Abstract
Flowering invasive plants can have dramatic effects on the resource landscape available to pollinators. Because many pollinators exhibit behavioral plasticity in response to competitor or resource density, this in turn can result in impacts on ecological processes such as pollination and plant reproduction. We examine how interactions between five common generalist eusocial bees change across an invasion gradient by examining how bee abundance and diet overlap changed with variation in both invasive plant abundance and competitor abundance in a temperate oak-savannah ecosystem. Specifically we focus on the bumblebees Bombus bifarius, B. mixtus, B. melanopygus and B. vosnesenskii, as well as the non-native honeybee Apis mellifera, and their interactions with the native flowering plants Camassia quamash, Camassia liechtlinii, and the invasive shrub Cytisus scoparius. We further examine whether changes in pollinator visits to the invasive and two common native plants can explain changes in diet overlap. Abundance of the invasive plant and other common floral resources had strong impacts on focal bee abundance, with certain species more likely to be present at highly invaded sites. This may be because highly invaded sites tended to be embedded in forested landscapes where those bees are common. Diet overlap was most affected by abundance of a common native plant, rather than the invasive plant, with diet overlap increasing non-linearly with abundance of the native plant. Furthermore, Apis mellifera, did not appear to have direct competitive effects on native bumblebees in this habitat. However, visit patterns suggest that bees most abundant at highly invaded sites may compete for access to native resources. Thus the impacts of this invasive plant on our focal bee species may be primarily indirect, via its’ competitive effects on native plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in local resource density driven by invasive plants can affect community composition at multiple trophic levels leading to emergent impacts on species interactions (Bezemer et al. 2014; McCary et al. 2016). For example, within fragmented ecosystems, invasive plants can have dramatic impacts on plant community composition (Pysek et al. 2012) that can in turn affect both plant-pollinator interactions by changing resource availability (Albrecht et al. 2016; Brown et al. 2002; Herron-Sweet et al. 2016) and potentially interactions between the pollinators themselves. This is because bees are known to modify their foraging behavior in response to resource abundance, resource quality (Robertson et al. 1999), competitor density (Brosi and Briggs 2013) and opportunity costs for foraging on complex flowers (Muth et al. 2015), all of which may be affected by an invasive plant.
Furthermore, resource quality can be a function of competitor abundance. Competing pollinators may deplete floral nectar or pollen and thus reduce resource quality (Heinrich 1976a; Robertson et al. 1999). As an invasive plant increases in density, it may reduce native plant abundance via resource competition (Jauni and Ramula 2015), increasing competition among pollinators for resources provided by native plants. However, a flowering invasive plant may provide an alternative resource for outcompeted pollinators (Harmon-Threatt and Kremen 2015), if they are capable of foraging on the invasive. This means the impact of competitors in invaded communities may depend on the foraging flexibility of pollinators, and whether they vary in ability to use the non-native resource.
These context-dependent foraging shifts have been studied extensively in bumblebees, where worker foraging behavior is a model system for optimal foraging and decision making. Some of the earliest studies of resource partitioning examined resource use relative to tongue length in bumblebees (Heinrich 1976b; Ranta and Lundberg 1980). Current research has demonstrated that bumblebees are responsive to both competitor density (Brosi and Briggs 2013) and resource abundance (Cartar 2009).
Our project focuses on interactions among five common generalist social pollinator species in the Oak Savannah of Southern Vancouver Island, a highly fragmented ecosystem spread across multiple urban parks and conservation areas. For pollinators, we focus on four native, short tongued bumblebee species: Bombus bifarius (Cresson), Bombus mixtus Cresson, Bombus melanopygus Nylander and Bombus vosnesenskii Radoszkowski. These species are the most abundant bumblebees at our sites, and have similar (short) tongue lengths and a high degree of overlap in floral resource use (Wray et al. 2014). We also include the non-native honeybee, Apis mellifera Linnaeus, because it is a common floral visitor with high resource use overlap with our focal bumblebee species. Furthermore, some evidence suggests that non-native honeybees may compete with wild pollinators for resources (Dohzono and Yokoyama 2010).
