Abstract
Amynthas agrestis and Metaphire hilgendorfi are being distributed across North America with unknown ecosystem impacts. Forest soils in urban areas sequester trace elements and earthworms may be bioaccumulating them. This study examined Cd, Cr, Co, Cu, Mn, Ni, Pb, V, and Zn in soils and earthworm tissues at 28 urban forest sites in and surrounding Poughkeepsie, NY, USA. Megascolecidae were present at 22 sites with means of 12 to 27 individuals m−2 and 4 to 12 dry weight g m−2. Urban forest soils within commercial uses had Mn, Pb, and Zn concentrations higher than within residential and agricultural uses. Earthworm trace element concentrations were poorly predicted by their respective soil concentrations, except for Pb. Urban forests in commercial uses and land-preserves, earthworm Cd and Pb concentrations were at or above concentrations known to negatively impact small mammal and bird health ( > 10 mg kg−1) with Co and V approaching toxic concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Exotic and invasive earthworms, such as Megascolecidae, have been introduced to forests across Europe and North America as a result of human activities, particularly agriculture and horticulture (Görres and Melnichuk 2012; Chang et al. 2017). Although earthworms can be beneficial in agricultural systems (e.g. Bityutskii and Kaidun 2008), their effects on forest ecosystems have been negative, with considerable consequences for plant communities, macrofauna, and soil biogeochemistry (Gundale et al. 2005; Dobson et al. 2017, 2018). An understudied aspect of the earthworm invasion are their effects on soil sequestration of potentially toxic metals (e.g. Richardson et al. 2016, 2017).
Urban forest soils have sequestered elevated concentrations of trace elements, such as Pb, Cd, Cu, and Zn, due to atmospheric deposition of local pollution by smelting, industrial activities, automobiles, and coal combustion (Adriano 2001; Steinnes and Friedland 2006; Pouyat et al. 2010). These pollutant metals are sequestered in urban forest soils due to their strong sorption to organic matter and secondary oxides. The introduced Megascolecidae pose a threat to this retention and sequestration capacity, by either disturbing organo-metal complexes or by bioaccumulating trace elements for biomagnifcation in terrestrial organisms (Richardson et al. 2015, 2017).
Measurements and field observations of trace element concentrations in Megascolecidae in North America are limited but are needed to identify where and how earthworms impact urban forest ecosystems. In this study, trace elements in urban forests adjacent to four land uses (commercial, residential, agricultural, preserved) were examined to determine if soil concentrations or land use influenced the bioaccumulation of metals by two Megascolecidae: Amynthas agrestis and Metaphire hilgendorfi. It is hypothesized that commercial pollution will cause the highest soil and earthworm tissue trace element concentrations while the less polluted residential, agricultural, and preserved-lands will have lower soil and earthworm tissue concentrations.
Materials and Methods
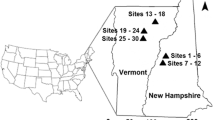
Twenty-eight sampling sites in and surrounding Poughkeepsie, New York, USA were studied (Fig. 1). The city and surrounding areas were chosen due to the known observation of Megascolecidae earthworms. Poughkeepsie, New York is considered humid continental with hot summers and cold winters (Dfa) according to the Köppen-Geiger classification with approximately 1182 mm precipitation and average high temperature of 16.2°C and low of 3.6°C. Twenty-five of the sites were chosen to approximately form a 10 km × 6 km grid, with a forest site every 2 km, to ensure both spatial variations as well as land-uses. The final three sites were preserved-lands at least 2 km away from the city, chosen as controls from urban effects on soil metal concentrations. In total, four land-use classes were sampled based upon current land-use: residential (i.e. single-family homes, apartments), commercial (i.e. near or between current businesses), agricultural (i.e. between horticultural and agricultural fields), and land-preserves (i.e. within wooded landscapes reserved as ecological habitat). Vegetation was secondary-growth, uneven-aged mixed composition stands consisting of northern hardwoods (e.g. Quercus, Acer, Fraxinus) with coniferous vegetation (e.g. Pinus) and introduced tree species (e.g. Ailanthus altissima). The soil parent material was basal glacial till modified by human actions (e.g. tillage, debris) underlain by shale-slate. The soils were well-drained, upland Inceptisols. Soil textures were all silt loams, with clay fractions < 25% for all sites.
