrestris), effects on surface soils can be trivial (Edwards 2004). Finally, incubation time might be another factor. Our study focused on effects during one growing season, and thus revealed short-term effects. Established earthworm populations with a long history of invasion might lead to consequences that differ fundamentally from short-term effects (Bohlen et al. 2004a; Zhang et al. 2013).
Our hypotheses that invasion of A. agrestis and A. tokioensis increases soil inorganic N and DOC concentrations were supported (Figs. 4, 7). Increases in inorganic N are likely due to enhanced mineralization in earthworm guts, and addition of organic materials into soils that provide substrates for mineralization (Scheu and Parkinson 1994; Costello and Lamberti 2009; Lubbers et al. 2011). Other studies also found elevated inorganic N in topsoils due to invasive earthworms under experimental (Hale et al. 2008; Greiner et al. 2012) and field conditions (Szlavecz et al. 2006). The fact that inorganic N increased at greater depths than previously reported suggests that Asian jumping worms might have stronger effects on N transformations and pools than other earthworm species.
Soil DOC concentrations may be altered by A. agrestis and A. tokioensis through both biotic and/or abiotic mechanisms. Biotic factors include (1) enhanced mineralization by earthworm activities that promote DOC production (Kalbitz et al. 2000); (2) earthworm-induced increases in soil organic matter that acts as prime sources of DOC (Currie and Aber 1997; Tipping et al. 1999; Sackett et al. 2013); and (3) secretion of labile C in mucus and casts of earthworms. Burrowing activities of earthworms could also create macropores and facilitate DOC movement across soil profiles. In addition, increased macropores could reduce contact time between DOC and mineral soils as water percolates, reducing DOC adsorption and increasing DOC levels at deeper soils (Kalbitz et al. 2000). We suspect that biotic factors are primarily responsible for production of DOC in soils, and abiotic changes caused by earthworms are probably the main reason for DOC increases at deeper soils.
Increased inorganic N and DOC may lead to nutrient losses through different pathways. One possibility is leaching. Increased nitrate and DOC in soils may exceed plant uptake, and can be susceptible to potential leaching and transport into aquatic systems, which may degrade water quality. Also, large soil aggregates and macropores could further facilitate preferential flows and leaching process (Greiner et al. 2012). Other studies have also founded increased nutrient leaching in forest and agricultural soils as a consequence of earthworm invasion (Bohlen et al. 2004b; Dominguez et al. 2004).
Comparison between forest and prairie ecosystems
Stronger effects in forests than prairies could be explained by differences in litter (quality, quantity, composition) and soil (physical–chemical structure, nutrient) properties. Deciduous forest litter normally has lower C:N ratio and is a preferable food source as compared to prairie graminoid litter. Differences in litter stoichiometry can further cascade into effects on soil nutrients (Fahey et al. 2013). In addition, forest soils are relatively easier to burrow than prairie soils that are often more compact and contain dense root systems. Forest soils are also more moist than prairie soils (averaged moisture of 35 ± 4 % in forest vs. 25 ± 3 % in prairie mesocosms over the experimental period), which may facilitate microbial activities in forest soils.
Nonetheless, the significant effects on prairie soils are consistent with the stimulated ligninolytic enzyme activities by A. agrestis (Belliturk et al. 2015), and provide evidence that A. agrestis and A. tokioensis—native to Asian grasslands—may survive and affect prairies. It further suggests that, while U.S. forests are considered susceptible to invasion of non-native earthworms, prairies that support diverse life forms and ecosystem functioning (Samson and Knopf 1994; Werling et al. 2014) may also be vulnerable to invasion of Asian jumping worms. One caveat is that our study only examined effects on prairies under experimental conditions but not in the field, mostly because no populations were detected in the prairies at the time of this work. Future studies should monitor multiple habitats, including prairies, to quantify rates and patterns of the spread of Asian jumping worms and understand their ecological effects under natural conditions.
Temporal dynamics of earthworm effects
Effects of A. agrestis and A. tokioensis did not change linearly over time and were most pronounced towards the end of the growing season (Figs. 6, 7). This temporal pattern aligns well with the life history of many epigeic Amynthas spp. that emerge from cocoons in spring, grow rapidly to sexual maturity and peak in biomass in the fall (Greiner et al. 2012; Ikeda et al. 2015). Another factor that might reinforce this pattern is the new litter fall occurring from September to October, which adds food sources for earthworms.
