Abstract
Sugar maple (Acer sacharrum Marsh.) in the western Upper Great Lakes region has recently been reported with increased crown dieback symptoms, prompting investigation of the dieback etiology across the region. Evaluation of sugar maple dieback from 2009 to 2012 across a 120 plot network in Upper Michigan, northern Wisconsin, and eastern Minnesota has indicated that forest floor disturbance impacts from exotic invasive earthworms was significantly related to maple dieback. Other plot level variables tested showed significant relationships among dieback and increased soil carbon, decreased soil manganese, and reduced herbaceous cover, each of which was also be correlated to earthworm activity. Relationships between possible causal factors and recent growth trends and seedling counts were also examined. Maple regeneration counts were not correlated with the amount of dieback. The recent mean radial increment was significantly correlated with various soil features and nutrients. This study presents significant evidence correlating sugar maple dieback in the western Upper Great Lakes region with earthworm activity, and highlights the need for considering the impacts of non-native earthworm on soil properties when assessing sugar maple health and productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Declines in the health of sugar maple (Acer saccharum Marsh.) have been documented in many portions of its range, especially in the last 50–60 years (Kessler 1965; Mader and Thompson 1969; McLaughlin et al. 1987; Millers et al. 1989; Kolb and McCormick 1993; Horsley and Long 1999; Drohan et al. 2002). Recent research has indicated unexpected, widespread decline in tree growth in northern North America, despite steady warming (Silva et al. 2010). Characteristics of ‘decline’ may include crown dieback, reduced growth, stressed trees, and regeneration failure, during which the causes may be unknown or attributed to a complexity of predisposing, inciting, and contributing factors (Manion and Lachance 1992). During periods of sugar maple dieback and decline episodes, the relationships among stand level measurements, management history, defoliator outbreaks, climate, and nutrient status have been examined (Bernier and Brazeau 1988; Allen et al. 1992; Houston 1992; Manion and Lachance 1992; Horsley et al. 2000; Côté and Ouimet 1996; St. Clair et al. 2008; Bal et al. 2015). Studies on sugar maple dieback have mostly focused on stands in New England and Canada, with fewer studies located in the Midwestern United States. Sugar maple decline in the eastern part of its range appears to be driven by local conditions, such as soil nutrients, and incited by heavy defoliation, severe drought, or a decade of bad winters (Horsley and Long 1999).
In the Great Lakes Region, the largest reported dieback and decline was during the late 1950s to early 1960s with high water tables and heavy cutting implicated as the cause (Millers et al. 1989). Other reports also found dieback in drier sites and undisturbed areas as well, but were not further examined (Anderson and Schmiege 1959). In northern Wisconsin and southern Upper Michigan, pockets of severe dieback and mortality were reported in the late 1950s and termed ‘maple blight’. The ‘blight’ began to subside after 1958 with heavy defoliation by maple webworm (Tetralopha asperatella Clemens) and early frosts being implicated as the causative agents (Anderson and Schmiege 1959; Schmiege and Anderson 1960; Allen et al. 1999; Houston 1999). Anecdotal evidence in Upper Michigan suggested that heavy harvesting on private lands was increasing the amount of tree mortality due to fungal pathogens in wounds. While approximately 8% of 90 sugar maple trees on industry lands were found to be infected with the sugar maple sapstreak fungus, (Ceratocystis virescens (Davidson) Moreau), the presence of the sapstreak fungus did not correlate with dieback incidence or crown condition (Bal et al. 2013).
Another novel factor that may be related to canopy health is the presence of invasive species. Reports and studies in maple stands have noted differences in the sugar maple forest floor condition across northern Michigan, Minnesota, and Wisconsin caused by non-native earthworms, though we are unaware of any studies that have directly compared canopy dieback to earthworm presence. The earthworm species distribution and their impacts does vary across the region (Sackett et al. 2012; Shartell et al. 2012). Earthworm activity alters soil physical, chemical, and biological properties, especially in the upper mineral soil horizons and forest floor (Groffman et al. 2004; Bohlen et al. 2004; Hale et al. 2005, 2006, 2008). Recent research has attributed earthworm invasion to sugar maple growth rate disturbances (Larson et al. 2010) and seedling survival (Drouin et al. 2014), but little is known about the impact of earthworms on mature sugar maple tree health. Invasive, exotic earthworms are an additional stress impacting tree species in this region that necessitated closer examination.
Severe dieback of sugar maple has been reported in the western Upper Peninsula of Michigan and other areas in the North Central region in recent years (MDNR 2009, 2010, 2012) with high proportions of valuable timber trees being affected in some areas. The extent to which the current dieback is related to biotic or abiotic factors is unclear. The objective of the work presented here was to determine the etiology of the current sugar maple dieback in the Western Upper Great Lakes region by characterizing relationships among basic canopy health and biotic and abiotic stand variables. Relationships among current crown dieback levels, recent radial growth, and seedling regeneration were investigated along with baseline forest plot information, tree condition, soil characteristics and nutrients, and forest floor condition to assess factors that may be related to the observed decline in canopy condition.
Methods
Plot establishment and base measurements
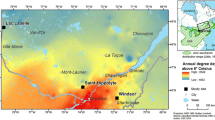
A network of 120 forest evaluation plots were established on private industry land in the western Upper Peninsula in 2009 (Marquette, Baraga, Houghton, and Keweenaw Counties), and public lands in northern Wisconsin, eastern Minnesota, and Upper Michigan in 2010 (Fig. 1). Plots were stratified so that half were on each of two ownership types (private industry and public) in the region. The private industry owned lands were expected to have the most intensive management regime with characterized by more frequent harvests and higher volumes of wood removed relative to public lands.
