Abstract
Alien invasive plant species can affect pollination, reproductive success and population dynamics of co-flowering native species via shared pollinators. Consequences may range from reproductive competition to facilitation, but the ecological drivers determining the type and magnitude of such indirect interactions remain poorly understood. Here, we examine the role of the spatial scale of invader presence and spatially contingent behavioural responses of different pollinator groups as potential key drivers, using the invasive Oxalis pes-caprae and the self-incompatible native annual Diplotaxis erucoides as a model system. Three treatments were assigned to native focal plants: (1) invader present at the landscape scale (hectares) but experimentally removed at the floral neighbourhood scale (pa); (2) invader present at both scales (pp); (3) invader absent at both scales (aa). Interestingly, we found pronounced spatially contingent differences in the responses of pollinators: honeybees and bumblebees were strongly attracted into invaded sites at the landscape scale, translating into native plant visitation facilitation through honeybees, while bumblebees almost exclusively visited Oxalis. Non-corbiculate wild bees, in contrast, showed less pronounced responses in foraging behavior, primarily at the floral neighborhood scale. Average heterospecific (Oxalis) pollen deposition onto stigmas of Diplotaxis was low (<1 %), but higher in the pp than in the pa treatment. Hand-pollination of Diplotaxis with Oxalis and conspecific pollen, however, reduced seed set by more than half when compared to hand-pollination with only conspecific pollen. Seed set of Diplotaxis, finally, was increased by 14 % (reproductive facilitation) in the pp treatment, while it was reduced by 27 % (reproductive competition) in the pa treatment compared to uninvaded populations. Our study highlights the crucial role of spatial scale and pollinator guild driving indirect effects of invasive on co-flowering native plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sympatric plant species frequently interact with co-flowering species via shared pollinators, with potentially pronounced consequences for their pollination, reproductive success, population dynamics, and evolution (e.g. Rathcke 1983; Feinsinger 1987; Sargent and Ackerly 2008). Many alien invasive (hereafter invasive) plant species are characterized by large flower displays and abundant floral resources that are highly attractive to native pollinators (Morales and Traveset 2009 and references therein). Consequently, invasive plant species are usually well integrated in local native plant–pollinator networks via generalist pollinators (e.g. Padrón et al. 2009; Traveset et al. 2013; Albrecht et al. 2014). This suggests that pollinator-mediated impacts of invasive on native species in invaded communities are common and may represent an important pathway by which plant invasions alter the reproductive success and thus possibly population dynamics of native plant species. These consequences may be negative (competitive; e.g. Chittka and Schürkens 2001; Totland et al. 2006; Muñoz and Cavieres 2008; Kandori et al. 2009; Flanagan et al. 2010, 2011), neutral or mixed (Nielsen et al. 2008; Moragues and Traveset 2005; Sun et al. 2013; Ferrero et al. 2013) or positive (facilitative; Lopezaraiza-Mikel et al. 2007; Molina-Montenegro et al. 2008; Jakobsson and Padrón 2014). Competition for pollination services among plant species can occur if a focal plant is pollen limited and pollinator visitation and thus the amount of pollen received is reduced in the presence of other co-flowering species usurping pollinator visits and thereby aggravating pollen limitation (e.g. Chittka and Schürkens 2001; Flanagan et al. 2010). Co-flowering species sharing pollinators may also experience competition through heterospecific pollen transfer (reviewed in Morales and Traveset 2009), which can reduce female reproductive success through stigma clogging (Waser and Fugate 1986), pollen allelopathy (Kanchan and Chandra 1980) or male reproductive success through pollen loss (Campbell and Motten 1985). Conversely, the presence of co-flowering species may facilitate pollinator visitation, pollination and reproductive success of a focal plant species (Rathcke 1983; Moeller 2004). Mechanisms of facilitation of pollinator visitation includes the enhanced per capita visitation to relatively unattractive plant species in the presence of a more attractive species (“magnet-species” effect; Thomson 1978), increased pollinator attraction and visitation due to larger collective floral displays of co-flowering species (Schemske 1981) or higher floral resource diversity (Ghazoul 2006), and increased numerical response of pollinators to a focal plant species in the presence of co-flowering species across years (Moeller 2004). Visitation facilitation does not necessarily result in reproductive facilitation, which is also dependent on visit quality (Mitchell et al. 2009). Although such indirect interactions among plants via shared pollinators and some of their underlying mechanisms have been demonstrated for a series of plant–pollinator systems, understanding the ecological factors and processes (i.e. the ecological context; Mitchell et al. 2009) determining whether they will be competitive, neutral or facilitative remains a central challenge for ecologists.