Methodological considerations
Effects of A. agrestis and A. tokioensis from our mesocosm experiment and field study were consistent, but the magnitude of effects sometimes differed. Such differences are not surprising. First, plant uptake is an important nutrient flux in the field, but negligible in mesocosms. Second, mesocosms are semi-closed systems as compared to field conditions, with little or no external inputs such as litter fall. Third, differences in shading could also affect soil hydrologic conditions and earthworm performance. Although we installed shade cloth above mesocosms, soils were not as fully shaded as below the closed forest canopy or the dense, tall prairie vegetation. Last but not least, in the mesocosms, a fixed number of earthworms were introduced into a confined space. Under field conditions, earthworm movement is not constrained, and soils at a particular location can be affected by variable number of earthworms. Nevertheless, the consistent results from both methods provide confidence in the effects of A. agrestis and A. tokioensis detected in this study.
Conclusions
Non-native earthworm invasion is a major driver of ecological change. Effects of European invasive earthworms in North America are well understood, but non-native Asian earthworms are increasingly discovered. We found that A. agrestis and A. tokioensis significantly reduced litter and enriched nutrients in surface soils. Effects were observed in both forest and prairie soils and may rival those reported for other invasive earthworms. The rapid transformation of litter and soil nutrients into labile and soluble forms may make invaded ecosystems more susceptible to nutrient losses. Effects on litter and soils may further cascade to other taxa, such as understory herbs, soil micro- and macro-fauna, and affect important ecological processes like plant establishment and growth.
References
Alban DH, Berry EC (1994) Effects of earthworm invasion on morphology, carbon, and nitrogen of a forest soil. Appl Soil Ecol 1:243–249
Balser TC, Firestone MK (2005) Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73:395–415
Bates D, Maechler M, Bolker B (2015) Linear mixed-effects models using Eigen and S4. R package version 1.1–7. http://cran.r-project.org/web/packages/lme4/index.html. Accessed 6 April 2015
Belliturk K, Gorres JH, Kunkle J, Melnichuk RDS (2015) Can commercial mulches be reservoirs of invasive earthworms? Promotion of ligninolytic enzyme activity and survival of Amynthas agrestis (Goto and Hatai 1899). Appl Soil Ecol 87:27–31
Blouin M, Hodson ME, Delgado EA, Baker G, Brussaard L, Butt KR, Dai J, Dendooven L, Pérès G, Tondoh J (2013) A review of earthworm impact on soil function and ecosystem services. Eur J Soil Sci 64:161–182
Bohlen PJ, Groffman PM, Fahey TJ, Fisk MC, Suarez E, Pelletier DM, Fahey RT (2004a) Ecosystem consequences of exotic earthworm invasion of north temperate forests. Ecosystems 7:1–12
Bohlen PJ, Pelletier DM, Groffman PM, Fahey TJ, Fisk MC (2004b) Influence of earthworm invasion on redistribution and retention of soil carbon and nitrogen in northern temperate forests. Ecosystems 7:13–27
Bohlen PJ, Scheu S, Hale CM, McLean MA, Migge S, Groffman PM, Parkinson D (2004c) Non-native invasive earthworms as agents of change in northern temperate forests. Front Ecol Environ 2:427–435
Bray RH, Kurtz L (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Burtelow AE, Bohlen PJ, Groffman PM (1998) Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Appl Soil Ecol 9:197–202
Callaham MA, Hendrix PF, Phillips RJ (2003) Occurrence of an exotic earthworm (Amynthas agrestis) in undisturbed soils of the southern Appalachian Mountains, USA. Pedobiologia 47:466–470
Costello DM, Lamberti GA (2009) Biological and physical effects of non-native earthworms on nitrogen cycling in riparian soils. Soil Biol Biochem 41:2230–2235
Crumsey JM, Le Moine JM, Capowiez Y, Goodsitt MM, Larson SC, Kling GW, Nadelhoffer KJ (2013) Community-specific impacts of exotic earthworm invasions on soil carbon dynamics in a sandy temperate forest. Ecology 94:2827–2837
Currie WS, Aber JD (1997) Modeling leaching as a decomposition process in humid Montane forests. Ecology 78:1844–1860
Dominguez J, Bohlen PJ, Parmelee RW (2004) Earthworms increase nitrogen leaching to greater soil depths in row crop agroecosystems. Ecosystems 7:672–685
Edwards CA (2004) Earthworm ecology. CRC Press, Boca Raton