We examine pollinator foraging behavior and the potential for competition for resources, using two focal native plant species, as well as the introduced Cytisus scoparius (L.) Link (Fabaceae, scotch broom). These include two native perennials, Camassia quamash (Pursh) Greene, and Camassia leichtlinii (Baker) S. Watson (Asparagaceae). Both Camassia species produce multiple blue to purple flowers on a single stem with pollen and nectar rewards that are easily accessible to all species of bees as well as other floral visitors that are not the focus of this paper (solitary bees, flies, wasps). Cytisus scoparius has hundreds of nectarless yellow pea-shaped flowers on a single bush with access to rewards limited to large bodied bees that can open, or “trip” the flowers (Knuth 1906; Parker 1997; Traveset and Richardson 2014). Our pollinator species vary in their collection of C. scoparius pollen, potentially due to differences in worker body size (Gillespie et al. 2017), thus we may expect them to differ in their responses to C. scoparius density. We focus on Camassia as native species because they are extremely common, have easily accessible flowers, and are likely important resources for a diversity of floral visitors. They are also the most highly visited native plants by our focal bees, which may be competing for access to floral rewards (Based on data from Wray et al. 2014). Cytisus scoparius is a highly disruptive non-native plant in this ecosystem, capable of completely replacing the native flora in the absence of active management (Shaben and Myers 2010). It is also pollinator dependent, but limits its floral visitors to large-bodied bees due to flower morphology: larger bodied bees are most efficient at ‘tripping’ the flower keel and accessing pollen rewards (Parker 1997). At our sites, C. scoparius and both Camassia spp. have coinciding blooms occurring in late spring to early summer. Cytisus scoparius density varies across sites primarily due to removal efforts by volunteers.
We examine how variation in non-native plant density and abundance of other pollinator species affect interactions among these potential competitors, and interactions between pollinators and plant species. Specifically, we ask (1) How does bee abundance vary with invasive plant abundance and other site characteristics; (2) how does bee diet overlap change with focal plant and competitor abundance and (3) how do visit rates by bees to focal plants change with plant and competitor abundance?
Materials and methods
Field sites
Sites comprised 18 oak-savannah fragments in the greater Victoria area, British Columbia, Canada, 1.75 km or more apart (Supplemental Table 1). At each site, we delineated a 1 ha focal area, encompassing populations of all our focal plant species, where present. At several sites this was split into two 1/2 ha patches, due to the steep rocky nature of the habitat. Sites varied in C. scoparius density primarily due to removal efforts by local volunteers and land managers (S. Gillespie, pers. obs).
We divided sampling periods within a day into morning (10–12), mid-day (12–2), and afternoon (2–4) periods. Each site was sampled at least twice for each period (morning, mid and afternoon) in 2014 and 2015, for a total of 6 or more samples for each site for each year collected over the course of focal species bloom. We sampled only during pollinator appropriate weather (temperatures above 15 °C and low winds).
Plant community sampling
On each sampling date, we quantified the flowering community using four 50 m transects spaced 50 meters apart throughout the sampling area. We sampled 10, 0.25 × 2.0 m quadrats per transect, perpendicular to the transect at random distances ranging from 1 to 5 m. Within each quadrat we counted the number of flowering units of each plant species in bloom. For herbaceous plants, a flowering unit was defined as an individual stem, flower or inflorescence to which a bee must fly to access resources. A flowering unit for both Camassia spp. was defined as a single inflorescence (C. quamash: average 1.97 open flowers, range 1–10; C. leichtlinii: average 2.45 open flowers, range 1–11). For C. scoparius, a flowering unit was an individual branch on a bush with no further flowering branches arising from it (Average 2.29 open flowers, range 1–10). To estimate the density of our focal plants at the site by year level, we first summed the number of flowering units across plots for each sampling day, and then averaged the number of flowering units across sampling days for each site. Thus plant density is measured as flowering stems/40 m2. Due to time constraints, we estimate resource availability as the number of flowering stems, rather than nectar or pollen resources explicitly.
Pollinator community sampling
The pollinator community was quantified during each sampling date by two people netting insects directly off of flowers, each for 20 min, for a total of 40 min sampled per site on each sampling date (over 120 min of sampling each year). While processing insects, the timer was stopped. Netters sampled throughout the 1 ha area on all plant species with open flowers. We collected all flower visitors—defined as any insect that was contacting the sexual parts of the plant. Butterflies, queen bumblebees, and honeybees were netted, identified, noted, and released. Insects were pinned and identified to species, or genera or morphospecies where species identification was not possible (Supplemental Table 2); however only focal bee identity was relevant to our analysis. To calculate the site-level abundance of each bumblebee species we averaged the number collected across sampling dates, for each site in each year.