A potentially crucial missing link in the explanation of these inconsistent and partly conflicting results is considering the spatial scale of heterospecific plant interactions via shared pollinators. On a small floral neighbourhood scale, pollinators may get lured from native plants to more conspicuous invaders with larger rewards. Conversely, pollinators may switch from invasive to native plants; in such cases, increased visitation may come at the expense of increased heterospecific pollen transfer (Cariveau and Norton 2009; Mitchell et al. 2009; Morales and Traveset 2009 and references therein). On a larger (landscape) scale, however, the presence of such highly rewarding invasive plant species may attract pollinators into invaded communities (possibly enhancing local pollinator population sizes in the longer term), which may result in increased visitation levels to sympatric native species (Bjerknes et al. 2007; Jakobsson and Padrón 2014). The type and magnitude of these processes may therefore be driven by the relative spatial distribution of invasive and native plants and the foraging behaviour of the involved pollinator groups (Jakobsson et al. 2008; Cariveau and Norton 2009). For example, social honeybees and bumblebees are expected to respond stronger to resource availability on a landscape scale than non-corbiculate solitary bees, due to their larger-scale foraging decisions and recruitment abilities (Steffan-Dewenter et al. 2002; Albrecht et al. 2007; Jha and Vandermeer 2009), and their preference for large flowering resource patches (e.g. Westphal et al. 2003) compared to non-corbiculate solitary bees (Sih and Baltus 1987). Hence, facilitation and competition among plant species via shared pollinators and the reproductive consequences of plant invasions on native plants may crucially depend on the interplay of these spatially contingent processes driven by distinct responses of different pollinator guilds (Bjerknes et al. 2007; Jakobsson et al. 2009).
Here, we examined the role of spatial scale driving pollinator-mediated impacts of an invasive plant on pollination and reproductive success of a native plant, using the annual Diplotaxis erucoides native to the Mediterranean basin and the invasive Oxalis pes-caprae as a model system. To explore potential mechanisms and their interplay at multiple spatial scales driving these effects we combined manipulative field experimentation with pollinator observations, pollen load analysis and a hand pollination experiment. Specifically, we addressed the following questions:
-
1.
Do the pollinator guilds of D. erucoides display different foraging behaviour in the presence of the invasive plant O. pes-caprae, and if so, does the scale of invasion (floral neighbourhood vs. landscape) affect the magnitude of this difference?
-
2.
Is the effect of heterospecific (invasive) pollen deposition on stigmas of native plants contingent upon the spatial scale of invasion?
-
3.
Does the presence of the invasive O. pes-caprae alter the reproductive success of the native D. erucoides by sharing pollinators with it, and is this effect contingent on the spatial scale of invader presence? How do the processes addressed in (1) and (2) interact across spatial scales and contribute to native plant reproductive success?
Materials and methods
Study species
Diplotaxis erucoides L. (Brassicaceae; hereafter Diplotaxis) is a weedy annual that is common throughout the Mediterranean basin frequently found in disturbed sites such as extensively managed orchards, olive groves and crop fields, and ruderal habitats. Plants are usually 0.2–1 m tall and form racemes of white flowers, with 4-8 flowers open at a given time. Each actinomorphic flower lasts around 3 days and consists of four petals arranged diagonally to other, six stamens (two short and four long ones) and one central pistil with swollen stigma. Fruits consist of thin dehiscent siliqua (Sans and Bonet 1993). The species requires cross-pollination to set seed (Kunin 1992; Sans and Bonet 1993; see also “Results” section). Oxalis pes-caprae (Oxalidaceae; hereafter Oxalis) is a bulbous annual herb originating from South Africa. It is among the most aggressive invasive plants of the Mediterranean region (Peirce 1997). It is typically 0.3–0.4 m in height mainly reproduces vegetatively via bulbs, and can form large clonal colonies (Vila et al. 2006). The cup-shaped flowers are relative large and distinctively bright yellow. Flowers are light-sensitive and close during very cloudy days and in late afternoon (Jakobsson et al. 2009). Although it is possible that Diplotaxis flowers received pollinator visits after Oxalis flowers had closed, it is unlikely that this disproportionally small proportion of visits could have strongly contributed to our findings. Both species offer nectar rewards to flower visitors (Gulyás and Czimber 1990; Costa et al. 2014). On the Balearic Islands, the two species share the same habitats, mainly in agricultural, ruderal and disturbed areas, and most of their principal flower visitors are the honeybee [Apis mellifera L. (Hymenoptera: Apidae)] and non-corbiculate bees, such as Anthophora sp., Eucera sp. and Andrena sp. (Jakobsson et al. 2009 for the Balearic Islands; Ferrero et al. 2013; Costa et al. 2014 for other study regions in the Western Mediterranean basin). Other, less frequent flower visitors are bumblebees, such as Bombus terrestris L. (Hymenoptera: Apidae) and Psithyrus sp. (Ferrero et al. 2013; Costa et al. 2014), as well as flies (including hoverflies, such as Eristalis tenax L. (Diptera: Syrphidae)) and butterflies, such as Pieris brassicae L. (Lepidoptera: Pieridae) (Jakobsson et al., 2009). Moreover, the flowering periods of both species largely overlap with their peak flowering time usually in January–February. Due to these flowering and reproductive traits, together with the fact that during their flowering period only few other plant species in these habitats are flowering, these two species represent an ideal study system for pollinator-mediated effects on the reproductive success of invasive on native plant species (Jakobsson et al. 2009).
Study design
The study was carried out on the island of Mallorca (Balearic Islands, Spain). Using a paired design, 12 sites were selected from six regions (two sites per region) in the eastern part of the cultivated plateau of the island. Each pair within a region consisted of a site that contained a population of Diplotaxis that was invaded by Oxalis (hereafter invaded sites) and a uninvaded site holding a population of Diplotaxis, but without Oxalis being present within a perimeter of at least 200 m (uninvaded sites). Populations of Diplotaxis within a region were separated by at least 2.4 km (mean ± SE: 4.1 ± 0.8 km), and regions were separated by 12.0 (±3.1) km on average. At each site we delimited 3 × 3 m plots: four plots at the invaded sites and two plots at the uninvaded sites. At invaded sites, Oxalis was experimentally removed at two plots, while at two plots it remained present. In each experimental removal plot, Oxalis flowers and buds, were continuously removed throughout the experiment (at least twice a week), leaving the vegetative parts intact. Thus, three different treatments as a function of the spatial scale at which the invasive Oxalis was present were established: (1) invader present at the landscape (ha) and the flowering neighbourhood scale (m2) (pp treatment); (2) invader present at the landscape scale but absent at the flowering neighbourhood scale (pa treatment); and (3) invader absent at both scales (aa treatment). Plots were chosen so that they were similar in the number of plants and flowers. In two regions we could establish only one plot per treatment due to small population sizes of Diplotaxis. Thus, a total of 30 plots were studied.