Eisenhauer N, Partsch S, Parkinson D, Scheu S (2007) Invasion of a deciduous forest by earthworms: changes in soil chemistry, microarthropds and vegetation. Soil Biol Biochem 39:1099–1110
Fahey TJ, Yavitt JB, Sherman RE, Maerz JC, Groffman PM, Fisk MC, Bohlen PJ (2013) Earthworm effects on the incorporation of litter C and N into soil organic matter in a sugar maple forest. Ecol Appl 23:1185–1201
Gates GE (1982) Farewell to North American megadriles. Megadrilogica 4:12–77
Greiner HG, Costello DM, Tiegs SD (2010) Allometric estimation of earthworm ash-free dry mass from diameters and lengths of select megascolecid and lumbricid species. Pedobiologia 53:247–252
Greiner HG, Kashian DR, Tiegs SD (2012) Impacts of invasive Asian (Amynthas hilgendorfi) and European (Lumbricus rubellus) earthworms in a North American temperate deciduous forest. Biol Invasions 14:2017–2027
Groffman PM, Bohlen PJ, Fisk MC, Fahey TJ (2004) Exotic earthworm invasion and microbial biomass in temperate forest soils. Ecosystems 7:45–54
Hale CM (2007) Earthworms of the great lakes. Kollath-Stensaas, Duluth
Hale CM, Frelich LE, Reich PB, Pastor J (2008) Exotic earthworm effects on hardwood forest floor, nutrient availability and native plants: a mesocosm study. Oecologia 155:509–518
Hendrix PF, Callaham MA, Drake JM, Huang CY, James SW, Snyder BA, Zhang WX (2008) Pandora’s box contained bait: the global problem of introduced earthworms. Annu Rev Ecol Syst 39:593–613
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363
Ikeda H, Callaham MA, O’Brien JJ, Hornsby BS, Wenk ES (2015) Can the invasive earthworm, Amynthas agrestis, be controlled with prescribed fire? Soil Biol Biochem 82:21–27
Ishizuka K (2001) Taxanomic study of the genus Pheretima s. lat. (Oligochaeta, Megasolecidae) from Japan. B Seikei Univ 33:1–125
Ishizuka K, Minagoshi F (2014) Pictorial book of earthworm. Zenkoku Noson Kyoiku Kyokai Co., Ltd., Tokyo (In Janpanese)
James SW (1995) Systematics, biogeography, and ecology of Nearctic earthworms from eastern, central, southern, and southwestern United States. In: Hendrix P (ed) Earthworm ecology and biogeography in North America. Lewis Publishers, Boca Raton, pp 29–52
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Kuznetsova A, Brockhoff PB, Christensen RHB (2014) lmerTest: tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version 2.0–6. http://cran.r-project.org/web/packages/lmerTest/index.html. Accessed 6 April 2015
Lavelle P, Decaens T, Aubert M, Barot S, Blouin M, Bureau F, Margerie P, Mora P, Rossi JP (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42:S3–S15
Lubbers IM, Brussaard L, Otten W, van Groenigen JW (2011) Earthworm-induced N mineralization in fertilized grassland increases both N2O emission and crop-N uptake. Eur J Soil Sci 62:152–161
Masamichi T, Masatoshi Y, Fumio Y (2011) Earthworm fauna (Annelida: Clitellata) of the main campus and Chiyoda experimental station of Forestry and Forest Products Research Institute. B FFPRI 4:281–289
McLean MA, Migge-Kleian S, Parkinson D (2006) Earthworm invasions of ecosystems devoid of earthworms: effects on soil microbes. Biol Invasions 8:1257–1273
R Development Core Team (2009) R A language and environment for statistical computing. http://www.R-project.org
Resner K, Yoo K, Sebestyen SD, Aufdenkampe A, Hale C, Lyttle A, Blum A (2015) Invasive earthworms deplete key soil inorganic nutrients (Ca, Mg, K, and P) in a northern hardwood forest. Ecosystems 18:89–102
Reynolds JW, Wetzel MJ (2012) Terrestrial Oligochaeta (Annelida: Clitellata) in North America, including Mexico, Puerto Rico, Hawaii, and Bermuda. III. Megadrilogica 15:191–211
Richardson DR, Snyder BA, Hendrix PF (2009) Soil moisture and temperature: tolerances and optima for a non-native earthworm species, Amynthas agrestis (Oligochaeta: Opisthopora: Megascolecidae). Southeast Nat 8:325–334
Richardson JB, Gorres JH, Friedland AJ (2016) Forest floor decomposition, metal exchangeability, and metal bioaccumulation by exotic earthworms: Amynthas agrestis and Lumbricus rubellus. Environ Sci Pollut Res. doi:10.1007/s11356-016-6994-5
Robertson GP, Sollins P, Ellis BG, Lajtha K (1999) Exchangeable ions, pH, and cation exchange capacity. In: Robertson GP, Biedsoe CS, Coleman DC, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 106–114
Sackett TE, Smith SM, Basiliko N (2013) Indirect and direct effects of exotic earthworms on soil nutrient and carbon pools in North American temperate forests. Soil Biol Biochem 57:459–467
Samson F, Knopf F (1994) Prairie conservation in North America. Bioscience 44:418–421