Soil sampling occurred in July and August of 2017 and 2018. Earthworm collection occurred in October of 2018 when their populations have reached maturity. At each sampling site, one taxonomic soil pit and one soil monolith was excavated (e.g. Dobson et al. 2017). Only the A horizon (mean depth 8.7 ± 0.8 cm) from the monolith at each site was analyzed in this study. Soil samples were oven dried and sieved < 2 mm. To collect earthworms, five 0.5 × 0.5 m soil pits were dug down to 20 cm depth. To avoid edge effects, selection of soil pit and earthworm excavations locations was greater than 15 m from any human development, road, trail and asphalt or concrete walkways. In addition, poorly drained soils and microtopographic lows were not sampled to avoid influences from redoximorphic conditions. This likely biased against hydrophilic earthworms. The soil profile for each site was described from the morphological pits following the USDA taxonomic guide. Earthworms were stored alive in their horizon material and identified live using guidelines from Chang et al. (2017). Their live mass and length were measured, rinsed and depurated for 48 h, frozen at − 40°C and freeze-dried to a constant weight. Four or five individual earthworms from each site were ground into a powder and 0.5 g subsamples of dry mass was digested following the same protocols as soil samples.
Soil samples from the morphological soil pit at each site were analyzed for physicochemical properties. Soil pH was determined using a 2:5, soil:water slurry in 0.01 M CaCl2. The % SOM was determined using loss on ignition, in which 4 g of soil was held at 550°C for 8 h. Soil samples from the morphological soil pit were analyzed for strong-acid extractable Cd, Cr, Co, Cu, Mn, Pb, V, Zn, following EPA method 3051A. Strong-acid digestion allows for quantification of trace elements in non-primary mineral phases that may become dissolved or exchanged. In brief, 1 g of air-dried, sieved material was digested at 100°C for 45 min in 5 mL of strong acid (9:1, HNO3:HCl), degassed overnight, and analyzed with an Agilent 7700 × ICP-MS (Agilent Technologies, Santa Clara, CA). NIST Montana Soil SRM and NIST Mussel Tissue SRM were within 80% of their reported values for Cd, Cr, Co, Cu, Mn, Pb, V, Zn. The < 90% recovery was due to non-dissolution of silicate mineral phases, as expected with strong acid digestions of soil material. Duplicates were within 10% of each other and blanks were at or below limits of quantification. Limits of quantification for the 7700 × ICP-MS were approximately 0.2 ng g−1 for all of the elements.
Descriptive statistics were calculated using Matlab (Matlab Inc, Natick, MA). Trace element concentrations in soils and earthworms were compared among land-uses using the non-parametric Kruskal–Wallis test and between earthworm species using the non-parametric Wilcoxon signed-ranks test and linear regressions. Soil physical and chemical properties were compared across land-uses using the Kruskal–Wallis tests and linear regressions. Bioaccumulation factors (BAF) were calculated as the earthworm concentration divided by the soil concentration. BAF values are unitless and BAF values > 1.0 suggest bioaccumulation of an element while BAF values of ≤ 1.0 suggests active expulsion of metals from the earthworm. Earthworm tissue concentrations and BAF values were compared among the two Megascolecidae.