Pollinator visits
In the center of each plot, three Diplotaxis were marked (hereafter focal plants) and flower visitation by pollinators was observed during 30 min in each of four sampling rounds from the beginning of February until the end of May 2011, always on sunny and calm days. Each plot was censused twice in the morning (10:00–12:30) and twice in the afternoon (12:30–15:00; no observations were made later in the afternoon after flowers of Oxalis closed). In the experimental removal treatment, we left at least 1 day between the removal of Oxalis flowers and censusing of pollinators of Diplotaxis. Whenever possible, all plots and treatments of a region and sampling round were censused on the same day, or, if not possible, censuses were made within two consecutive days. In total, 20 h of observation time were spent for each treatment. In each census we recorded the number of pollinator visits (i.e. only those flower visits contacting the reproductive organs of the flower), distinguishing between different pollinator groups: non-corbiculate wild bees, honeybees, bumblebees (Bombus sp.), flies, beetles (these groups represent the important pollinator groups of Diplotaxis and Oxalis (Jakobsson et al. 2009; Fig. 3) and other flower visitors. Although some of these groups, such as non-corbiculate wild bees, encompass a range of species differing in morphology and potentially also foraging behaviour, these broader pollinator guilds have proven useful in many studies to address research questions such as those in this study (e.g. Steffan-Dewenter et al. 2002). For practical reasons, tiny flower visitors (<3 mm; e.g. thrips) were not censused. However, these very small insects only rarely visit Diplotaxis in the study area and are unlikely of any functional importance for the pollination of the study plant species (Jakobsson et al. 2009). Due to low numbers, flower visitor groups other than the two main pollinator groups, non-corbiculate wild bees and honeybees, could not be separately analysed. The open flowers of the focal plants were marked with a small piece of ribbon and recorded in each census.
To further assess and compare the pollinator communities at the landscape scale, transect walks (Westphal et al. 2008) were conducted once along five 10 m transects with a width of 2 m (100 m2 total census area) at each site and all flower visiting insects recorded and assigned to the following groups: non-corbiculate wild bees, honeybees, bumblebees, flies, beetles, others.
Pollen deposition
In order to quantify conspecific and heterospecific (from Oxalis) pollen deposition to Diplotaxis under the different treatments, two randomly chosen matured stigmas (of approximately the same stage of maturation) were collected from observed marked flowers of each of the three focal plants of each plot (giving 60 samples per treatment and a total of 180 samples). All stigmas of a site were collected on the same day, placed on a slide using mounting medium and stained with fuchsine glycerogelatin (Trigo et al. 2007) and covered with a cover slip immediately after collection in the field. The cover slip was firmly pressed over the stigmas so that pollen grains were distributed in a single layer, facilitating their identification (Kearns and Inouye 1993). In the laboratory, stigmas were observed under a microscope at 400×, and pollen grains were counted and identified with the help of a reference collection. Counts of conspecific pollen included self-pollen since flowers were not emasculated. To further investigate effects of heterospecific (Oxalis) pollen deposition on the reproductive success of Diplotaxis, we performed a hand-pollination experiment at one of the uninvaded study sites. Each of three randomly selected inflorescences of each of 20 randomly chosen plants were bagged with a white nylon bag of c. 1 mm mesh size to prevent insects. One flower of each inflorescence was assigned to one of three hand pollination treatments: (1) conspecific pollen treatment: the flower was pollinated with an anther (either short or long stamen) of another Diplotaxis individual present in the same population but not in the direct neighbourhood of the treated plant. Flowers were hand-pollinated by rubbing the freshly collected anther of the donor plant four times onto the lobes of the stigmata (four applications); (2) heterospecific pollen treatment with a low amount of Oxalis pollen: the Diplotaxis flower was pollinated with a randomly chosen anther of a randomly selected Oxalis flower previously collected (less than 1 h before) from another locality with one application of the anther onto the stigma. Subsequently, Diplotaxis pollen was applied onto the same stigma [Kwak and Jennersten 1991; four applications as in treatment (1)]; (3) heterospecific pollen treatment with a high amount of Oxalis pollen: same as treatment (2) but four applications instead of one of Oxalis pollen. Each treated flower was marked (by means of a permanent pen) with a unique code according to the applied hand pollination treatment and bagged again until fruits were ripe and siliqua were collected and the number of seeds in each was recorded. Because six of the 20 plants died from drought before collection, a total of 14 plants (42 fruits) could be analysed.
Plant reproductive success
Ripe but still closed fruits of three randomly chosen inflorescences of each focal Diplotaxis plant of each plot were collected and the number of seeds a fruit contained was recorded. Only fruits from flowers that were open during the experimental treatments (marked flowers) were considered for the analysis of seed set. A total of 890 fruits were analyzed. Since Diplotaxis is an annual plant, seed set directly reflects life-time reproductive success.