The western Upper Great Lakes region can be highly varied due to the distance from the Great Lakes themselves, proximity to inland lakes, and topographical variation. The region is humid continental, characterized by severe winters, heavy snowfall, and cool summers. The growing season runs from the last frost in spring, usually May, until the first frost in fall, usually September. Minimum average monthly temperature is −1.1 °C and maximum average monthly temperature is 10.4 °C. Rainfall averages 81.5 cm per year, with most rain falling between April and September. Across the region, average annual snowfall is 2.58 m (ranging from 1.1 to 5.9 m) (climate data averaged from NOAA weather stations in proximity to field sites; National Oceanic Atmospheric Administration, National Climatic Data Center, Ashville, NC. http://www.ncdc.noaa.gov/ (accessed October 2012).
Regional foresters were consulted to identify areas of northern hardwoods with and without significant levels of sugar maple dieback. Most of the circular 0.04 ha plots included at least 10 sugar maple trees, and were at least 40 m from established roadways to minimize edge effects. All plots were reevaluated each year until 2012, and trees that had grown to 10 cm dbh were added into the plot measurements. All species of trees greater than 10 cm within the plots were identified to species, measured (diameter at breast height, dbh), and underwent full canopy and bole assessments following standard Forest Health Monitoring Protocol (USDA 1999).
All field crews responsible for crown assessments, including dieback, underwent a minimum week long training with the researchers, standardizing assessments. A crew measuring trees in the field consisted of at least two persons, in order to view canopies more fully. Field sites were also visited regularly throughout the season during all 4 years to validate and discuss canopy assessment with crews.
In addition to crown dieback, other crown variables assessed included crown transparency (considers the sunlight penetrating through the crown), crown density (mean foliage density), the number of sides of the crown exposed to full light, the uncompacted live crown ratio (percentage of live crown length in the actual length of tree) (USDA 1999). The estimated percentage of crown containing dwarf, discolored, defoliated, curled, or otherwise damaged foliage was estimated, though cause was not attributed.
Crown dieback for all trees was estimated as the percentage of the whole crown that was dead (0–99%), including recent dead branches, peeling branches or bark, or fine branches and twigs without buds. Trees that were harvested were not included in yearly plot level, sugar maple dieback averages. Trees that were 100% dead during plot establishment were noted, but not included in the plot average dieback of sugar maple for that year. However, subsequent natural mortality was included to capture dead and dying trees. Damage to trees by cankers (Nectria or Eutypella spp.), sugar maple borer (Glycobius speciosus (Say)), visible decay, woodpecker activity, logging wounds, or other causes were recorded.
Elevation, slope, aspect, and landscape position (shoulder or summit, mid-slope, bench, lower slope, bottomland or flat) were recorded as baseline plot information. Other plot information (overall herbaceous percent aerial cover, soil density, and forest floor condition) was recorded approximately 12 m from plot center in the four cardinal directions to provide a plot mean. Soil density was estimated with a Dickey soil penetrometer at each 7.5 cm to a soil depth of 45 cm, unless prevented by rock or fragipan. Percent aerial cover of herbaceous plants was visually assessed by species in 1 m2 quadrats (Coffman et al. 1984). The forest floor condition or earthworm impact rating was determined using a 1–5 scale (Table 1) similar to that used by the Great Lakes Worm Watch in Minnesota, (greatlakeswormwatch.org) (Hale 2007; Loss et al. 2013). The earthworm impact rating scale quantifies the condition of the forest floor in a 1 m2 quadrat with a rating of 1 having only the most recent litter and the presence of multiple middens and castings, and 5 having no evidence of earthworms and a completely intact forest floor (Shartell et al. 2013). Approximately 5 m from plot center in the four cardinal directions, a 3.6 m radius sapling (1.27–9.9 cm dbh) plot and a 1.12 m radius seedling (<1.27 cm dbh) regeneration plot were established, with all species counted.
Tree cores
In each year of plot evaluation, a subset of plots had increment cores collected from three sugar maple trees in a similar size class (ideally within 5 cm dbh), which included one with little to no dieback, one with intermediate dieback, and one with the highest dieback (but not dead yet) on the plot. All cores were from dominant or co-dominant canopy trees to reduce the likelihood of partial or missing rings (Lorimer et al. 1999). Two increment cores were collected at 90° angles at breast height (1.4 m) from each tree for determination of the annual diameter increment. Cores were refrigerated until being mounted and sanded for reading annual ring widths on a digital measuring stage to the nearest 0.001 mm with Measure J2X software (Voor-Tech Consulting LLC, Nolderness, NH, 1999). Cores were visually cross-dated, and cores with significant decay or that were otherwise unreadable were excluded. Data from the two cores were averaged per year for each tree. Recent mean radial increments (RMRI) were calculated as the most complete, recent 5 year (2003–2008) mean radial increment (mm) per tree, which were averaged per plot for regression analysis.
Soil sampling and analysis
Soil cores (2.5 cm diameter), including the litter layer when present, were collected 3 m from plot center in the four cardinal directions to a soil depth of 30 cm during August. In the laboratory the four samples from each plot were combined, oven dried at 50 °C, ground, and sieved (2 mm) prior to analysis. Soil pH was determined in a 1:1 weight to deionized water ratio using a pH meter (Digi-Sense, pH/mV/ORP 5938-00). Soil particle size (sand, silt and clay) was determined using the hydrometer method (Day 1965), and soil organic matter (SOM) was measured using loss-on-ignition (LOI) by heating 10 g samples in a muffle furnace at 290 °C for 4 h (David 2008).
