Abstract
Many invasive alien plants occur in large populations with abundant flowers which are highly attractive to pollinators, and thus might affect pollination of co-occurring native species. This study focuses on the invasive Heracleum mantegazzianum and distance-dependent effects on pollination of Mimulus guttatus in abandoned grassland over 2 years. First, we examined pollinator abundance in yellow traps at 0, 10, 30 and 60–200 m from H. mantegazzianum. We then placed M. guttatus plants at the same distances to monitor effects of the invasive species on pollinator visitation and seed set of neighbouring plants. Finally, we conducted a garden experiment to test if deposition of H. mantegazzianum pollen reduces seed set in M. guttatus. No distance effect was found for the number of bumblebees in traps, although the invasive species attracted a diverse assemblage of insects, and visitation of M. guttatus was enhanced close to H. mantegazzianum. This positive effect was not reflected by seed set of M. guttatus, and heterospecific pollen decreased seed set in these plants. Overall there is little evidence for negative effects of the invasive species on pollination of neighbouring plants, and flower visitation even increases close to the invaded patches. The functional role of the invader and suitable control strategies need further clarification, since removal of H. mantegazzianum may actually damage local pollinator populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien plants are a focal area of ecological research due to the negative effects of these species on native biodiversity (Drake et al. 1989; Lodge 1993; Sakai et al. 2001). However, there is still little published information about the consequences of invasive alien plants on pollination in the invaded communities (see, e.g., Brown and Mitchell 2001; Brown et al. 2002; Totland et al. 2006; Lopezaraiza-Mikel et al. 2007). Such competitive effects have been demonstrated among native species (Willems and Lahtinen 1997), and staggered flowering phenologies, for example, are interpreted as adaptations to avoid interspecific competition for pollinators (Appanah 1993; Lobo et al. 2003). Invasive alien species can build up large populations with abundant or prolonged flowering (Traveset and Richardson 2006), and the flowers of some aliens are highly attractive for pollinators which might have considerable effects on pollination of co-occurring natives (Brown et al. 2002; Larson et al. 2006). Thus, pollinator competition can lead to reduced seed set in native species as observed for Impatiens glandulifera in herbaceous communities (Chittka and Schürkens 2001). Competition for pollinator services could occur through changes in pollinator behaviour (Bergman 1996), leading to pollen-limited seed set (cf. Rathcke 1988). Thus, the presence of an invasive alien plant could affect pollinators to a degree that seed set of the neighbouring (native) species becomes reduced compared to non-invaded sites. However, the introduction of alien plants could also have positive effects since they may act as ‘magnet species’ enhancing pollinator visits to co-flowering plants, as observed by Rathcke (1983) for native plants. Moreover, the alien species might provide resources that support larger pollinator populations over prolonged time periods (Waser and Real 1979 for natives). Thus, invasive alien plants that are integrated in a native plant-pollinator network may have implications for management, and new studies are needed to make progress in this controversial research field.
Besides possible affects on pollen quantity, changes in pollinator visits may lead to deposition of heterospecific pollen (Grabas and Laverty 1999). Heterospecific pollen is recognized as an important mechanism through which plants can compete for pollinator services. Heterospecific pollen can reduce fruit and seed production in several ways, for instance, by pollen allelopathy, stigma clogging, stigma closing or stylar inhibition (Brown and Mitchell 2001). Unless the native plant species is highly specific with respect to pollination, heterospecific pollen is likely to occur, whether an invasive alien species is present or not. However, invasive plants may have flower and pollen traits to which the co-flowering native species are not adapted, and they may be problematic because of their high local abundance. Such negative effects may decrease with distance from the invaded area.
Interactions between alien and native plants might be particularly strong in species-poor communities with few pollinators. In the temperate zone, abandoned riparian grasslands, for example, are low-diversity communities due to a combination of nutrient-rich moist habitat conditions and rare disturbance (Grime 2001); this corresponds with rather poor pollinator communities (Westrich 1989). However, some riparian plants and the associated insect species are rare, and near-natural floodplains are among the most endangered European ecosystems (Ellenberg 1988). A serious threat to these ecosystems is invasive alien plants which are particularly abundant in riparian habitats (de Waal et al. 1994). It is typical that species-poor communities have a high degree of generalization, i.e. each plant and pollinator species has a relatively high number of interactions (Olesen and Jordano 2002; Olesen et al. 2002). This may favour the integration of alien plants into the native pollination web, which increases the possibility of interaction between the alien and the native plants, mediated through pollinator activity (Lopezaraiza-Mikel et al. 2007).