Statistical analyses
All analyses were performed with the statistical software R version 2.13.1 (R Development Core Team 2013). Linear mixed effect models were fitted to test differences among flower visitors (response variables: total number of visits of non-corbiculate wild bees, honeybees, bumblebees (log-transformed), flies (square-root transformed), beetles and other flower visitors (both log-transformed)) at the plant community between invaded and uninvaded sites (transect sampling), with treatment as fixed effect and region as random effect. A model was fitted for each different flower visitor group (data pooled across transects for each site) using the lme-function of the nlme package (Pinheiro et al. 2009). To test treatment effects on total pollinator visitation [response variable total number of pollinator visits to flowers of Diplotaxis (pooled over all three focal plants per 120 min); research question (1)], a linear mixed effect model with treatment, fitted after the covariate number of flowers of the observed focal plants, and plot, site and region as nested random effects was fitted. Time of day (morning vs. afternoon) did not explain significant variation in pollinator visitation and the data were therefore pooled. To further investigate potential differences in visitation patterns among pollinator groups, additional (separate) models were fitted with the response variables total number flower visits by honeybees and the total number of flower visits by non-corbiculate wild bees (square-root transformed)—the two most important pollinator groups of Diplotaxis (Fig. 3). The covariate number of flowers did not explain significant variation in these latter two response variables and was therefore omitted from the model (Zuur et al. 2009). Differences among treatment levels (three levels) were analysed based on the model contrasts following recommendations by Moran (2003).
Except for one stigma, no pollen of Oxalis was found on stigmas of Diplotaxis in uninvaded sites; hence, only the data of invaded sites (pp vs. pa treatments) were considered for the analysis of (1) the number of pollen grains of Oxalis (square-root-transformed) and (2) the proportion of Oxalis pollen (logit-transformed, Warton and Hui 2011) on stigmas of Diplotaxis [research question (2)]. Accordingly, linear mixed effect models with treatment as fixed effect and region, plot and focal plant as nested random effects were fitted to analyse variation in these two response variables. Significant differences of the pp and pa treatment from 0 indicated significant differences between these treatments and the aa treatment. To test for treatment differences in the hand pollination experiment, linear mixed model analysis was used; donor stamen (long vs. short) and plant were included as nested random effects and the number of seeds produced per hand-pollinated flower as response variable. To test treatment effects on seed set of Diplotaxis [research question (3)], linear mixed effect models with focal plant, plot, site and region as nested random effects were fitted.
Linear model assumptions of normality and homoscedasticity of residuals were visually verified by normal Q–Q plots and by plotting residuals against the predicted values (Zuur et al. 2009). Graphs were created using the software GraphPad Prism 5.04. Arithmetic means (±1 SE) are reported.
Results
Flower visitation by pollinators
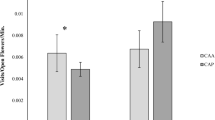
At the entire plant community level of invaded and uninvaded sites (transect sampling), the number of honeybees (F 1,5 = 5.18, P < 0.072) and bumblebees (F 1,5 = 4.89, P < 0.078) tended to be higher at invaded compared to uninvaded sites, whereas the numbers of flower-visiting non-corbiculate wild bees and other groups were not significantly different among invaded and uninvaded sites (Fig. 1). On focal Diplotaxis plants, the number of visits did not differ between treatment aa and either the pp or the pa treatment after accounting for variation in flower number, but tended to be higher when Oxalis was present at the flowering neighbourhood scale (pp) than when experimentally removed (pa; t = 1.94, df = 17, P = 0.069) (Fig. 2a). Separate analyses of the two most important groups of pollinators of Diplotaxis, non-corbiculate wild bees and honeybees, revealed that these two groups responded differently to the treatments: the number of flower visits by honeybees was higher in the pp (t = 2.85, df = 5, P = 0.036), and, slightly less so in the pa treatment (t = 2.64, df = 5, P = 0.046), compared to the aa treatment (Fig. 2b). However, it did not differ between the pp and the pa treatment in invaded sites (Fig. 2b). In contrast, flower visits by non-corbiculate bees was not significantly increased in the pp or the pa treatment in invaded sites compared to uninvaded ones (Fig. 2c). Moreover, the slightly higher average number of non-corbiculate bee visits in the pp treatment compared to the pa treatment in invaded sites (Fig. 2c) was statistically not significant (P > 0.1).
Mean (+1 SE) number of visitors of flowering plants present within belt transects (100 m2 total census area) at invaded and uninvaded sites. Different letters (regular font) indicate significant differences among treatments tested separately for each flower visitor group [P < 0.05; italic letters indicate marginally significant results (P < 0.1)]
a Total number of pollinator visits (mean + 1 SE) to flowers of the native Diplotaxis erucoides, b mean (+1 SE) number of honeybee visits and c mean (+1 SE) number of non-corbiculate wild bee visits per 120 min as a function of the spatial scale of the presence of Oxalis pes-caprae: the invasive species was present at both the landscape scale and the flowering neighborhood scale (pp); the invader was present at the landscape scale but absent at the flowering neighborhood scale (pa); the invader was absent at both spatial scales (aa). Different letters (regular font) indicate significant differences among treatments tested separately for each flower visitor group [P < 0.05; italic letters indicate marginally significant results (P < 0.1)]
Heterospecific pollen deposition on native stigmas
The number of conspecific pollen grains did not differ significantly among treatments (Fig. 3). Heterospecific pollen deposition, however, was higher in the pp compared to the pa treatment (absolute number of pollen grains: t = 5.31, df = 13, P < 0.001; proportion: t = 5.34, df = 13, P < 0.001) at invaded sites (Fig. 3). Although only contributing a small proportion of the total stigmatic pollen load (Fig. 3), both the absolute number (pp: t = 16.41, df = 62, P < 0.001; pa: t = 10.88, df = 62, P < 0.001) and the proportion (pp: t = 27.20, df = 62, P < 0.001; pa: t = 32.65, df = 62, P < 0.001) of heterospecific Oxalis pollen grains on the stigmas of Diplotaxis differed significantly from 0. The number of pollen grains of other species on stigmas of Diplotaxis was low and not significantly different among treatments (Fig. 3).