Observations of visiting pollinators
To quantify visits by our focal pollinators independently of netting, we observed pollinator visits to our focal plants for two 10 min periods per focal plant species per sampling date for a total of at least 120 min of observation per plant species per site per year. For C. leichtlinii and C. quamash, we watched up to 20 flowering units of each plant, depending on the numbers present at locations chosen haphazardly for observation. For C. scoparius, because of its structure and very low visit rates, we observed up to 40 flowering units (branches) on an individual shrub. We identified bumblebees and honeybees to species on the wing, and other visitors to morphospecies groups (Supplemental Table 2). For each insect that visited flowers within our patch, we counted the number of stems visited and the number of flowers visited per stem. From this data, for each sample date, we calculated the number of individuals of each focal pollinator seen per flowering unit observed. This accounts for variable numbers of flowering units observed across some samples. We then averaged this value for each observation date, then across observation dates for each site in each year.
Analysis
All analyses were conducted in R. We examined explanatory variables using variance inflation factors (VIF). Unless otherwise stated, VIF were all less than 3.0, indicating that covariance is not sufficient to confound our analysis (Zuur et al. 2007). In all analyses, focal plant densities were log transformed to improve normality. Response variables were log transformed when necessary, as indicated in statistical tables. All variables were calculated at the site-by-year level to account for variable sample sizes among sites and years. One site had to be discarded for 2015 (Little Saanich Mountain), due to highly disruptive invasive species control efforts. Data is averaged within site and year by necessity, as individual dates may have low numbers of observations of our focal insects. Our study spans a 1 month period in early spring. While the plants are highly structured in their phenology, all of our focal insects are all social insects that have long-lived colonies that should be common within the landscape during that time, though their local abundance may change in response to resource availability.
Bumblebee site abundance
We used general linear mixed models to examine which site characteristics explained the abundance of each focal bee species (Bates et al. 2015). Our model examined what effect C. scoparius, C. leichtlinii, and C. quamash density and year had on relative focal bee abundance, with site included as a random variable. For all analyses, type 3 sums of squares were calculated using the “Anova” function in the car package (Fox and Weisber 2011), while the slope of each linear relationship was extracted using the summary function (R-Development-Core-Team 2009).
Diet overlap
Diet overlap was calculated using the Morista Index (Krebs 1999) for each focal bee combination at each site. For example, we calculated the diet overlap of B. bifarius with B. melanopygus at each site, based on the resource use they have in common, and then similarly calculated overlap of B. bifarius with all other focal bee species at that site. We used our netting data to approximate resource use as it estimates the proportion of bees at a site that are using each visited flowering plant. Low values of the Morista index indicate that two bee species are using disparate resources, while high values indicate use of similar resources. The Morista index was chosen specifically because it handles smaller, and unbalanced sample sizes with low bias (Smith and Zaret 1982); however to be conservative we removed any samples with fewer than 5 individual bees in a combination at that specific site.
For each focal bee species, we asked whether its’ diet overlap with competitors changed with any site characteristics. To test this, we asked whether C. scoparius, C. leichtlinii, or C. quamash density, partner identity, year, total pollinator abundance at that site, and the partner bee-by-plant interactions, affected the Morista index for each focal bee species, with site included as a random effect. Non-significant interactions were dropped from the final model.
Focal pollinator visit patterns
To analyze the effects of site characteristics on visit patterns to focal plants, we subdivided data by whether we observed a focal species in bloom on a given observation date. Then, to calculate focal pollinator and plant density for analyses of focal pollinator visit patterns, we only included those observations where the focal plant in question was blooming. Thus, calculations for C. quamash analyses only include those dates where we could observe pollinator visits to C. quamash. This means for example that the flowering community during C. quamash bloom will be different than that for C. scoparius, as they do not have perfect overlap in flowering time.
When examining VIF for these subdivided data, we find that they are greater 3.0 for focal plant densities in the C. leichtlinii and C. scoparius samples, suggesting that plant densities were more correlated in these slightly later-flowering plant samples. To address this covariance, we summarized focal plant densities into a single gradient using principal component analysis. For both C. leichtlinii and C. scoparius visit rates, PC1 represents a gradient from high C. scoparius (negative values) to high Camassia density (positive values), and we refer to it as the C. scoparius to Camassia gradient. This gradient (PC1) explains ~ 57% of the variation in the variables included in the analysis. Other PCs explain 20% or less additional variation each, and examination of scree plots shows that only PC1 explains sufficient variation to be retained in further analyses (Supplemental Table 3).