Results and Discussion
Megascolecidae were present at 22 of the 28 urban forest sites with A. agrestis present at all 22 sites and M. hilgendorfi present at 20 of the 22 sites. Megascolecidae earthworms were present at 60% of the agricultural sites and 73% of residential urban forest sites while 100% of commercial and land-preserve sites had both earthworm species. Only two sites (one residential and one agricultural) had European earthworm species (Aporrectodea spp. and Dendrobaena spp.) but due to their limited abundance, they were not discussed in this study. The number of earthworms and total dry mass was greater at commercial urban forest sites (27 ± 3 individuals; 12 ± 3 g m−2) than residential (14 ± 4 individuals; 6 ± 2 g m−2) and agricultural sites (13 ± 6 individuals; 4 ± 2 g m−2) but not land-preserve sites (19 ± 4 individuals; 9 ± 2 g m−2). These results highlight extensive distribution of the exotic Amynthas genus across this metropolitan area. Moreover, the initial hypothesis of greater populations associated with residential areas was not supported. The dispersal mechanism of domestic horticultural products may be the most important transport for these animals (Görres and Melnichuk 2012), which includes landscaping for commercial businesses and within land-preserves.
Soil concentrations of Cd, Mn, Ni, Pb, V and Zn were significantly above background soil concentrations across land-use types (Table 1, Adriano 2001). Urban forests within commercial uses had Mn, Pb, and Zn concentrations three times higher than average U.S. background soil concentrations (Mn = 560 mg kg−1, Pb = 19 mg kg−1, Zn = 90 mg kg−1 in Adriano 2001). Linear regressions were used to relate trace metals to soil properties and each other. Trace element concentrations were not significantly related to measured soil pH or %SOM. Soil pH was negatively related to %SOM however (r = − 0.59, p < 0.05). Cadmium, Co, Ni, Cu, Zn, Pb, and V soil concentrations were positively related with each other (r > 0.50, p < 0.05).Soil metal concentrations varied across land-uses; urban forests in residential areas typically had lower trace element concentrations than commercial, agricultural, and land-preserve sites (p < 0.01, Table 1), except for Cd, which were significantly greater in residential areas (p < 0.01). Urban forests near commercial areas had significantly higher metal concentrations than urban forests near agricultural and land-preserve sites (p < 0.01; Table 1), except for Cd, Co, and Cu. These results partly coincide with existing knowledge, which demonstrated urban emissions of trace metals are greatest near areas with high road traffic, manufacturing, and industrial activities (Adriano 2001; Steinnes and Friedland 2006; Laidlaw et al. 2012). However, the elevated soil concentrations of Cr, Co, Mn, Ni, Pb, V and Zn at the land-preserve compared to residential areas were not expected due to their distance from the metropolitan area. One possibility is historical deposition of trace elements has remained in land-preserves while local nutrients or physical disturbances have mobilized trace elements from residential forest soils (see Groffman et al. 2006).
Earthworm concentrations of Cd and Pb were above concentrations known to negatively impact the health of small mammals and birds (10 mg kg−1 for both, U.S. National Research Council 2006) in urban forests within all four land-uses. Moreover, Co and V were approaching concentrations known to negatively impact animals (25 mg kg−1 for both, U.S. National Research Council 2006) in urban forests in commercial uses and land-preserves. Earthworm trace element concentrations were significantly different among land-uses, with higher trace element concentrations in urban forest sites in commercial uses and in the land-preserves compared to agricultural and residential areas (p < 0.05; Table 2), except Cd.