Mean (+1 SE) number of conspecific and heterospecific (invasive Oxalis pes-caprae) pollen grains on stigmas of Diplotaxis erucoides as a function of the spatial scale of the presence of Oxalis: the invasive species was present at both the landscape scale and the flowering neighborhood scale (pp); the invader was present at the landscape scale but absent at the flowering neighborhood scale (pa); the invader was absent at both spatial scales (aa). Different letters indicate significant differences among treatments (P < 0.05)
Flowers of Diplotaxis exclusively pollinated with conspecific pollen resulted in higher (more than twice as high) seed set than flowers pollinated with both conspecific and Oxalis pollen, regardless of whether the amount of this heterospecific pollen was high (t = 2.85, df = 26, P = 0.009) or low (t = 3.26, df = 26, P = 0.001) (Fig. 4). No differences were detected in seed set when comparing only flowers pollinated with high and low amounts of Oxalis pollen (Fig. 4).
Mean (+1 SE) number of seeds per fruit of the native Diplotaxis erucoides resulting from three hand-pollination treatments: (1) pollination with conspecific (Diplotaxis) pollen only, (2) pollination with conspecific and high amounts of heterospecific (invasive Oxalis pes-caprae) pollen and (3) pollination with low amounts of Oxalis pollen (for details of the hand-pollination protocol see “Materials and methods” section). Different letters indicate significant differences among treatments (P < 0.05)
Plant reproductive success
Seed set of Diplotaxis differed among treatments (F 2,16 = 35.11, P < 0.001; Fig. 5): it was lower in uninvaded sites (aa) compared to sites invaded by Oxalis, when the invasive species was present at both the landscape scale and the flowering neighbourhood scale (pp; t = 2.60, df = 5, P = 0.048). However, seed set of plants in the aa treatment was higher than that of plants in the pa treatment (t = 4.89, df = 5, P = 0.005; Fig. 5). Seed set of Diplotaxis in the pa treatment was lower than in the pp treatment (t = 8.28, df = 17, P < 0.001; Fig. 5).
Mean (+1 SE) number of seeds per fruit of Diplotaxis erucoides as a function of the spatial scale of the presence of Oxalis pes-caprae: the invasive species was present at both the landscape scale and the flowering neighborhood scale (pp); the invader was present at the landscape scale but absent at the flowering neighborhood scale (pa); the invader was absent at both spatial scales (aa). Different letters indicate significant differences among treatments (P < 0.05)
Discussion
Effect of the spatial scale of invasion on flower visitation
Invasions by attractive and highly rewarding invasive plant species, such as Oxalis pes-caprae, can result in a pronounced local increase in floral resources. The consequences on the quantity of native flower visits will depend on the behavioural and population dynamical responses of pollinators, which may involve different spatially hierarchical processes (Jakobsson et al. 2008). The addition of abundant floral resources may attract pollinators from non-invaded areas into the invaded ones (Albrecht et al. 2014), potentially leading to visitation facilitation, if per-capita visitation of one or more co-flowering species also is increased (Feldman et al. 2004). Indeed, our transect walk results indicate a strong increase (34 %) in flower visitor numbers of invaded communities at the landscape scale, in particular of the social honeybees and bumblebees (Fig. 1; Online Resource 1). Interestingly, the different pollinator groups responded distinctively to the presence of the invasive plant at different spatial scales. The honeybee, an important pollinator species of Diplotaxis (see also Jakobsson et al. 2009), was highly attracted by and a frequent visitor of Oxalis. This translated into visitation facilitation at the landscape (entire plant population) scale: honeybees more frequently visited Diplotaxis flowers in sites invaded by Oxalis compared to uninvaded ones, irrespective of whether the invader was present at the floral neighbourhood scale or not. While literally absent at uninvaded sites, bumblebees (mainly Bombus terrestris species complex, M. Albrecht, pers. obs.) almost exclusively visited Oxalis—and, in contrast to honeybees, only very rarely Diplotaxis flowers—at invaded sites. Thus, despite the high temporal and spatial overlap of the two co-flowering plant species at invaded sites, bumblebees showed a strong preference and a high degree of flower constancy for the invasive species, a phenomenon that has also been observed for other highly attractive plant species invading native communities (e.g. Bartomeus et al. 2008, and A. Traveset, unpubl. data for Carpobrotus edulis). Non-corbiculate wild bees, the most frequent flower visitors of Diplotaxis, showed yet another response in foraging behavior as a function of the invader presence at different spatial scales: they tended to visit more Diplotaxis flowers when these were close to Oxalis flowers, although no visitation facilitation was found on the landscape scale. This suggests some visitation spillover restricted to the immediate floral neighbourhood (individual plant patch) scale (Cariveau and Norton 2009). Indeed, we regularly observed such switching behavior of non-corbiculate bees, in particular of Eucera sp. bees (the most frequent shared flower visitors of Diplotaxis and Oxalis; see also Jakobsson et al. 2009), from Oxalis to adjacent Diplotaxis flowers and vice versa. Switching bees may perceive adjacent plants as a single patch of resources (Klinkhamer et al. 2001), even if composed of different species. Even if pollinators do discriminate among co-flowering species and exhibit high levels of flower constancy, other flowering species are regularly probed and compared in resource levels to the preferred species, and if rewards are low, switching is predicted by optimal foraging theory (Goulson 1999). Interestingly, Jakobsson et al. (2009) studying the same invader-native plant model system, found, contrary to our study, increased visitation rates by pollinators—mainly solitary