Five soil samples were also collected to 20 cm soil depth with a 2.5 cm diameter soil corer from beneath the drip lines of the two trees with the least and the most dieback in each plot that were cored for growth ring analysis. In the laboratory, the five soil samples under each tree were pooled by horizon, dried at 70 °C, and passed through a 2 mm sieve. Total C and total N were analyzed by the dry combustion method used by Forest Inventory and Analysis (FIA) (O’Neill et al. 2005). Plot samples were also analyzed for exchangeable cations (K, Ca, Mg, Al, Na,), extractable trace elements (Mn, Fe, Ni, Cu, Zn, Cd, Pb, Cr, B, Sr, Ba), and extractable SO4-S by the US Forest Service, Logan Forestry Sciences Laboratory in Logan, Utah. A 1:10 extraction ratio was used (0.5 g in 25 mL of 1 M NH4Cl). Extracts were diluted 1:10 in deionized water to prepare a better background matrix for ICP analysis (Amacher et al. 1990; O’Neill et al. 2005). Soil nutrient concentrations were combined by horizon for use in the baseline regression models to represent overall site conditions (including from under healthy and unhealthy trees). Total soil nutrient pools were not determined.
Soil series information was obtained from Soil Survey Geographic Online Database (Minnesota plots not available), including soil order, great group, drainage class (poorly drained, moderately well drained, well drained, somewhat excessively drained), presence of saturated conditions in subgroup, oxides in subgroup, presence of fragipan, shallowest depth to bedrock (cm), depth to high water table (m), surface run off class (rapid, high, medium, slow, low), potential for frost action (high, moderate, low), potential for seedling mortality, (high, moderate, low), sugar maple site index, and drainage index from the U.S. Forest Service National Drainage Index (http://foresthealth.fs.usda.gov/soils/, accessed 12-1-12). The seedling mortality factor rating found in soil survey manuals considers soil conditions that impact planted or natural regeneration, such as drainage class, aspect, and slope overall for the soil series, but it does not assess plant competition (Soil Survey Division Staff 1993).
Statistical analysis
Mean sugar maple dieback (2009–1012), mean sugar maple seedling counts, and the RMRI were evaluated separately using unweighted stepwise linear regression models with non-forced variables (α = 0.05 or 0.1 for approaching significance) and Pearson correlation coefficients. For regression purposes, the individual tree percentages of crown dieback were averaged for each plot and trees that were 100% dead the initial year of data collection were excluded. Subsequent mortality was captured by inclusion in the plot average unless trees were harvested and removed from the dataset. Plot averages were not transformed as the mean and the variances were independent (Allen et al. 1992). Models were tested with measured plot-level variables and soil variables. Average soil nutrient concentrations used in the regression analysis were: C, N, K, Ca, Mg, Al, Na, Ca/Al, Mn, Fe, Ni, Cu, Zn, Cd, Pb, Cr, B, Sr, Ba, SO4-S, C/N ratio, Ca/Al ratio, and exchangeable cation capacity (ECEC). Other soil measurements in the regression models were pH, % sand, % silt, % clay, and % organic matter (LOI). Though large sets of ecosystem-related variables tend to show high levels of colinearity (Zurr et al. 2009; Larcombe et al. 2013), this was not an issue for these models, as the variance inflation factors (VIF) ranged from 1.0 to 1.5. Relationships among earthworm impacts and other significant plot variables, such as recent growth (RMRI), were also investigated using linear regression. All analyses were conducted with the software program Statistix 9.1 (©1985–2008).
Results
Of the 2763 trees in the 120 plots evaluated (the majority annually), 75% were sugar maple. Other live tree species commonly found included red maple, Acer rubrum L. (9.8%), yellow birch, Betula alleghaniensis Britton (3.1%), and American basswood, Tilia americana L. (2.7%). The following results refer only to sugar maple. Descriptors of plot characteristics are located in Table 2. Sugar maple mean crown dieback from 2009 to 2012 was 12.5%, though mean dieback values ranged considerably across the study area and among years (14.0% ± 1.3 SE in 2009, 11.4% ± 0.7 SE in 2010, 16.3% ± 0.9 SE 2011, 9.0% ± 0.7 SE in 2012; p < 0.001) with mean transparency following a similar pattern as dieback and crown density with an inverse pattern, as was expected with how these variables are measured in the field (Bal and Storer 2015). There was no significant difference in dieback values between ownership types (Bal and Storer 2015).
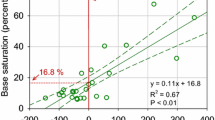
The stepwise regression of plot-level and soil variables with the total average sugar maple dieback (Table 3) retained four variables: earthworm impact rating as measured by the forest floor condition (p = 0.009), soil Mn, soil C, and herbaceous cover (all p < 0.001). Although average mineral soil pH was not significantly related to sugar maple crown dieback in the regression models, soil pH was significantly related to the average earthworm impact rating (p < 0.001; Fig. 2). As dieback increased, average soil pH was less acidic. The Recent Mean Radial Increment (RMRI) also significantly decreased with increasing soil pH (p = 0.006; Fig. 2). The RMRI was not significantly correlated with mean dieback (p = 0.56) or forest floor condition (p = 0.46) during the study period. However, plots with mean forest floor ratings with the most impacts of earthworm activity (virtually exposed mineral soil) also had the highest mean percentages of maple crown dieback over all years of the study (Table 4).
Within plots across the study range, sugar maple seedling numbers were low (Godman et al. 1990), averaging just 16,000 per hectacre (ranging from 0 to 200,000 plus), while seedlings of other tree species combined averaged less than 1 per hectare. Sugar maple seedling counts were significantly correlated with average sugar maple diameter at breast height, dbh (r = 0.395; p = 0.006) and the seedling mortality factor from Soil Surveys (r = 0.10; p = 0.016), but not with any other variables, including dieback. No factors in the model with sugar maple seedling counts were significant with α set at 0.1.