Competition for pollinator services by invasive alien plants may reduce the reproductive capacity of neighbouring species. In this study, the overall question is whether or not competition for pollinators is a mechanism by which an invasive species negatively affects other plants at a local scale. A prominent invasive alien in northwestern and central Europe is the tall herb Heracleum mantegazzianum (Apiaceae). Flowers of Apiaceae are simple and known to be visited by a wide range of pollinators (Grace and Nelson 1981; Proctor et al. 1996). We studied the effects of H. mantegazzianum on pollinator abundance and pollination of the target species Mimulus guttatus (Scrophulariaceae), focussing on the following hypotheses:
-
1.
Pollinator abundance decreases with distance from the invasive alien species.
-
2.
Seed set in co-flowering plants is reduced with distance to the invasive species.
-
3.
Heterospecific pollination by the invasive species reduces seed set of co-flowering plants.
Materials and methods
Study species
The invasive alien Heracleum mantegazzianum Sommier & Levier (Apiaceae) is a tall, monocarpic, perennial herb with small white flowers arranged in compound umbels. The open hermaphrodite and protandrous flowers are attractive to a wide range of insects which visit flowers for pollen and nectar collection (Grace and Nelson 1981). In our study area, we observed Apis mellifera, Bombus spp. (mainly B. terrestris species complex, B. hortorum, B. pascuorum, and different red-tailed species such as B. lapidarius, B. pratorum and B. ruderarius), syrphids, other Diptera and beetles of the families Nitidulidae, Cantharidae and Scarabaeidae as flower visitors. Since its introduction from the western Caucasus in the 19th century, H. mantegazzianum has become a successful invader in many European countries, with a marked increase in distribution during the past decades (de Waal et al. 1994; Nielsen et al. 2005). Heracleum mantegazzianum possesses several traits thought to characterize a successful invasive species: high fecundity, early germination, rapid growth and high rates of spread (Nielsen et al. 2005).
Mimulus guttatus Fischer ex DC (Scrophulariaceae) was used as a target species for exploring pollinator services affected by H. mantegazzianum. It is an annual or perennial herb that occurs in wet or semidry grasslands and along small streams. The plant produces pairs of showy yellow flowers in sequential progression up flowering stems. The flowers are bee-pollinated (Apis mellifera, Bombus spp. and solitary bees), but various Diptera and certain species of butterflies contribute to pollination (Macnair et al. 1989). Mimulus guttatus has a mixed mating system with insect pollination and up to four modes of self-pollination (selfing rates 21–75%; Ritland 1990), and in absence of pollinators anthers brush by the stigma at the time of corolla abscission (Dole 1990). The species has been frequently studied due to its complex breeding system (e.g. Macnair et al. 1989; Ritland 1990; Dole 1992) and being at the centre of an actively evolving species complex (Vickery 1978; Ritland and Ritland 1989). Mimulus guttatus is native to North America and the first records in Europe date back to the 19th century (Weidema 2000; Truscott et al. 2006). The species is invasive in some western and central European countries (Truscott et al. 2006), but occurs only sporadically in northern Europe. Although not a native, Mimulus guttatus was chosen because (1) it shares pollinators with H. mantegazzianum, (2) its pollination system is well known, (3) the flowers are suitable for manipulative experiments, (4) it was not found in the study area and thus the field experiments were not affected by local populations, and (5) the plant was sufficiently robust for transplant experiment.
Field sites and experimental design
Five study sites with large stands of H. mantegazzianum were identified in June 2006 in southern Sealand (≈100 km south of Copenhagen, 0–20 m a.s.l.; 55°15′22′′–05′27′′ N, 12°5′50′′–5′60′′ E). The sites represented abandoned riparian grasslands dominated by tall herbs and Phragmites australis; they were <2 ha in size and located within an agricultural landscape. The vegetation of all sites was relatively poor in insect-pollinated species, with only 50–100 flowering plants per 100 m2 [e.g. Cirsium arvense, C. palustre, Epilobium hirsutum and Symphytum × uplandicum; nomenclature follows Hansen (1999)]. Common to all sites were linear dense populations of H. mantegazzianum along water courses, but there were no other systematic differences with distance from H. mantegazzianum, e.g. in terms of the native flowering community.