bees—when Oxalis was absent at the flowering neighbourhood scale but present at the landscape scale. It remains open whether this discrepancy is due to site specific factors—Jakobsson et al. (2009) studied a series of plots at a single site—across year variation in pollinator community composition or other factors driving these scale effects. It would have been interesting to compare the consequences on plant reproductive success among the two studies, but unfortunately this was not measured in Jakobsson et al. (2009). Our findings, however, suggest that the social honeybees and bumblebees mainly perceived and responded to the resource boost by the invasive Oxalis at the landscape scale, being strongly attracted from the surrounding landscape into the invaded sites, while the non-corbiculate wild bees appeared to show changes in foraging behavior primarily at the floral neighborhood scale. This is in a line with studies showing larger-scale foraging decisions and recruitment abilities of honeybees and bumblebees compared to non-corbiculate solitary bees (e.g. Steffan-Dewenter et al. 2002; Albrecht et al. 2007; Jha and Vandermeer 2009) and stronger preference for large and abundantly flowering patches, including mass-flowering crops (e.g. Westphal et al. 2003), than solitary bees (Sih and Baltus 1987). Larger foraging ranges, facilitated by on average larger body sizes, in particular of bumblebees (Steffan-Dewenter et al. 2002), advanced communication skills and often higher levels of flower constancy of honeybees and bumblebees (Goulson 1999) may have contributed to these distinct patterns among the two pollinator guilds.
The role of heterospecific pollen deposition across spatial scales
In agreement with the observed switching of pollinators among Oxalis to Diplotaxis at invaded sites, the analysis of the stigmas of focal Diplotaxis plants proofed the occurrence of heterospecific (invasive) pollen deposition onto native stigmas in invaded populations. In agreement with our expectations, the proportion of Oxalis pollen transferred to Diplotaxis stigmas was higher when the invader was also present in the direct vicinity of focal Diplotaxis. Although we could not quantitatively analyse switching behavior here, our observations suggest that non-corbiculate bees accounted for most of the switching, and may therefore have primarily contributed to the observed higher rates heterospecific pollen deposition at the floral neighborhood scale in the presence of Oxalis. Indeed, non-corbiculate solitary bees appear to less often show high levels of flower constancy than social honeybees or bumblebees (e.g. Goulson 1999).
Consequences on native plant reproductive success
Pollinator mediated consequences on plant reproduction are driven by both the quantity and quality of pollinator visits. We found the impacts of Oxalis on the reproductive success of the native species in our model system crucially depending on the spatial scale of the invader presence: reproductive success of Diplotaxis was higher in invaded sites only if Oxalis was present at the floral neighborhood scale, but decreased if the invader was absent from such floral neighborhood scale. This finding of reproductive facilitation is in agreement with the tendency towards higher numbers of visits by the most frequent flower visitors, non-corbiculate wild bees and honeybees. Indeed, our findings suggest that primarily these spatially contingent changes in visitation frequency driven by spatially dependent behavioural responses of different pollinator guilds have important consequences on native plant reproductive success. In addition to visitation facilitation through spillover effects from co-flowering Oxalis plants, the influence of “effective mutualism” sensu Waser and Real (1979) may have also contributed to the pronounced reproductive facilitation effects. Thus, pollinators foraging on Oxalis in invaded Diplotaxis sites could have switched to the latter after Oxalis flowers have closed in late afternoon (approximately after 15:00 hours). However, since the proportion of flower visits Diplotaxis receives after this time of the day was disproportionally small (Albrecht, personal observation), the latter pathway was probably of minor importance in contributing to the observed reproductive facilitation. Heterospecific (invasive) pollen deposition, although potentially important at high levels (as shown by the hand-pollination experiment) appeared to play a less important role in reproductive success at the low levels observed in our study under field conditions (<1 % on average). Our findings may reconcile some of the contradicting findings of facilitative versus competitive effects of invasive on native plants via shared pollinators. For example, our results are in agreement with several studies documenting facilitative effects on a floral neighbourhood scale (e.g. Molina-Montenegro et al. 2008; Ferrero et al. 2013) as well as with studies reporting competitive effects on native focal plants further away from invasive plants in invaded populations (Brown and Mitchell 2001; Brown et al. 2002; Cariveau and Norton 2009). Factors other than spatial scale such as the relative abundance of invasive and native species (Muñoz and Cavieres 2008; Flanagan et al. 2010) or similarity in flower phenotype (Morales and Traveset 2009) have been identified as further predictors of pollinator-mediated consequences of invasive on native plant pollination, but predictions may not always match observations (e.g. Sun et al. 2013). Our study highlights that—in addition to spatial scale—also pollinator guild and their distinct behavioural responses to invader presence across spatial scales (affecting both quantity and quality of visits to native plants) appear to play a crucial role in understanding and predicting such consequences. This may actually help explain the contrasting findings of studies using similar or even identical invasive-native plant study systems but likely different compositions of the involved pollinator communities (e.g. Jakobsson et al. 2009; Ferrero et al. 2013; this study).