For C. scoparius and C. leichtlinii samples, we asked whether the frequency of observation of each focal bee on each focal plant was affected by the C. scoparius to Camassia gradient (PC1), B. mixtus, B. melanopygus, B. bifarius, A. mellifera or B. vosnesenskii abundance, and sampling year, with site included as a random effect.
For bee visits to C. quamash, VIF values were less than 3 and focal plants were analyzed separately without using PCA. Thus for C. quamash our model examines whether visit patterns to each focal plant are affected by C. scoparius, C. leichtlinii, C. quamash, B. mixtus, B. melanopygus, B. bifarius, Apis mellifera or B. vosnesenskii abundance, and sampling year, with site included as a random effect.
Explanatory and response variables were transformed as necessary (Tables 1, 2, 3) to improve assumptions of normality. Where focal bee abundance data was sufficient for linear analysis, we used the lmer function with a logistic distribution (Bates et al. 2015). Where data were highly zero-skewed we used negative binomial models to examine whether explanatory variables affected the likelihood of observing a bee on a plant at a site. Then where there were sufficient numerical data (> 10 positive observations) we used general linear mixed models on the non-zero observations to test for an impact of site characteristics and focal bee abundance on the number of bees observed. To visualize significant effects, we used the Effect package in R (Fox and Hong 2009) to plot partial regressions with 95% confidence limits. Partial regression plots show the effect of adding the variable of interest to a model.
Results
Bumblebee site abundance and plant community patterns
We netted a total of 2840 focal pollinators across both years. Of these, 1355 were A. mellifera, 672 were B. mixtus, 296 were B. melanopygus, 294 B. vosnesenskii and 223 were B bifarius. Camassia quamash density varied from an average of 0.04–6 stems per m2; C. leichtlinii varied from zero to 5.1 stems per m2, while C. scoparius varied from zero to 25.7 stems per m2.
While not sufficiently correlated to confound most analyses, sites with high C. scoparius have lower densities of both Camassia species (spearman correlation coefficient: − 0.43 for C. leichtlinii, − 0.50 for C. quamash), likely due to physical displacement, particularly at high C. scoparius densities. This pattern was stronger during observations of C. leictlinii (i.e. later in the season when more C. scoparius was in flower).
Bombus bifarius and B. melanopygus both increased in abundance with C. scoparius abundance. Bombus mixtus was more common at sites with more C. leichtlinii, and A. mellifera increased with both Camassia species. Bombus melanopygus and A. mellifera were more common in 2015, while B. bifarius and B. vosnesenskii were more common in 2014 (Figs. 1, 2; Table 1; full statistics in Supplemental Table 4).
Impacts of site characteristics on focal bee abundance. a Bombus bifarius abundance increased with Cytisus scoparius density (stems/m2); b Bombus mixtus abundance increased with Camassia leichtlinii density (stems/m2); c Bombus melanopygus density increased with C. scoparius density (stems/m2); d Apis mellifera abundance increased with both Camassia quamash and C. leichtlinii density (stems/m2)
Diet overlap
Diet overlap increased with C. quamash density for all focal bees. This relationship was significantly non-linear for B. mixtus, B. melanopygus and A. mellifera, saturating at high C. quamash abundance (Table 2, Fig. 3a, Supplemental Table 5). For B. vosnesenskii diet overlap was also higher in 2015. Finally, for B. melanopygus, diet overlap was lower with B. vosnesenskii than with other partners (Table 2, Supplemental Table 5). There was also a significant interaction between partner bee identity and C. leichtlinii density for B. melanopygus. Diet overlap between B. melanopygus and B. bifarius declined with C. leichtlinii abundance while overlap with other partner bees was stable (Fig. 3b). Diet overlap declined with competitor abundance for B. bifarius only (Table 2). Finally, diet overlap for A. mellifera declined with C. scoparius density (Fig. 3c).