At each site, five parallel transects (60–200 m) were established perpendicular to the H. mantegazzianum stands; the distance among transects was random to avoid any systematic effect within the study sites. To analyse natural abundance of pollinators and pollination of the target species, stations with individual potted M. guttatus plants were placed at distances of 0, 10 and 30 m along all transects, i.e. 15 stations for each site.
The experiment was repeated in June 2007, albeit with a modified design where stations were set up 0, 30 and 60–100 m from H. mantegazzianum to cover larger distance effects. Due to control of H. mantegazzianum in two of the 2006 sites, two additional sites were chosen in 2007 in the same catchment.
The experiment was initiated at the time of H. mantegazzianum flowering, but because of a warm winter and spring, the plants flowered 3 weeks earlier in 2007. In Denmark, flowering usually starts in mid June and lasts for about 4 weeks. Flowering phenology of H. mantegazzianum was recorded at 5 days intervals in 3 weeks throughout the experiment. The weather was warm and dry in 2006 (mean monthly temperature and precipitation in June were 14.9°C and 7.6 mm, respectively; in July, 20.4°C and 13.9 mm), whereas lower temperatures and days with heavy rain occurred in 2007 (June: 16.0°C, 119.6 mm). Watering of the M. guttatus plants was necessary every second or third day.
Local pollinator abundance in traps
To examine local pollinator abundance yellow cross barrier traps (‘Unitraps’, Plant Research International, Wageningen) were established at all experimental stations. The traps were available in yellow, transparent or green colours; yellow was chosen as this colour is effectively attracting insects (Southwood and Henderson 2000). The traps consisted of a horizontal yellow cross (diameter: 16 cm; height: 18 cm) fitted onto a bucket of water (1.6 l) with detergent. The traps were installed on 15 July 2006 and 22 June 2007, respectively, and removed 4 days later.
The aim of the study was to use the same locations as for the other experiments without any interference among experimental set-ups. However, by installing the insect traps after the flowering period of M. guttatus, insect abundance might have been overestimated. Pollinators were preserved in 70% ethanol, and bee identification followed Hammer and Holm (1970), Prŷs-Jones and Corbet (1991) and Amiet (1996) and Bertsch et al. (2005).
Transplantation of M. guttatus and pollinator observations on flowers
Vegetative plants of M. guttatus were obtained from the botanical garden of the Faculty of Life Sciences, Copenhagen in May 2006 and 2007. Plants were grown separately in 1.2 l plastic pots filled with a peat-based substrate (N, 70 g m−3; P, 91 g m−3; K, 200 g m−3; pH 5.6–6.0) in the botanical garden of the Faculty (55°41′ N, 12°33′ E, 15 m a.s.l.); once a week the position of the pots was randomised. To make the target plants as uniform as possible they were cut down to 5 cm height; ≈3 weeks later the plants started flowering.
To assess if H. mantegazzianum affected visitation frequency of potential pollinators we transplanted 75 M. guttatus plants to the five field sites (with five transects and three distances, each). To match the relatively small test plants (20–40 cm) with the surrounding vegetation they were placed on stakes of different height (i.e. not all test stations were at the same height, varying from 60 to 140 cm) in plastic buckets which allowed drainage of excess water. Thus, relative height of the target plants was not confounded with respect to other treatments (e.g., distance). In 2007, yellow plastic plates (20 × 20 cm) were established beneath the buckets to attract more pollinators.
We monitored 25 H. mantegazzianum and 75 M. guttatus plants for 10 min intervals between 10 am and 5 pm over 5 days with dry and sunny weather, and counted the number of pollinator visits to compound umbels of H. mantegazzianum and to flowers of M. guttatus (n = 170 periods for all sites combined, except one site in 2007 due to rain). Before each period started, we counted the number of open flowers in M. guttatus. Based on counts of the number of umbellets per umbel (n = 20) and the number of flowers per umbellet (n = 255) in H. mantegazzianum, an average number of 3,600 and 2,400 flowers per umbel were calculated for terminal and satellite umbels, respectively.