Conclusions
We conclude that processes shaping the pollinator mediated effects of plant species invasions on the pollination and reproductive success of native plant species critically depends on (1) the spatial scale of invader presence and (2) the net effect of distinct, spatially contingent behavioral responses of the involved pollinator groups. Our study shows that both reproductive facilitation and competition can occur within the same invasive-native plant species system depending on the spatial location of the native focal plant relative to invasive plants. These findings highlight that both the type of pollinator-mediated indirect plant–plant interactions (facilitation vs. competition) and the spatial scale (floral neighborhood vs. population or landscape level) of such interactions are crucially contingent on the involved pollinator guilds.
References
Albrecht M, Duelli P, Müller C, Kleijn D, Schmid B (2007) The Swiss agri-environment scheme enhances pollinator diversity and plant reproductive success in nearby intensively managed farmland. J Appl Ecol 44:813–822
Albrecht M, Padrón B, Bartomeus I, Traveset A (2014) Consequences of plant invasions on compartmentalization and species’ roles in plant–pollinator networks. Proc R Soc B 281:20140773. doi:10.1098/rspb.2014.0773
Bartomeus I, Bosch J, Vilà M (2008) High invasive pollen transfer, yet low deposition on native stigmas in a Carpobrotus-invaded community. Ann Bot 102:417–424
Bjerknes AL, Totland Ø, Hegland SJ, Nielsen A (2007) Do alien plant invasions really affect pollination success in native plant species? Biol Conserv 138:1–12
Brown BJ, Mitchell RJ (2001) Competition for pollination: effects of pollen of an invasive plant on seed set of a native congener. Oecologia 129:43–49
Brown BJ, Mitchell RJ, Graham SA (2002) Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83:2328–2336
Campbell DR, Motten AF (1985) The mechanism of competition for pollination between two forest herbs. Ecology 66:554–563
Cariveau DP, Norton AP (2009) Spatially contingent interactions between an exotic and native plant mediated through flower visitors. Oikos 118:107–114
Chittka L, Schürkens S (2001) Successful invasion of a floral market. Nature 411:653
Costa J, Ferrero V, Castro M, Jorge A, Afonso A, Loureiro J, Castro S (2014) Pollen flow between flowers of the same morph in invasive populations of Oxalis pes-caprae L. in the western Mediterranean region. Plant Biosyst. doi:10.1080/11263504.2014.991363
Feinsinger P (1987) Effects of plant species on each other’s pollination: is community structure influenced? Trends Ecol Evol 2(5):123–126
Feldman TS, Morris WF, Wilson WG (2004) When can two plant species facilitate each other’s pollination? Oikos 105(1):197–207
Ferrero V, Castro S, Costa J, Acuña P, Navarro L, Loureiro J (2013) Effect of invader removal: pollinators stay but some native plants miss their new friend. Biol Invasions 15:2347–2358
Flanagan RJ, Mitchell RJ, Karron JD (2010) Increased relative abundance of an invasive competitor for pollination Lythrum salicaria reduces seed number in Mimulus ringens. Oecologia 164:445–454
Flanagan RJ, Mitchell RJ, Karron JD (2011) Effects of multiple competitors for pollination on bumblebee foraging patterns and Mimulus ringens reproductive success. Oikos 120:200–207
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304
Goulson D (1999) Foraging strategies of insects for gathering nectar and pollen and implications for plant ecology and evolution. Perspect Plant Ecol Evol Syst 2:185–209
Gulyás S, Czimber G (1990) Apicultural importance of Diplotaxis erucoides (Torner) DC. Acta Ovar 32(1):12–17
Jakobsson A, Padrón B (2014) Does the invasive Lupinus polyphyllus increase pollinator visitation to a native herb through effects on pollinator population sizes? Oecologia 174:217–226
Jakobsson A, Padrón B, Traveset A (2008) Pollen transfer from invasive Carpobrotus spp to natives—a study of pollinator behaviour and reproduction success. Biol Conserv 141:136–145
Jakobsson A, Padrón B, Traveset A (2009) Competition for pollinators between invasive and native plants: effects of spatial scale of investigation. Ecoscience 16:138–141
Jha S, Vandermeer JH (2009) Contrasting bee foraging in response to resource scale and local habitat management. Oikos 118:1174–1180
Kanchan S, Chandra J (1980) Pollen allelopathy—a new phenomenon. New Phytol 84:739–746
Kandori I, Hirao T, Matsunaga S, Kurosaki T (2009) An invasive dandelion unilaterally reduces the reproduction of a native congener through competition for pollination. Oecologia 159:559–569
Kearns CA, Inouye DW (1993) Techniques for pollination biologists. University Press of Colorado, Colorado
Klinkhamer PG, De Jong TJ, Linnebank LA (2001) Small-scale spatial patterns determine ecological relationships: an experimental example using nectar production rates. Ecol Lett 4:559–567
Kunin WE (1992) Density and reproductive success in wild populations of Diplotaxis erucoides (Brassicaceae). Oecologia 91:129–133
Kwak MM, Jennersten O (1991) Bumblebee visitation and seed set in Melampyrum pratense and Viscaria vulgaris: heterospecific pollen and pollen limitation. Oecologia 86:99–104
Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J (2007) The impact of an alien plant on a native plant–pollinator network: an experimental approach. Ecol Lett 10:539–550
Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD (2009) New frontiers in competition for pollination. Ann Bot 103:1403–1413
Moeller DA (2004) Facilitative interactions among plants via shared pollinators. Ecology 85:3289–3301
Molina-Montenegro MA, Badano EI, Cavieres LA (2008) Positive interactions among plant species for pollinator service: assessing the ‘magnet species’ concept with invasive species. Oikos 117:1833–1839
Moragues E, Traveset A (2005) Effect of Carpobrotus spp on the pollination success of native plant species of the Balearic Islands. Biol Conserv 122:611–619
Morales CL, Traveset A (2009) A meta-analysis of impacts of alien vs native plants on pollinator visitation and reproductive success of co-flowering native plants. Ecol Lett 12:716–728
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
Muñoz AA, Cavieres LA (2008) The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. J Ecol 96:459–467
Nielsen C, Heimes C, Kollmann J (2008) Little evidence for negative effects of an invasive alien plant on pollinator services. Biol Invasions 10:1353–1363
Padrón B, Traveset A, Biedenweg T, Díaz D, Nogales M, Olesen JM (2009) Impact of alien plant invaders on pollination networks in two archipelagos. PLoS ONE 4:e6275. doi:10.1371/journal.pone.0006275
Peirce JR (1997) The biology of Australian weeds: 31 Oxalis pes-caprae L. Plant Protect Q 12:110–119
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2009) nlme: linear and nonlinear mixed effects models. R package version, 31–96
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rathcke BJ (1983) Competition and facilitation among plants for pollination. In: Real L (ed) Pollination biology. Academic, New York, pp 305–329
Sans XF, Bonet A (1993) Producción de frutos y semillas en Diplotaxis erucoides (L.) DC sometida a diferentes tratamientos de polinización. Collect Bot 22:49–54
Sargent RD, Ackerly DD (2008) Plant–pollinator interactions and the assembly of plant communities. Trends Ecol Evol 23:123–130
Schemske DW (1981) Floral convergence and pollinator sharing in two bee-pollinated tropical herbs. Ecology 62:946–954
Sih A, Baltus MS (1987) Patch size, pollinator behavior and pollinator limitation in catnip. Ecology 68:1679–1690
Steffan-Dewenter I, Münzenberg U, Bürger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83:1421–1432
Sun SG, Montgomery BR, Li B (2013) Contrasting effects of plant invasion on pollination of two native species with similar morphologies. Biol Invasions 15:2165–2177
Thomson JD (1978) Effects of stand composition on insect visitation in two-species mixtures of Hieracium. Am Midl Nat 100:431–440
Totland Ø, Nielsen A, Bjerknes AL, Ohlson M (2006) Effects of an exotic plant and habitat disturbance on pollinator visitation and reproduction in a boreal forest herb. Am J Bot 93:868–873
Traveset A, Heleno R, Chamorro S, Vargas P, McMullen CK, Castro-Urgal R, Nogales M, Herrera HW, Olesen JM (2013) Invaders of pollination networks in the Galápagos Islands: emergence of novel communities. Proc R Soc B 280:20123040. doi:10.1098/rspb.2012.3040
Trigo MM, Melgar M, García J, Recio M, Docampo S, Cabezudo B (2007) El polen de la atmósfera de Vélez-Málaga. Concejalía de Medio Ambiente Ayuntamiento de Vélez, Málaga
Vila M, Bartomeus I, Gimeno I, Traveset A, Moragues E (2006) Demography of the invasive geophyte Oxalis pes-caprae across a Mediterranean island. Ann Bot 97:1055–1062
Warton DI, Hui FK (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Waser NM, Fugate ML (1986) Pollen precedence and stigma closure: a mechanism of competition for pollination between Delphinium nelsonii and Ipomopsis aggregata. Oecologia 70:573–577
Waser NM, Real LA (1979) Effective mutualism between sequentially flowering plant species. Nature 281:670–672
Westphal C, Steffan-Dewenter I, Tscharntke T (2003) Mass flowering crops enhance pollinator densities at a landscape scale. Ecol Lett 6:961–965
Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SPM, Szentgyörgyi H, Tscheulin T, Vaissière BE, Woyciechowski M, Biesmeijer JC, Kunin WE, Settele J, Steffan-Dewenter I (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78:653–671
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank Cristina Tur, Veriozka Azenas, Clara Vignolo, Rocío Castro and Xavi Rotllán, for their help in the field and the laboratory. Moreover, we thank María del Mar Trigo Pérez for valuable help with pollen identification and Charles Novaes for statistical advice. We are also grateful to two anonymous referees for valuable comments on earlier versions of the manuscript. This work was supported by a fellowship for prospective researchers by the Swiss National Science Foundation (Grant No. PBZHP3-131020).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albrecht, M., Ramis, M.R. & Traveset, A. Pollinator-mediated impacts of alien invasive plants on the pollination of native plants: the role of spatial scale and distinct behaviour among pollinator guilds. Biol Invasions 18, 1801–1812 (2016). https://doi.org/10.1007/s10530-016-1121-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1121-6