Factors affecting resource overlap between different bee species. Higher values on the Y axis indicate more diet overlap. a Diet overlap for all species increased with Camassia quamash density (stems/m2), but the increase was non-linear for Bombus mixtus, Bombus melanopygus and Apis mellifera; b residual B. melanopygus diet overlap with Bombus bifarius declined with Camassia leichtlinii density (stems/m2), but did not change with other partner bees c A. mellifera diet overlap declined with Cytisus scoparius abundance (stems/m2). Residuals for b were calculated using a model fit with all model variables except partner bee identity and C. leichtlinii abundance (stems/m2)
Visit patterns
There was no effect of either floral resource or competitor abundance on visit rates of B. bifarius to C. quamash in the binomial model. For those sites where we observed B. bifarius visiting C. quamash, observations increased at sites with more C. leichtlinii (Fig. 4a). For B. bifarius visiting C. leichtlinii nothing was significant in the binomial model; however, for those sites where we observed B. bifarius, observations decreased with B. melanopygus site abundance (Fig. 4b). There was no effect of either floral resource or competitor abundance on observations of B. bifarius visiting C. scoparius (Table 3A, Supplemental Table 6).
Partial regressions showing factors significantly related to visit rates of Bombs bifarius to focal plant species. Grey bars represent upper and lower 95% confidence limits. a Observations of B. bifarius on Camassia quamash increased with log Camassia leichtlinii density (stems/m2). Model: B. bifarius observations ~ Camassia leichtlinii density + C. quamash density + C. scoparius density+ B. mixtus abundance + B. melanopygus abundance + B. bifarius abundance + A. mellifera abundance + B. vosnesenskii abundance, + sampling year + site (random effect). b observations of B. bifarius on C. leichtlinii decreased with Bombus melanopygus abundance. Model: B. bifarius observations ~ PC1 + B. mixtus abundance + B. melanopygus abundance + B. bifarius abundance + A. mellifera abundance + B. vosnesenskii abundance, + sampling year + site (random effect)
Bombus mixtus visits to C. leichtlinii and C. quamash increased with B. mixtus site abundance. There was insufficient data to analyze B. mixtus visits to C. scoparius (Table 3B).
Nothing significantly affected B. melanopygus visits to C. leichtlinii or C. quamash. For visits to C. scoparius, nothing was significant in the binomial model, and there was insufficient data to apply a linear model (n = 8 observations; Table 3C).
Nothing affected B. vosnesenskii visits to C. quamash or C. scoparius. We were more likely to observe B. vosnesenskii visiting C. lelichtlinii at sites with more B. vosnesenskii overall (binary model) (Table 3D).
We observed more A. mellifera visiting C. quamash at sites with less C. scoparius, more B. bifarius and more A. mellifera (Fig. 5). More A. mellifera visited C. leichtlinii in 2015 and at sites with less B. melanopygus. For A. mellifera visits to C. scoparius, nothing was significant in the binomial model. When we examined patterns in A. mellifera at sites where it was present on C. scoparius, we saw more A. mellifera at sites with more B. vosnesenskii, and higher values of PC1 (less C. scoparius and more Camassia; Fig. 6). We observed Apis mellifera less frequently on C. scoparius at sites with more B. bifarius, and more frequently in 2014 (Table 3E).
Partial regressions showing variables significantly related to visit rates of Apis mellifera to Camassia quamash. Grey bars represent upper and lower 95% confidence limits. a Observations of A. mellifera declined with Cytisus scoparius density (stems/m2). and b increased with Bombus bifarius abundance and c increased with B. vosnesenskii abundance. Model: A. mellifera observations ~ Camassia leichtlinii density + C. quamash density + C. scoparius density+ B. mixtus abundance + B. melanopygus abundance + B. bifarius abundance + A. mellifera abundance +B. vosnesenskii abundance, + sampling year + site (random effect)
Partial regressions showing variables significantly related to visit rates of Apis mellifera to Cytisus scoparius. Grey bars represent upper and lower 95% confidence limits. a A. mellifera on C. scoparius declined with PC1 (C. scoparius to Camassia gradient); b declined with Bombus bifarius abundance and c increased with Bombus vosnesenskii abundance. Model: A. mellifera observations ~ PC1 + B. mixtus abundance + B. melanopygus abundance + B. bifarius abundance + A. mellifera abundance +B. vosnesenskii abundance, + sampling year + site (random effect)
Discussion
Focal bee abundance
Focal plant density was associated with variable changes in focal bee abundance. Bombus bifarius and B. melanopygus were both associated with sites with higher C. scoparius abundance, whereas B. mixtus and A. mellifera were more abundant at sites with more C. leichtlinii, and more C. quamash in the case of A. mellifera. Apis mellifera was also the most abundant of our focal bees and several species varied in abundance across years (Fig. 2). Year to year variation in focal bee abundance is likely related to changes in spring weather. The months preceding bloom in 2015 were considerably warmer and dryer than in 2014, with higher average temperatures and far fewer days without precipitation in February and March (mean temperature in February, March: 3.6, 7.4 °C in 2014; 8.3, 8.9 °C in 2015, Environment Canada, http://climate.weather.gc.ca/historical_data/search_historic_data_e.html). This may favor colony growth of different species, or cause shifts in bee phenology relative to flower phenology.