We defined a ‘flower visit’ as an insect contacting the reproductive parts of H. mantegazzianum or entering the flower of M. guttatus. Common pollinators of the study species included Apis mellifera, Bombus sp., other Hymenoptera, syrphid flies and other Diptera. Pollen beetles (Nitidulidae: Meligethes aenus) were not registered as they were considered minor pollinators, and floral herbivory by these species actually can reduce pollination (Krupnick et al. 1999). Although the M. guttatus plants were grown under similar conditions in both years, there were more flowers on the plants at the time of pollinator observations in 2007 (7.72 ± 0.70, mean ± SE) than in 2006 (2.14 ± 0.19).
Seed set experiments
Potential effects of changed pollination on seed set were studied in the same M. guttatus plants used for pollinator observations. Before the plants were transferred to the field, one randomly selected flower was marked as control and a second flower was emasculated with forceps to avoid autogamy. Given the mixed mating system of M. guttatus the emasculation allowed pollination by out-crossing or geitonogamy in the field, while untreated flowers were pollinated either by out-crossing, geitonogamy or autogamy. The treatments were labelled with colour-coded cotton threads on the flowering stems. Mimulus guttatus has sensitive stigmatic lobes which clasp upon touch and only re-open in case of no or little pollen on the stigma (Macnair et al. 1989; Ritland and Ritland 1989). Thus, flowers with closed stigmatic lobes were not used in the experiment. In June 2007, a third treatment was applied to estimate seed set due to out-crossing. We simulated insect pollination by hand-pollinating one flower on each of the 75 plants prior to transfer to the field, using a fine brush and a mixture of pollen from mature anthers of >15 individuals.
Mimulus guttatus terminated flowering earlier than H. mantegazzianum, and thus some plants had to be replaced. By the end of the experiment, 141 and 140 M. guttatus plants were used in 2006 and 2007, respectively. There was no bias in plant replacement with respect to distance from H. mantegazzianum, transect or site (data not shown). In 2006, the experiment was initiated on 28 June and lasted for 18 days, whereas the experiment in 2007 went over 15 days, from 8 to 22 June. Capsules of M. guttatus were collected at maturity and seed set was determined using a dissecting microscope and a fine-scale balance. The correlation between seed numbers and seed mass was verified by counting and weighing 15 samples of 100–2,500 seeds and performing linear regressions (r = 0.95 and 0.83 in 2006 and 2007, respectively).
Heterospecific pollen deposition
Qualitative changes in pollen deposition may cause stigma clogging and thus reduced seed set in M. guttatus. To test this we marked two flowers on each of 15 plants in the botanical garden of the Faculty. Test flowers were unmasculated but only flowers with open stigmatic lobes were used in the experiment. One flower on each plant was pollinated with pollen of H. mantegazzianum using a fine brush and a second one was left untreated. Pollen of mature H. mantegazzianum anthers was collected 2 days before the experiment and stored at 5°C. The experiment was started on 26 June 2006 and terminated at seed set. Mature capsules of M. guttatus were collected, and seed set was determined as described above.
Statistical analyses
We used Poisson regression with random effects (Glimmix macro in SAS 9.1) to analyse variation in pollinator abundance. The effects of distance (0, 10, 30 and 50–200 m from H. mantegazzianum) was considered to be fixed, and experimental site (five sites) and transects nested within site (five transects) to be random. Data from each year were analysed separately because the design was not fully crossed (not all distances were represented in both years). All pollinators listed in Supplementary Material, Appendix 1 were included in the analysis.
A Poisson regression was also applied for analysing the pollinator visits in 10 min periods on M. guttatus considering the same fixed and random effects as above. The number of open flowers in M. guttatus and the interaction between open flowers and distance were used as explanatory variables in the initial model but not included in the final model due to lack of significance. Again, the experimental design was not fully crossed (not all distances were represented in both years) and data for the two years analysed separately. Observations of pollinators on H. mantegazzianum were compared using a t-test.
Variation in seed set of M. guttatus in the field was analysed separately for each year with Poisson regression (not all distances and treatment were represented in both years). The number of plants used per station varied from 1 to 4 per station (1.69 ± 0.04, mean ± SE), because plants were replaced in case of termination of flowering. Thus, we calculated mean seed set per flower for each experimental station (n = 75) prior to the analysis of variance. Again the effects of treatment (three treatments of the flowers) and distance were considered to be fixed, and site and transects to be random.