The control of soils trace element concentrations on the bioaccumulation by earthworms was explored. The linear regression results demonstrated that bioaccumulation of trace elements was not explicitly controlled by pseudototal soil concentrations, except Pb (Fig. 2). Most trace element concentrations in earthworms were poorly predicted by their respective soil concentration among the twenty-two sites. These results demonstrate that other factors must be taken into account when predicting bioaccumulation of trace elements by earthworms. First, A. agrestis and M. hilgendorfi are considered epi-endogeic (Chang et al. 2017) and thus can consume fresh litter that typically have low trace element concentrations or consume mineral soil with high trace element concentrations. This study only considered the A horizon as most sites lacked an organic horizon to sample. Second, the bioaccumulation of trace elements may be more dependent on its bioavailability and mobility. Trace elements that exist as salts are more readily bioavailable while those strongly complexed to secondary minerals are often less bioavailable (Dai et al. 2004; Richardson et al. 2015). Further, strong acid and other pseudototal extractions can underestimate potentially bioavailable metals from silicate minerals and overestimate bioavailable organic-bound metals. Lastly, the variations may be site-specific and controlled by the type and abundance of food available for the earthworms (Latif et al. 2013), properties not measured in this study. Using bioaccumulation factors (BAF), the data showed a consistent bioaccumulation or exclusion of trace elements from earthworm tissues across land-uses. Cadmium and Cu were bioaccumulated across land-uses (BAF ≥ 1.0) while Cr, Co, Mn, Ni, Pb, V, and Zn were not consistently bioaccumulated (BAF < 1.0) (Table 3). The Cd and Ni results agree with previous studies (Morgan and Morgan 1999; Dai et al. 2004; Nannoni et al. 2011; Latif et al. 2013; Richardson et al. 2015) but Pb and Zn results disagree with other studies (Ernst et al. 2008; Richardson et al. 2015). This demonstrates that bioaccumulation of metals can be strongly influenced by their urban environment, as trace element concentrations or available food for earthworms may potentially influence their exposure relative to soil concentrations. Moreover, BAF values can be misleading as low BAF values can still result in potentially hazardous earthworm tissue concentrations, such as Pb in this study (Table 3).
There were significant trace metal differences between the two Megascolecidae. Overall, the A. agrestis had higher trace element concentrations than M. hilgendorfi, except for Pb and Zn. A. agrestis had higher tissue concentrations than M. hilgendorfi (Fig. 3) and since they inhabited the same soils, BAF values were significantly higher for A.agrestis than M. hilgendorfi. Both earthworms are considered epi-endogeic, feeding on plant litter and mineral soil (Chang et al. 2017). This may have been due greater A. agrestis in commercial areas with higher elemental concentrations, or potentially A. agrestis may be a more effective colonizer and have first access to trace elements. There may also be unknown physiological differences between A. agrestis and M. hilgendorfi that result in different tissue metal retention. Their preferred type of food scavenged and their ecophysiological group are unclear and may be important for bioaccumulation prediction (Morgan and Morgan 1999; Nannoni et al. 2011).
In conclusion, the study showed that land-use and species impacted soil and earthworm trace element concentrations within urban forests. Urban forests had significantly different trace element concentrations within a local area, with highest concentrations in commercial areas and lowest in residential areas. However, elevated trace element concentrations within soils did not necessarily control concentrations of inhabiting earthworms. Instead, earthworm metal bioaccumulation was metal and site specific. Most importantly, tissue Cd and Pb concentrations reached potentially hazardous concentrations across land-uses. Predicting trace element bioaccumulation is important for urban ecosystems since earthworms attained concentrations potentially hazardous to small mammals and birds and soil concentrations are the most common method to predict areas of likely high metal transfer. Instead, we suggest capturing and analyzing earthworms to accurately measure the ecological risk associated with predators consuming them.