The stepwise regression model for sugar maple RMRI retained four significant variables: dbh (r = 0.28; p < 0.001), elevation (r = 0.19; p < 0.001), soil Pb (r = 0.12; p < 0.001), and plot slope (r = 0.245; p = 0.008). An additional four variables in the model approached significance (α = 0.1): soil pH (r = 0.06; p = 0.059), soil Cu (r = 0.11; p = 0.089), and soil Zn (r = 0.10; p = 0.09). Considering the other significant variables associated with dieback, the RMRI was not significantly correlated with soil C (p = 0.14). However, recent growth did significantly increase with increasing soil Mn with linear regression (r = 0.30; p < 0.001). The RMRI also significantly increased with increased herbaceous cover (r = 0.01; p < 0.001), which was possibly related to earthworm disturbance, though this was not significant in the stepwise regression model for growth.
Discussion
Occurrence of maple dieback in the Western Upper Great Lakes region appears more prevalent than historically reported (Bal et al. 2015), although it is variable across the landscape (Fig. 1). One of the largest maple forest health monitoring research projects, the North American Maple Project, found lower mean levels of maple crown dieback from 1988 to 1990 (7% ± 0.4 SE in 1988, 6% ± 0.3 SE in 1989, 6% ± 0.3 SE in 1990; Allen et al. 1992) than in the current study. However, there were some differences in how the crown dieback was categorized (Millers et al. 1991; USDA 1999), and how plots were categorized for forest land use.
Evidence of earthworm activity and variables influenced by earthworm activity (such as herbaceous abundance and soil chemistry) were significant in models of dieback and recent growth. Although we did not measure specific worm species assemblages or biomass, which could shed further light on the mechanics of worm infuence on mature tree health, the forest floor assessment is an efficient, accurate method for predicting the presence of Lumbricus spp. (Loss et al. 2013). Study areas with the greatest visible impact on the forest floor from earthworm presence appear to be located around Houghton and Keweenaw Counties (Fig. 3), which have some of the largest and longest European settlement history in the MI Upper Peninsula (Stearns 1997). A key factor of the spread and distribution of earthworms are roadways and waterways (Cameron and Bayne 2015), which correlates with the less intensive impacts of worms in the more remote National Forests. However, we do not have a specific measure of the timing of earthworm invasions in this study.
Worm activity causes soils to be more intermixed, and also exposes the soil to periods of warmer and drier conditions or more freezing temperatures (Groffman et al. 2004; Hale et al. 2006; Frelich et al. 2006; Hale et al. 2008). As the O horizon disappears with increasing worm biomass, total organic matter and bulk density in the A horizon increase (Hale et al. 2005; Dempsey et al. 2011). Maple dieback was positively correlated with soil C, and generally reflects increased earthworm activity (Wironen and Moore 2006). However, earthworm impacts on soil C levels can vary as a result of numerous factors, particularly the time since invasion, which we do not know. Invasive earthworms also alter soil nutrient availability, especially Ca, Mg, K, and P (Li et al. 2002; Resner et al. 2015), but none stood out in the maple dieback regression models (Table 3), possibly due to the broad range of soil types in our study.
In a mesocosm study with Lumbricus terrestris and other worm species, 87–98% of sugar maple leaf litter mass were lost by the end of 1 year (Holdsworth et al. 2012). Maple is one of the northern hardwood species with relatively higher foliage Ca content, as compared to oak, Quercus spp., and beech, Fagus spp.). Lumbricid earthworms preferentially feed on higher quality litter, and therefore may have greater impacts on maple stands than other northern hardwood stands (Holdsworth et al. 2012; McTavish et al. 2013). Maple roots may be very sensitive to changes in soil conditions, as the majority of fine roots are located in the O horizon and upper 10 cm of soil (Fisk et al. 2004). Mycorrhizal colonization in maple roots have also been shown to decrease due to worm activity (Lawrence et al. 2001; Dempsey et al. 2011).
Earthworms are calciferous organisms and tend to avoid more acid soils, especially the larger Lumbricid species (Reich et al. 2005; Moore et al. 2013; Shartell et al. 2013), and were more evident in our higher pH soils (Fig. 2). This suggests that liming soil to improve sugar maple health may have unanticipated consequences by also improving conditions for earthworm activity (Moore et al. 2013, 2015). However, some of the lowest foliar concentrations of Ca and Mg and highest Al and Mn were found on our sites with the most earthworm activity in the forest floor (Bal 2013). Foliar Ca may not always be a good indicator of available soil Ca, as Ca uptake is influenced by soil Al concentrations. There is strong evidence for depleted soil Ca effecting maple dieback in its more eastern range (Bal et al. 2015). Soil pH was not a significant predictor of maple dieback in our study, though it is correlated with worm activity (Fig. 2). It may be that in the Upper Midwest, earthworm activity is having a more pronounced influence on maple health due to other environmental or climatic factors, or soil Ca has not been perturbed or depleted as in eastern soils.
Earthworm activity may create a “pulse” or short term increase in nutrients (Hale et al. 2008; Larson et al. 2010) that rapidly leach out, or are fixed as soil oxides, as belowground nutrient dynamics are disturbed (Resner et al. 2015). This pattern is likely why earthworm garden experiments often report increases in available soil nutrients (Hale et al. 2008), which is contradictory to longer term field observations. Our dieback model also indicated a significant negative relationship with soil Mn, which may be a result of less earthworm activity in acid soils with greater Mn availability. However, tree growth appears to be positively correlated with soil Mn, and low Mn levels have been previously reported in declining sugar maple stands (Adams and Hutchison 1992), though excess Mn in acidic soils is much more commonly reported (Bal et al. 2015). The transitory nature of nutrient availability between early and later stages of earthworm colonization are likely influencing this dynamic.