To determine whether seed set of M. guttatus was influenced by heterospecific pollination in the garden experiment, we used a t-test for matched pairs to compare seed set of flowers pollinated with H. mantegazzianum pollen with untreated flowers. Two stems of the flowering plants broke, thus reducing the total sample size to 13 plants.
Results
Local pollinator abundance in traps
There was no significant difference in pollinator abundance among traps placed at 0, 10, 30 and 50–200 m to H. mantegazzianum in neither 2006 (F 2,47 = 0.45, P = 0.64; Fig. 1) nor 2007 (F 2,44 = 0.91, P = 0.41). Catch rate in 2007 was markedly different from 2006. The insects in the cross barrier traps consisted mainly of nine species of bumblebees (5–8 spp. per site), but also Apis mellifera, solitary bees, Vespidae and Syrphidae (Supplementary Material, Appendix 1). The nitidulid beetle Meligethes aenus and unidentified Diptera (not syrphid flies) were found in all samples.
Pollinator observations on flowers
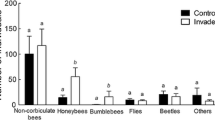
More insects were recorded on M. guttatus plants located next to H. mantegazzianum stands than at any other distances in both 2006 (F 2,38 = 42732.2, P < 0.0001) and 2007 (F 2,36 = 5.57, P = 0.01). Overall, the mean (±SE) number of visits to M. guttatus was 0.09 ± 0.06 per plant per 10 min in 2006, and 1.00 ± 0.21 in 2007 (Fig. 2). Dipterans were the most abundant insect group observed (67%; Fig. 3). Only few pollinators were registered on M. guttatus in 2006, and the average number of visits was different between the study years (F 1,62 = 17.7, P < 0.0001).
Pollinator visits to Heracleum mantegazzianum (per umbel; means ± SE) and the test species Mimulus guttatus (per plant; means ± SE) recorded in 10 min intervals. Mimulus guttatus plants were placed at 0, 10, 30 and 50–200 m from H. mantegazzianum; numbers above bars refer to the number of observation periods
In contrast, umbels of H. mantegazzianum were highly attractive to the pollinators and received 8.80 ± 1.45 visits per umbel during 10 min in 2006, and 8.95 ± 0.94 in 2007 (Fig. 2). The pollinator assemblage in the invasive alien species was dominated by A. mellifera (70% visits; Fig. 3), and the mean number of insects visiting H. mantegazzianum was not different between years (t 43 = −0.08, P > 0.10). The pollinator community of the two species was significantly different (Chi-square test of independence; χ2 = 98.5, df = 5, P < 0.01).
Seed set of M. guttatus
Distance to H. mantegazzianum had no effect on seed set of M. guttatus neither in 2006 (F 2,116 = 1.12, P = 0.33) nor in 2007 (F 2,193 = 2.44, P = 0.09), while the flower treatments had marked effects in both years (2006: F 1,116 = 78.69, P < 0.0001; 2007: F 2,193 = 7.02, P = 0.001; Fig. 4). The interaction between treatment and distance was also tested in the initial models but was not significant (data not shown). Overall, the M. guttatus plants produced more seeds in 2007 than in 2006 (z = 4.06, P < 0.01). In 2006, seed set of emasculated flowers was reduced compared to untreated flowers (t 116 = −8.81, P < 0.001), while this was not true in 2007 (t 193 = 0.52, P = 0.60). In 2007, hand-pollinated flowers produced more seeds than emasculated (t 193 = −2.89, P = 0.004) or untreated flowers (t 193 = 3.40, P = 0.001).
Heterospecific pollen deposition
Heterospecific pollination reduced seed set of M. guttatus (t = 4.03, df = 12, P < 0.01). Mimulus guttatus flowers hand-pollinated with H. mantegazzianum produced 203.6 ± 32.6 seeds per flower (mean ± SE), while seed set of untreated flowers was 363.9 ± 39.2.