References
Adriano DC (2001) Trace elements in terrestrial environments. Springer, New York, NY
Bityutskii NP, Kaidun PI (2008) The influence of earthworms on the mobility of microelements in soil and their availability for plants. Eurasian Soil Sci 41(12):1306–1313
Dobson AM (2018) Impacts of Deer and Earthworms on understory forest plants. Dissertation, Cornell University. https://doi.org/10.7298/X4P84948
Chang CH, Johnston MR, Görres JH, Dávalos A, McHugh D, Szlavecz K (2017) Co-invasion of three Asian earthworms, Metaphire hilgendorfi, Amynthas agrestis and Amynthas tokioensis in the USA. Biol Invasions 20:1–6
Dai J, Becquer T, Rouiller JH, Reversat G, Bernhard-Reversat F, Nahmani J, Lavelle P (2004) Heavy metal accumulation by two earthworm species and its relationship to total and DTPA-extractable metals in soils. Soil Biol Biochem 36(1):91–98
Dobson AM, Blossey B, Richardson JB (2017) Invasive earthworms change nutrient availability and uptake by forest understory plants. Plant Soil 421(1–2):175–190
Ernst G, Zimmermann S, Christie P, Frey B (2008) Mercury, cadmium and lead concentrations in different ecophysiological groups of earthworms in forest soils. Environ Pollut 156(3):1304–1313
Görres JH, Melnichuk RD (2012) Asian invasive earthworms of the genus Amynthas Kinberg in Vermont. Northeast Nat 19(2):313–323
Groffman PM, Pouyat RV, Cadenasso ML, Zipperer WC, Szlavecz K, Yesilonis ID, Band LE, Brush GS (2006) Land use context and natural soil controls on plant community composition and soil nitrogen and carbon dynamics in urban and rural forests. For Ecol Manag 236(2–3):177–192
Gundale MJ, Jolly WM, Deluca TH (2005) Susceptibility of a northern hardwood forest to exotic earthworm invasion. Conserv Biol 19(4):1075–1083
Laidlaw MA, Zahran S, Mielke HW, Taylor MP, Filippelli GM (2012) Re-suspension of lead contaminated urban soil as a dominant source of atmospheric lead in Birmingham, Chicago, Detroit and Pittsburgh, USA. Atmos Environ 49:302–310
Latif R, Malek M, Mirmonsef H (2013) Cadmium and lead accumulation in three endogeic earthworm species. Bull Environ Contam Toxicol 90(4):456–459
Morgan JE, Morgan AJ (1999) The accumulation of metals (Cd, Cu, Pb, Zn and Ca) by two ecologically contrasting earthworm species (Lumbricus rubellus and Aporrectodea caliginosa): implications for ecotoxicological testing. Appl Soil Ecol 13(1):9–20
Nannoni F, Protano G, Riccobono F (2011) Uptake and bioaccumulation of heavy elements by two earthworm species from a smelter contaminated area in northern Kosovo. Soil Biol Biochem 43(12):2359–2367
National Research Council (2006) Mineral tolerance of animals. National Academies Press, Washington, DC
Pouyat RV, Szlavecz K, Yesilonis ID, Groffman PM, Schwarz K (2010) Chemical, physical, and biological characteristics of urban soils. Urban Ecosystem Ecology 210:119–152
Richardson JB, Görres JH, Jackson BP, Friedland AJ (2015) Trace metals and metalloids in forest soils and exotic earthworms in northern New England, USA. Soil Biol Biochem 85:190–198. https://doi.org/10.1016/j.soilbio.2015.03.001
Richardson JB, Renock DJ, Görres JH, Jackson BP, Webb SM, Friedland AJ (2016) Nutrient and pollutant metals within earthworm residues are immobilized in soil during decomposition. Soil Biol Biochem 101:217–225
Richardson JB, Görres JH, Friedland AJ (2017) Exotic earthworms decrease Cd, Hg, and Pb pools in upland forest soils of Vermont and New Hampshire USA. Bull Environ Contam Toxicol 99(4):428–432
Steinnes E, Friedland AJ (2006) Metal contamination of natural surface soils from long-range atmospheric transport: existing and missing knowledge. Environ Rev 14(3):169–186
Acknowledgements
I would like to thank Brendan Braithwaite and Jonah G. Jordan for field support and laboratory assistance. This research was funded by the University of Massachusetts Amherst through research funds to Justin Richardson.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richardson, J.B. Trace Elements in Surface Soils and Megascolecidae Earthworms in Urban Forests Within Four Land-Uses Around Poughkeepsie, New York, USA. Bull Environ Contam Toxicol 103, 385–390 (2019). https://doi.org/10.1007/s00128-019-02669-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02669-z