Biotic soil disturbance (mainly by earthworms) have likely occurred in other areas reporting maple dieback, but the impacts by these organisms are usually not the study focus. Few, if any, reports of current sugar maple decline mention earthworms, or attempts to measure them as a variable impacting tree health along with nutrients, climate, or other influences. Earthworms have long been established in areas of the eastern U.S. and therefore may not have been considered as an exotic species (Coderre et al. 1995; Hendrix and Bohlen 2002).
Earthworm activity has been previously shown to have a negative impact on sugar maple regeneration (Hale et al. 2006; Corio et al. 2009; Drouin et al. 2014), but this effect was not significant in this study. This could be a result of our assessing forest floor condition rather than measuring specific earthworm biomass or species assemblages, as was done in other studies. An extremely thick litter layer can prohibit sugar maple seedling emergence (Cleavitt et al. 2011; Patterson et al. 2012), but reductions and changes in the litter layer also impacts seedling emergence and survival (Hale et al. 2006; Holdsworth et al. 2007a).
The observations of growth trends are a concern for such an economically and ecologically important species in the hardwood forests of the Midwest. The lack of a correlation between the RMRI and dieback in the model could be due to our broad geographic study area, the large number of sampled trees, stand age, and harvest rotation schedule (Bal et al. 2015), all of which are not likely to be captured using RMRI as an indicator of growth. If the etiology of dieback is gradual, dead branches eventually snap off, reducing reported dieback levels (Watmough et al. 1999), or annual variation in precipitation or data collection timing (mid-June–August) may influence the amount of foliage in crowns and mask dead twigs. Consequently, recent growth alone does not appear to be a good predictor of maple dieback or decline.
The relationship we found between RMRI and herbaceous cover is likely representative of reduced herb cover in plots with high earthworm impacts. Our measure of herbaceous cover did not account for habitat class or invasive species, as plots could be fully covered in exotic graminoids or native forbs and shrubs. Sedges (Carex spp., such as the upland-occurring Carex pensylvanica, Carex plantiginea, and Carex albursina) and shield ferns (mainly Dryopteris spinulosa), the two most abundant plant groups recorded in our study, are known to increase with earthworm abundance by likely altering abiotic conditions (Holdsworth et al. 2007b; Corio et al. 2009; Fisichelli et al. 2012; Roth et al. 2015). Since site variables potentially influenced by earthworms were significantly correlated with RMRI, but not the forest floor condition, it seems earthworm activity can disturb nutrient uptake enough to alter tree ring widths (Larson et al. 2010).
As the forest floor is consumed and tree roots die or are eaten (Gilbert et al. 2014), trees go through a stress period and have to reestablish their fine root network deeper into the mineral soil. Removal of the forest floor results in more xeric conditions and nutrient disturbance in the upper layers of soil, and may exacerbate other factors that can affect tree growth, such as decreased canopy cover. Hypothetically, once roots are reestablished, tree growth would stabilize, and stress effects of earthworms on the trees are diminished. However, the complexity of changes and lingering indirect effects from earthworms would likely continue to impact the forest ecosystem (Bohlen et al. 2004; Frelich et al. 2006; Sackett et al. 2013). As soil processes and nutrient dynamics are impacted, it is clear that earthworm invasions need to be considered when assessing maple forest health. Analysis over a longer time period would be beneficial to understanding the impacts of earthworm disturbance on sugar maple growth and health in this region.
Conclusion
The sugar maple crown dieback and plot variables modeled here provide indirect correlative evidence of earthworm influence on deteriorating mature tree health. The relationship of earthworm activity and concurrent impacts associated with sugar maple dieback and growth highlights the need for studies to be comprehensive when determining sugar maple health etiologies, especially key soil factors and conditions. Site requirements and earthworm impacts should be further assessed for other northern hardwood species in addition to sugar maple, especially if a management recommendation is to promote species diversity. Best management practices should be evaluated and followed regarding reducing the continual spread of exotic earthworms. Sugar maple health and growth in the Great Lakes warrants further investigation and monitoring.
References
Adams CM, Hutchison TC (1992) Fine-root growth and chemical composition in declining Central Ontario sugar maple stands. Can J For Res 22:1489–1503. doi:10.1139/x92-199
Allen DC, Barnett CJ, Millers I, Lachance D (1992) Temporal change (1988–1990) in sugar maple health, and factors associated with crown condition. Can J For Res 22:1776–1784. doi:10.1139/x92-232
Allen DC, Molloy AW, Cooke RR, Pendrel BA (1999) A ten-year regional assessment of sugar maple mortality. In: Proceedings of sugar maple ecology and health: an international symposium. Warren, MI. June 2–4, 1998. USDA Forest Service Gen Tech Rep NE-261 pp 27–45