Previous work in our lab showed that B. bifarius and B. melanopygus are both associated with forested sites (Wray et al. 2014). In our data, high broom sites tended to be more highly forested in the surrounding matrix, while lower broom sites are surrounded by other habitat types, mostly urban areas (correlation between forest cover within 400 m buffer, and log C. scoparius density: Pearson coefficient = 0.57; p < 0.001). This correlation may help to explain the pattern of B. bifarius and B. melanopygus co-occurrence at high C. scoparius sites in our data. Sites with high C. scoparius density also tended to have less Camassia—likely due to competitive impacts of C. scoparius (Shaben and Myers 2010). Wray et al. (2014) also found that C. quamash was associated with urban sites. In our data, we find that A. mellifera is positively associated with C. quamash, perhaps driven by a higher abundance of both species in urban areas (due to urban beekeeping and volunteer C. scoparius control efforts, respectively).
Diet overlap and foraging behavior
Diet overlap increased with C. quamash density for all focal pollinators. However, this increase saturates at high C. quamash density for B. mixtus, B. melanopygus and A. mellifera. This pattern is not driven by changes in focal pollinator abundance, as only A. mellifera increases in site-level abundance with C. quamash density (Fig. 1d).
While high diet overlap was traditionally used as evidence for high levels of interspecific competition, real-world interpretation is more complex (Holt 1987). At the small spatial and temporal scales measured here, high overlap may indicate that specific floral resources are common enough for pollinators to share, while lower overlap may indicate that one species is unable to compete with another, and has thus shifted onto alternative resources to avoid competition (Brosi and Briggs 2013).
Given that C. quamash provides abundant nectar and pollen with no barriers to access these rewards, it seems likely that an increase in diet overlap reflects increased shared use of C. quamash. As C. quamash becomes more common, more and more individual foragers of different species may swap onto this resource, leading to high overlap at moderate densities. This would match the predictions of optimal diet models, which suggest that individual diets should broaden in low quality environments, leading to lower diet overlap (MacArthur and Pianaka 1966). As C. quamash increases in density, more high quality resources are available, the environmental quality is increasing, and competition may be lower, allowing for higher diet overlap. However, if exploitation of C. quamash reaches some maximum level where its pollen or nectar are depleted, some pollinators may diversify their resource use, causing diet overlap to saturate or even decline. Previous research has shown that reward depletion can lead to pollinators foraging on lower quality resources. For example, when pollen is depleted on high quality Mimulus guttatus plants, pollinators will switch to the lower-quality pollen morph (Robertson et al. 1999).
Bombus melanopygus also had lower diet overlap with B. vosnesenskii and declining overlap with B. bifarius with increasing C. leichtlinii abundance (Fig. 3b). For B. vosnesenskii, a simple explanation may be that B. melanopygus and B. vosnesenskii differ in abundance across years, and are thus less likely to be caught on similar resources when measured at the site by year level.
The decline in diet overlap between B. melanopygus and B. bifarius as C. leichtlinii abundance declines is less likely to be explained by changes in site abundance of bees. Neither bee species changes in density with C. leichtlinii abundance. We also observe B. bifarius less frequently on C. leichtlinii at sites with abundant B. melanopygus (Table 3A; Fig. 4b). This decline in diet overlap could partly be explained by the fact that B. melanopygus was more common in 2015, while B. bifarius was more common in 2014. However, we do not find a significant year effect for B. bifarius observations on C. leichtlinii, and B. melanopygus is actually less abundant on C. leichtlinii in 2015 when it is more common overall. Given that both B. bifarius and B. melanopygus are also more common at sites with more C. scoparius the decrease in overlap may be partially driven by competition for C. leichtlinii. This latter result may reflect interspecific interactions on C. leichtlinii, where the two bumblebees most common at sites invaded by C. scoparius may compete for resource.