Scheu S, Parkinson D (1994) Effects of earthworms on nutrient dynamics, carbon turnover and microorganisms in soils from cool temperate forests of the Canadian Rocky Mountains—laboratory studies. Appl Soil Ecol 1:113–125
Snyder BA, Boots B, Hendrix PF (2009) Competition between invasive earthworms (Amynthas corticis, Megascolecidae) and native North American millipedes (Pseudopolydesmus erasus, Polydesmidae): effects on carbon cycling and soil structure. Soil Biol Biochem 41:1442–1449
Snyder BA, Callaham MA, Hendrix PF (2011) Spatial variability of an invasive earthworm (Amynthas agrestis) population and potential impacts on soil characteristics and millipedes in the Great Smoky Mountains National Park, USA. Biol Invasions 13:349–358
Snyder BA, Callaham MA, Lowe CN, Hendrix PF (2013) Earthworm invasion in North America: food resource competition affects native millipede survival and invasive earthworm reproduction. Soil Biol Biochem 57:212–216
Speratti AB, Whalen JK (2008) Carbon dioxide and nitrous oxide fluxes from soil as influenced by anecic and endogeic earthworms. Appl Soil Ecol 38:27–33
Suarez ER, Pelletier DM, Fahey TJ, Groffman PM, Bohlen PJ, Fisk MC (2004) Effects of exotic earthworms on soil phosphorus cycling in two broadleaf temperate forests. Ecosystems 7:28–44
Suarez ER, Fahey TJ, Yavitt JB, Groffman PM, Bohlen PJ (2006) Patterns of litter disappearance in a northern hardwood forest invaded by exotic earthworms. Ecol Appl 16:154–165
Szlavecz K, Placella SA, Pouyat RV, Groffman PM, Csuzdi C, Yesilonis I (2006) Invasive earthworm species and nitrogen cycling in remnant forest patches. Appl Soil Ecol 32:54–62
Tipping E, Woof C, Rigg E, Harrison AF, Ineson P, Taylor K, Benham D, Poskitt J, Rowland AP, Bol R, Harkness DD (1999) Climatic influences on the leaching of dissolved organic matter from upland UK Moorland soils, investigated by a field manipulation experiment. Environ Int 25:83–95
Uchida T (2004) Feeding strategies of earthworms (Megascolecidae Oligochaeta) in Japan. Jpn J Ecol:235–243 (in Japanese)
Uchida T, Kaneko N, Ito MT, Futagami K, Sasaki T, Sugimoto A (2004) Anlaysis of the feeding ecology of earthworms (Megascolecidae) in Janpanese forests using gut content fraction and δ15 N and δ13 C stable isotope natural abundance. Appl Soil Ecol 27:153–163
Underwood AJ (1994) On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecol Appl 4:3–15
Vickers AJ (2001) The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 1:6
Werling BP, Dickson TL, Isaacs R, Gaines H, Gratton C, Gross KL, Liere H, Malmstrom CM, Meehan TD, Ruan L, Robertson BA, Robertson GP, Schmidt TM, Schrotenboer AC, Teal TK, Wilson JK, Landis DA (2014) Pereenail grasslands enhance bidoversity and multiple ecosystme services in bioenergy landscapes. P Natl Acad Sci USA 111:1652–1657
Wiltshire G, Laubscher D (1989) Economical manual estimation of ammonium, nitrate and total inorganic nitrogen in soils. S Afr J Plant Soil 6:53–58
Zhang WX, Hendrix PF, Snyder BA, Molina M, Li JX, Rao XQ, Siemann E, Fu SL (2010) Dietary flexibility aids Asian earthworm invasion in North American forests. Ecology 91:2070–2079
Zhang WX, Hendrix PF, Dame LE, Burke RA, Wu JP, Neher DA, Li JX, Shao YH, Fu SL (2013) Earthworms facilitate carbon sequestration through unequal amplification of carbon stabilization compared with mineralization. Nat Commun 4. doi:10.1038/ncomms3576
Acknowledgments
We thank Chris Kucharik, Steve Carpenter, Randy Jackson, and Timothy Whitby for help with the experimental design of this research. We appreciate logistical support from Susan Carpenter and Bradley Herrick, and thank the University of Wisconsin–Madison Arboretum for providing the field facility. We thank Jeffrey Hatzel, Allison Lobue, Eric Booth, Hannah Friedrick, Sam Zipper, Melissa Motew and Jason Schatz for field assistance, and Lawrence Oates, Liz Runde and Jane Remfert for help with laboratory analyses. Susan Carpenter, Carly Ziter, Bradley Herrick and two anonymous reviewers provided helpful feedback on earlier versions of the manuscript. Funding was provided by the National Science Foundation under Grant DEB-1038759 and Northern Temperate Lakes Long-Term Ecological Research under grant DEB-1440297.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qiu, J., Turner, M.G. Effects of non-native Asian earthworm invasion on temperate forest and prairie soils in the Midwestern US. Biol Invasions 19, 73–88 (2017). https://doi.org/10.1007/s10530-016-1264-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1264-5