Amacher MC, Henderson RE, Breithaupt MD, Seale CL, LaBauve JM (1990) Unbuffered and buffered salt methods for exchangeable cations and effective cation-exchange capacity. Soil Sci Soc Am J 54:1036–1042. doi:10.2136/sssaj1990.03615995005400040018x
Anderson GW, Schmiege DC (1959) The forest insect and disease situation lake states 1958. USDA Forest Service Station Paper No 70
Bal TL (2013) Evaluation of sugar maple dieback in the Upper Great Lakes region and development of a forest health youth education program. Dissertation. Michigan Technological University
Bal TL, Storer AJ (2015) Evaluation of sugar maple dieback trends in the Upper Great Lakes region. In: Potter KM, Conkling BL (eds) Forest health monitoring: national status, trends, and analysis 2014 USDA Forest Service Gen Tech Rep SRS-209, pp 119–124
Bal TL, Richter DL, Storer AJ, Jurgensen MF (2013) The relationship of the sapstreak fungus, Ceratocystis virescens to sugar maple dieback and decay in Northern Michigan. Am J Plant Sci 4:436–443. doi:10.4236/ajps.2013.42A056
Bal TL, Storer AJ, Jurgensen MF, Doskey PV, Amacher MC (2015) Nutrient stress predisposes and contributes to sugar maple dieback across its northern range: a review. Forestry 88:64–83. doi:10.1093/forestry/cpu051
Bernier B, Brazeau M (1988) Nutrient deficiency symptoms associated with sugar maple dieback and decline in the Quebec Appalachians. Can J For Res 18:762–767. doi:10.1139/x88-116
Bohlen PJ, Groffman PM, Fahey TJ, Fisk MC, Suárez E, Pelletier DM, Fahey RT (2004) Ecosystem consequences of exotic earthworm invasion of north temperate forests. Ecosystems 7:1–12. doi:10.1007/s10021-003-0126-z
Cameron EK, Bayne EM (2015) Spatial patterns and spread of exotic earthworms at local scales. Can J Zool 93:721–726. doi:10.1139/cjz-2014-0197
Cleavitt NL, Fahey TJ, Battles JJ (2011) Regeneration ecology of sugar maple (Acer saccharum): seddling survival in relation to nutrition, site factors, and damage by insects and pathogens. Can J For Res 41:235–244. doi:10.1139/X10-210
Coderre D, Mauffette Y, Gagnon D, Tousignant S, Bessette G (1995) Earthworm populations in healthy and declining sugar maple forests. Pedobiologia 39:86–96
Coffman MS, Alyanak E, Kotar J, Ferris JE (1984) Field guide habitat classification system for upper Peninsula of Michigan and Northeast Wisconsin. Cooperative Research on Forest Soils; School of Forestry and Wood Products, Michigan Technological University, Houghton
Corio K, Wolf A, Draney M, Fewless G (2009) Exotic earthworms of Great Lakes Forests: a search for indicator plant species in maple forests. For Ecol Manag 258:1059–1066. doi:10.1016/j.foreco.2009.05.013
Côté B, Ouimet R (1996) Decline of the maple-dominated forest in southern Quebec: impact of natural stresses and forest management. Environ Rev 4:133–148. doi:10.1139/a96-009
David MB (2008) Use of loss-on-ignition to assess soil organic carbon in forest soils. Commun Soil Sci Plan 19:1593–1599. doi:10.1080/00103628809368037
Day PR (1965) Hydrometer methods of particle size analysis. Methods of Soil Anal Agron 9:562–566
Dempsey MA, Fisk MC, Fahey TJ (2011) Earthworms increase the ratio of bacteria to fungi in northern hardwood forest soils, primarily by eliminating the organic horizon. Soil Biol Biochem 43:2135–2141. doi:10.1016/j.soilbio.2011.06.017
Drohan PJ, Stout SL, Petersen GW (2002) Sugar maple (Acer saccharum Marsh.) decline during 1979–1989 in northern Pennsylvania. For Ecol Manag 170:1–17. doi:10.1016/S0378-1127(01)00688-0
Drouin M, Bradley R, Lapointe L, Whalen J (2014) Non-native anecic earthworms (Lumbricus terrestris L.) reduce seed germination and seedling survival of temperate and boreal trees species. Appl Soil Ecol 75:145–149. doi:10.1016/j.apsoil.2013.11.006
Fisichelli NA, Frehlich LE, Reich PB, Eisenhauer N (2012) Linking direct and indirect pathways mediating earthworms, deer, and understory composition in Great Lakes forests. Biol Invasions 15:1057–1066. doi:10.1007/s10530-012-0350-6
Fisk MC, Fahey TJ, Groffman PM, Bohlen PJ (2004) Earthworm invasion, fine-root distributions, and soil respiration in north temperate forests. Ecosystems 7:55–62. doi:10.1007/s10021-003-0130-3
Frelich LE, Hale CM, Scheu S, Holdsworth AR, Heneghan L, Bohlen PJ, Reich PB (2006) Earthworm invasion into previously earthworm-free temperate and boreal forests. Biol Invasions 8:1235–1245. doi:10.1007/s10530-006-9019-3
Gilbert KD, Fahey TJ, Marez JC, Sherman RE, Bohlen P, Dombroskie JJ, Groffman PM, Yavitt JB (2014) Exploring carbon flow through the root channel in a temperate forest soil food web. Soil Biol Biochem 76:45–52. doi:10.1016/j.soilbio.2014.05.005
Godman RM, Yawney HW, Tubbs CH (1990) Acer saccharum Marsh. Sugar Maple. In Burns, RM Honkala BH (eds) Silvics of North America, vol 2. Hardwoods. USDA Forest Service Agricultural Handbook 654. USDA Forest Service, Washington, pp 78–91
Groffman PM, Bohlen PJ, Fisk MC, Fahey TJ (2004) Exotic earthworm invasion and microbial biomass in temperate forest soils. Ecosystems 7:45–54. doi:10.1007/s10021-003-0129-9
Hale CM (2007) Earthworms of the Great Lakes. Kollath-Stensaas, Duluth, p 36
Hale CM, Frelich LE, Reich PB, Pastor J (2005) Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota, U.S.A. Ecosystems 8:911–927. doi:10.1007/s10021-005-0066-x