Bombus bifarius and B. melanopygus are most common at forested sites (Wray et al. 2014), where C. scoparius is most abundant in our data. We have also observed that extensive C. scoparius removal efforts are more likely to occur at more urban sites that are accessible to volunteers (personal observation). Given the negative effects that C. scoparius has on native plant abundance (Shaben and Myers 2010), it seems likely that both B. bifarius and B. melanopygus may be more resource stressed than our other focal bee species because of their association with forested landscapes. That is, increased C. scoparius removal and control efforts in forested habitats further from the urban core may be beneficial to these pollinators in particular.
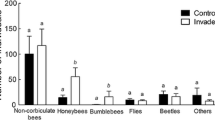
Despite being the most abundant of all focal pollinator species (Fig. 2), we find no evidence for unique direct impacts of non-native A. mellifera on bumblebees. Patterns in diet overlap were not different with A. mellifera versus other species (Table 2), nor did site-level A. mellifera abundance have significant impacts on visit rates by focal bees (Table 3). Rather, the strong bottom-up effects of C. quamash density on diet overlap suggests that the overall impacts of resource availability outweighs high A. mellifera abundance. However, the highest densities of A. mellifera occur at sites with the highest densities of C. quamash. Thus, high abundance of A. mellifera could contribute to resource depletion of C. quamash at high densities that could contribute to the saturation of diet overlap with C. quamash density (Fig. 3a). Overall, the landscape heterogeneity around our habitats may also buffer wild bees from competitive impacts of honeybees that have been documented in homogeneous mass-flowering crops (Lindstrom et al. 2016). These results suggests that maintaining floral diversity in these urban parks can help support local co-existence of urban beekeeping with wild pollinators.
Our results show that C. scoparius differs from other commonly studied flowering invasive plants in its effects on the visit patterns of the local pollinator community. For example, many generalist, highly attractive flowering invasives can facilitate pollination of native plants by attracting more pollinators to a local area (Albrecht et al. 2016; Feldman et al. 2004). We see no evidence of higher visit rates by any focal pollinator to our native plants at sites with high C. scoparius density (Table 3). This may be because C. scoparius has specialized floral morphology, no nectar production, low overall visit rates and differs greatly in floral syndrome from both Camassia species. Indeed, invasive plants are more likely to affect natives pollinator visits directly when they share similar floral traits (Gibson et al. 2012).
Conclusions
Despite being a potential pollen resource, C. scoparius did not have strong direct impacts on diet overlap or foraging patterns among our focal pollinators. Rather, we find that variation in diet overlap among focal pollinator species is driven by abundance of native plant resources rather than non-native plant abundance. This suggests that any impacts of C. scoparius on diet overlap are indirect—via resource competition with and displacement of native species—rather than via direct interactions with pollinators (Stout and Morales 2009). However, competition for native resources may be occurring between pollinators that are more common at highly invaded sites, partly due to the landscape context in which the non-native is more common. Furthermore, at least in this habitat, we see no evidence for direct competitive effects between non-native honeybees and native bumblebees. Overall our findings indicate that both landscape context and local resources can affect diet overlap and foraging behavior by generalist pollinators, and that a major impact of non-native flowering plants on pollinators may be indirect, and driven by displacement of native floral resources.
References
Albrecht M, Ramis MR, Traveset A (2016) Pollinator-mediated impacts of alien invasive plants on the pollination of native plants: the role of spatial scale and distinct behaviour among pollinator guilds. Biol Invasions 18:1801–1812
Bates D, Maechler M, Bolker BM et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bezemer TM, Harvey JA, Cronin JT (2014) Response of native insect communities to invasive plants. In: Berenbaum MR (ed) Annual review of entomology, vol 59. Annual Reviews, Palo Alto, p 119-U740
Brosi BJ, Briggs HM (2013) Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc Natl Acad Sci U S A 110:13044–13048
Brown BJ, Mitchell RJ, Graham SA (2002) Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83:2328–2336
Cartar RV (2009) Resource-tracking by bumble bees: What explains local responses to density of bergamot (Monarda fistulosa) flowers? Ecoscience 16:470–475
Dohzono I, Yokoyama J (2010) Impacts of alien bees on native plant-pollinator relationships: a review with special emphasis on plant reproduction. Appl Entomol Zool 45:37–47
Feldman TS, Morris WF, Wilson WG (2004) When can two plant species facilitate each other’s pollination? Oikos 105:197–207
Fox J, Hong J (2009) Effect displays in R for multinomial and proportional-odds logit models: extensions to the effects package. J Stat Softw 32:1–24
Fox J, Weisber S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Gibson MR, Richardson DM, Pauw A (2012) Can floral traits predict an invasive plant’s impact on native plant-pollinator communities? J Ecol 100:1216–1223
Gillespie SD, Bayley J, Elle E (2017) Native bumble bee (Hymenoptera: Apidae) pollinators vary in floral resource use across an invasion gradient. Can Entomol 149:204–213
Harmon-Threatt AN, Kremen C (2015) Bumble bees selectively use native and exotic species to maintain nutritional intake across highly variable and invaded local floral resource pools. Ecol Entomol 40:471–478
Heinrich B (1976a) Foraging specializations of individual bumble-bees. Ecol Monogr 46:105–128
Heinrich B (1976b) Resource partitioning among some eusocial insects—bumblebees. Ecology 57:874–889
Herron-Sweet CR, Lehnhoff EA, Burkle LA et al (2016) Temporal- and density-dependent impacts of an invasive plant on pollinators and pollination services to a native plant. Ecosphere 7:1–13
Holt RD (1987) On the relationship between niche overlap and competition—the effect of incommensurable niche dimensions. Oikos 48:110–114
Jauni M, Ramula S (2015) Meta-analysis on the effects of exotic plants on the fitness of native plants. Perspect Plant Ecol Evol Syst 17:412–420
Knuth P (1906) Handbook of flower pollination based on Hermann Mueller’s ‘The fertilisation of flowers by insects’. Clarendon Press, Oxford
Krebs CJ (1999) Ecological methodology, 2nd edn. Addison-Wesley Educational Publishers Inc
Lindstrom SAM, Herbertsson L, Rundlof M et al (2016) Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc R Soc B Biol, Sci, p 283
MacArthur RH, Pianaka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
McCary MA, Mores R, Farfan MA et al (2016) Invasive plants have different effects on trophic structure of green and brown food webs in terrestrial ecosystems: a meta-analysis. Ecol Lett 19:328–335
Muth F, Keasar T, Dornhaus A (2015) Trading off short-term costs for long-term gains: how do bumblebees decide to learn morphologically complex flowers? Anim Behav 101:191–199
Parker IM (1997) Pollinator limitation of Cytisus scoparius (Scotch broom), an invasive exotic shrub. Ecology 78:1457–1470
Pysek P, Jarosik V, Hulme PE et al (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Change Biol 18:1725–1737
R-Development-Core-Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ranta E, Lundberg H (1980) Resource partitioning in bumblebees—the significance of differences in proboscis length. Oikos 35:298–302
Robertson AW, Mountjoy C, Faulkner BE et al (1999) Bumble bee selection of Mimulus guttatus flowers: the effects of pollen quality and reward depletion. Ecology 80:2594–2606
Shaben J, Myers JH (2010) Relationships between Scotch broom (Cytisus scoparius), soil nutrients, and plant diversity in the Garry oak savannah ecosystem. Plant Ecol 207:81–91
Smith EP, Zaret TM (1982) Bias in estimating niche overlap. Ecology 63:1248–1253
Stout JC, Morales CL (2009) Ecological impacts of invasive alien species on bees. Apidologie 40:388–409
Traveset A, Richardson DM (2014) Mutualistic interactions and biological invasions. Annu Rev Ecol Evol Syst 45:89–113
Wray JC, Neame LA, Elle E (2014) Floral resources, body size, and surrounding landscape influence bee community assemblages in oak-savannah fragments. Ecol Entomol 39:83–93
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer, New York
Acknowledgements
We are grateful to Julie Wray, Lindsey Button, Ranah Chavoshi, Jessica Bayley, Severin Vallaincourt and Tiia Haapalainen for field assistance; the cities of Oak Bay, Victoria, and Esquimalt, the District of Saanich, the Capital Regional District, and the National Research Council of Canada for site access; and Simon Fraser University and the Natural Sciences and Engineering Research Council (NSERC) of Canada for funding (Discovery Grant to E.E. and Undergraduate Student Research Award to J.B.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gillespie, S., Elle, E. Non-native plants affect generalist pollinator diet overlap and foraging behavior indirectly, via impacts on native plant abundance. Biol Invasions 20, 3179–3191 (2018). https://doi.org/10.1007/s10530-018-1767-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1767-3