Hale CM, Frehlich LE, Reich PB (2006) Changes in cold-temperate hardwood forest understory plant communities in response to European earthworm introductions. Ecology 87:1637–1649. doi:10.1890/0012-9658(2006)87[1637:CIHFUP]2.0.CO;2
Hale CM, Frelich LE, Reich PB, Pastor J (2008) Exotic earthworm effects on hardwood forest floor, nutrient availability and native plants: a mesocosm study. Oecologia 155:509–518. doi:10.1007/s00442-007-0925-6
Hendrix PF, Bohlen PJ (2002) Exotic earthworm invasions in North America: ecological and policy implications. Bioscience 52:801–811. doi:10.1641/0006-3568(2002)052[0801:EEIINA]2.0.CO;2
Holdsworth AR, Frehlich LE, Reich PB (2007a) Effects of earthworm invasion on plant species richness in Northern hardwood forests. Conserv Bio 21:997–1008. doi:10.1111/j.1523-1739.2007.00740.x
Holdsworth AR, Frehlich LE, Reich PB (2007b) Regional extent of an ecosystem engineer: earthworm invasion in Northern hardwood forests. Ecol Appl 17:1666–1677. doi:10.1890/05-2003.1
Holdsworth AR, Frehlich LE, Reich PB (2012) Leaf litter disappearance in earthworm-invaded Northern hardwood forests: role of tree species and the chemistry and diversity of litter. Ecosystems 15:913–926. doi:10.1007/s10021-012-9554-y
Horsley SB, Long RP (eds) (1999) Sugar maple ecology and health: an international symposium. USDA Forest Service General Technical Report NE-261, Warren, June 2–4, 1998
Horsley SB, Long RP, Bailey SW, Hallett RA, Hall TJ (2000) Factors associated with the decline disease of sugar maple on the Allegheny Plateau. Can J For Res 30:1365–1378. doi:10.1139/x00-057
Houston DR (1992) A host–saprogen model for forest dieback-decline diseases. In: Manion PD, Lachance D (eds) Forest decline concepts. APS Press, New York, pp 3–25
Houston DR (1999) History of sugar maple decline. In: Horsley SB, Long RP (eds) Sugar maple ecology and health, USDA Forest Service General Technical Report NE-261, pp 19–26
Kessler KJ Jr (1965) Dieback of managed, old growth northern hardwoods in upper Michigan, 1954–1964: a case history. Plant Dis Rep 49:483–486
Kolb TE, McCormick LH (1993) Etiology of a sugar maple decline in four Pennsylvania stands. Can J For Res 23:2395–2402. doi:10.1139/x93-296
Larcombe MJ, Silva JS, Vaillancourt RE, Potts BM (2013) Assessing the invasive potential of Eucalyptus globulus in Australia: quantification of wildling establishment from plantations. Biol Invasions 15:2763–2781. doi:10.1007/s10530-013-0492-1
Larson ER, Kipfmueller KF, Hale CM, Frelich LE, Reich PB (2010) Tree rings detect earthworm invasions and their effects in Northern hardwood forests. Biol Invasions 12:1053–1066. doi:10.1007/s10530-009-9523-3
Lawrence B, Fisk MC, Fahey TJ, Suarez ER (2001) Influence of nonnative earthworms on mycorrhizal colonization of sugar maple (Acer saccharum). New Phytol 157:145–153. doi:10.1046/j.1469-8137.2003.00649.x
Li X, Fisk MC, Fahey TJ, Bohlen PJ (2002) Influence of earthworm invasion on soil microbial biomass and activity in a Northern hardwood forest. Soil Bio Biochem 34:1929–1937. doi:10.1016/S0038-0717(02)00210-9
Lorimer CG, Dahir SE, Singer MT (1999) Frequency of partial and missing rings in Acer saccharum in relation to canopy position and growth rate. Plant Ecol 143:189–202. doi:10.1023/A:1009847819158
Loss SR, Hueffmeier RM, Hale CM, Host GE, Sjerven G, Frelich LE (2013) Earthworm invasions in Northern hardwood forests: a rapid assessment method. Nat Areas J 33:21–30. doi:10.3375/043.033.0103
Mader DL, Thompson BW (1969) Foliar and soil nutrients in relation to sugar maple decline. Soil Sci Amer Proc 33:794–800. doi:10.2136/sssaj1969.03615995003300050046x
Manion PD, Lachance D (eds) (1992) Forest Decline concepts. American Phytopathological Society, St. Paul
McLaughlin DL, Linxon SN, Dimma DE, McIlveen WD (1987) Sugar maple decline in Ontario. In: Hutchison TC, Meema KM (eds) Effects of atmospheric pollutants on forests, wetlands, and agricultural ecosystems. Springer, New York, pp 101–116
McTavish MJ, Basiliko N, Sackett TE (2013) Environmental factors influencing immigration behavior of the invasive earthworm Lumbricus terrestris. Can J Zool 91:859–865. doi:10.1139/cjz-2013-0153
Michigan Department of Natural Resources and Environment, Forest Management Division, (2009) Michigan Forest Health Highlights. http://fhm.fs.fed.us/fhh/fhh_09/mi_fhh_09.pdf. Accessed 5 June 2015
Michigan Department of Natural Resources and Environment, Forest Management Division, (2010) Michigan Forest Health Highlights. http://fhm.fs.fed.us/fhh/fhh_10/mi_fhh_10.pdf. Accessed 5 June 2015
Michigan Department of Natural Resources, 2012. Forest Management Division, (2012) Forest Health Highlights https://www.michigan.gov/documents/dnr/ForestHH_409440_7.pdf. Accessed 5 June 2015
Millers I, Lachance D. Burkman WG. Allen DC (1991). North American sugar maple decline project: organization and methods. General Technical Report NE-154. USDA, Forest Service, Northeastern Forest Experiment Station, Radnor
Millers I, Shriner DS, Rizzo D (1989) History of hardwood decline in the Eastern United States. General Technical Report NE-126. USDA Forest Service, Northeastern Forest Experiment Station, Broomall
Moore JD, Ouimet R, Bohlen PJ (2013) Effects of liming on survival and reproduction of two potentially invasive earthworm species in a Northern hardwood forest Podzol. Soil Biol Biochem 64:174–180. doi:10.1016/j.soilbio.2013.04.013
Moore JD, Ouimet R, Long RP, Bukaveckas PA (2015) Ecological benefits and risks arising from liming sugar maple dominated forests in northeastern North America. Environ Rev 23:66–77. doi:10.1139/er-2014-0048
O’Neill KP, Amacher MC, Perry CH (2005) Soils as an indicator of forest health: a guide to the collection, analysis, and interpretation of soil indicator data in the forest inventory and analysis program. General Technical Report NC-258. USDA, Forest Service, North Central Research Station, St. Paul
Patterson SL, Zak DR, Burton AJ, Talhelm AF, Pregitzer KS (2012) Simulated N deposition negatively impacts sugar maple regeneration in a northern hardwood ecosystem. J App Ecol 49:155–163. doi:10.1111/j.1365-2664.2011.02090.x
Reich PB, Oleksyn J, Modryznski J, Mrozinski P, Hobbie SE, Eissesnstat DM, Chorover J, Chadwick OA, Hale CM, Tjoelker MG (2005) Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol Lett 8:811–818. doi:10.1111/j.1461-0248.2005.00779.x
Resner K, Yoo K, Sebestyen SD, Aufdenkampe A, Hale C, Blum A (2015) Invasive earthworms deplete key soil inorganic nutrients (Ca, Mg, K, and P) in a Northern hardwood forest. Ecosystems 18:89–102. doi:10.1007/s10021-014-9814-0
Roth AM, Whitefeld TJS, Lodge AG, Eisenhauer N, Frelich LE, Reich PB (2015) Invasive earthworms interact with abiotic conditions to influence the invasion of common buckthorn (Rhamnus cathartica). Oecologia 178:219–230. doi:10.1007/s00442-014-3175-4
Sackett TE, Smith SM, Basiliiko N (2012) Exotic earthworm distribution in a mixed-use northern temperate forest region: influence of disturbance type, development age, and soils. Can J For Res 42:375–381
Sackett TE, Smith SM, Basiliko N (2013) Indirect and direct effects of exotic earthworms on soil nutrient and carbon pools in North American temperate forests. Soil Biol Biochem 57:459–467. doi:10.1139/x11-195
Schmiege DC, Anderson GW (1960) The forest insect and disease situation, Lake States, 1959. USDA Forest Service Station Paper No 79
Shartell LM, Corace RG, Storer AJ (2012) Exotic earthworm communities within upland deciduous forests of national wildlife refuges in the upper Midwest. J Fish Wildl Manag 3:332–340. doi:10.3996/042012-JFWM-033
Shartell LM, Lilleskov EA, Storer AJ (2013) Predicting exotic earthworm distribution in the northern Great Lakes Region. Biol Invasions 15:1665–1675. doi:10.1007/s10530-012-0399-2
Silva LR, Anand M, Leithead MD (2010) Recent widespread tree growth decline despite increasing atmospheric CO2. PLoS ONE 5:e11543. doi:10.1371/journal.pone.0011543
Soil Survey Division Staff (1993) Soil survey manual. Soil conservation service. USDA Handbook 18
Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Soil Survey Geographic (SSURGO) database for counties in WI and MI. Available online at http://soildatamart.nrcs.usda.gov. Accessed 12 Jan 2012
St. Clair S, Sharpe WE, Lynch JP (2008) Key interactions between nutrient limitation and climatic factors in temperate forests: a synthesis of the sugar maple literature. Can J For Res 38:401–414. doi:10.1139/X07-161
Stearns FW (1997) History of the Lake States Forests: natural and human impacts. In: Vasievich JM, Webster HH (eds) Lake States regional forest resources assessment: technical papers. USDA Forest Service, North Central Forest Experiment Station, Gen-Tech-Rep NC-189. pp 8–29
U.S. Department of Agriculture, Forest Service (1999) Forest Health Monitoring 1999 Field Methods Guide. USDA Forest Service, National Forest Health Monitoring Program, Research Triangle Park, North Carolina
Watmough SA, Brydges T, Hutchison T (1999) The tree-ring chemistry of declining sugar maple in central Ontario, Canada. Ambio 28:613–618. doi:10.2307/4314967
Wironen M, Moore TR (2006) Exotic earthworm invasion increases soil carbon and nitrogen in an old-growth forest in southern Quebec. Can J For Res 36:845–854. doi:10.1139/x06-016
Zurr AF, Ieno EN, Walker NJ, Saveliey AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This research was funded by GMO, LLC and the USDA Forest Service–Forest Health Monitoring Program (Project NC-EM-B-10-02). The foresters at American Forest Management, Inc, the USDA Forest Service, and Michigan DNR were invaluable in their assistance in locating sugar maple stands with and without dieback. Eric Lilleskov, USFS, provided us with the earthworm impact rating scale. We also are indebted to Michael Amacher at the US Forest Service Rocky Mountain Research Station for his assistance with soil processing. Thanks to Christopher Webster, Michigan Tech, for providing use of the tree ring reader. Dana Richter, Eric Lilleskov, Robert Heyd, Joseph O’Brien, and Manfred Mielke provided many early comments and assistance throughout the course of this project. Finally, thanks to the field technicians that helped core trees and process samples: Sally Sanderson, Amy Berns, James Klapperich, Chad Fortin, Christine Jones, Melissa Porter, Donavon Young, Will Schultz, Eric Hollenbeck, Andrew Beebe, Sunshine Love, Alex Larsen, Kurt Lehman, and Jonathon Malette.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bal, T.L., Storer, A.J. & Jurgensen, M.F. Evidence of damage from exotic invasive earthworm activity was highly correlated to sugar maple dieback in the Upper Great Lakes region. Biol Invasions 20, 151–164 (2018). https://doi.org/10.1007/s10530-017-1523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1523-0