Discussion
Local pollinator abundance in traps
Bumblebees exhibit some constancy to particular sites both within and between foraging trips (Heinrich 1975; Osborne and Williams 2001). In our study, the invasive H. mantegazzianum seemed to be an important resource for pollinators in abandoned grasslands with only few other forage plants. However, there were no differences in pollinator abundance caught within a range of 0–300 m, contrary to hypothesis 1. This might be because bumblebees are relatively mobile pollinators (Dramstad 1996; Osborne et al. 1999), and because the large yellow traps were highly attractive.
The rather species-poor pollinator communities expected for abandoned moist grassland (Westrich 1989) was not reflected by pollinator diversity within the traps. Except for a few missing Psithyrus species (appearing later in summer), diversity of bumblebees and cuckoo bees corresponded to what is known for this region of Denmark (H.B. Madsen, pers. comm.) and northern Europe generally (Teräs 1985 and references therein). Thus, in the fragmented agricultural landscape of this study H. mantegazzianum may influence pollination interactions by supporting populations of native pollinators in the abandoned grasslands.
Considerable numbers of bumblebees were caught in 2006 although the traps were installed for only 4 days. The lower numbers in 2007 might be due to an earlier start of the trapping, and thus lower seasonal abundance of Bombus sp., and more adverse weather conditions.
Pollinator observations on flowers
For a wide variety of pollinators it has been shown that short flight distances (<1.4 m) among sequentially visited flowers are most common (Grabas and Laverty 1999 and references therein). The potential of one plant to influence pollination of co-flowering species should therefore be greatest in immediate vicinity of the target species. Such patterns were also found in our study where visits to M. guttatus plants adjacent to H. mantegazzianum were significantly more frequent than for plants at 10, 30 or 50–200 m distance, although pollinator visitation was low for all plants. This indicates a weak facilitative interaction, i.e. a ‘magnet effect’ of the invasive species which corresponds to higher pollinator abundance close to the invasive plant as predicted by hypothesis 1.
The magnitude of this facilitative effect depends on the extent of pollinator overlap between H. mantegazzianum and M. guttatus, flower attractiveness and pollinator behaviour. First, bumblebees, syrphid flies and other dipterans were observed on both species, while Apis mellifera, the most frequent pollinator on H. mantegazzianum, was not recorded on M. guttatus. Thus, pollinator overlap between the two species was restricted to insects that comprised only a minor proportion of the pollinators on the invasive species. Second, features that signal flower attractiveness to pollinators include nectar and pollen availability, a showy corolla and fragrance (Harder et al. 2001); and attractiveness also increases with the number of flowers displayed (Ohashi and Yahara 2001). Given the huge number of H. mantegazzianum flowers, foraging on a marginal resource such as M. guttatus might be less rewarding. Third, pollinators that otherwise make random choices may exhibit passive flower constancy as they enter a monospecific patch of flowers such as H. mantegazzianum (Thomson 1981). A combination of these factors may explain the sparse facilitative effect.
Positive effects on pollinator visits to native species facilitated by an invasive plant have been found in some other studies (Grabas and Laverty 1999; Moragues and Traveset 2005; Larson et al. 2006), although most publications report negative effects or no significant difference (Grabas and Laverty 1999; Chittka and Schürkens 2001; Brown et al. 2002; Aigner 2004; Ghazoul 2004; Moragues and Traveset 2005; Larson et al. 2006; Totland et al. 2006). These results suggest that the impact of invasive species on pollination of native plants may be species-specific, with additional effects of the inherent temporal variability of pollination systems (Moragues and Traveset 2005; Larson et al. 2006; Bjerknes et al. 2007). The annual variation in visitation of M. guttatus found in our study could be explained by differences in the number of flowers per plant, which may have attracted more insects in 2007.
Seed set of M. guttatus
Although the presence of the invasive H. mantegazzianum had a weak positive effect on pollinator visitation of adjacent M. guttatus it did not affect seed production, thus contradicting hypothesis 2. Previous experiments have also failed to detect effects on fruit or seed set of natives (Grabas and Laverty 1999; Aigner 2004; Moragues and Traveset 2005; Totland et al. 2006), and seed set may be more robust than pollinator visitation to alien invasion (Totland et al. 2006). However, the published results are ambiguous as negative effects on seed set have been found as well (Grabas and Laverty 1999; Chittka and Schürkens 2001; Brown et al. 2002), while no studies have observed increased reproduction.
Because of the mixed mating system of M. guttatus, seed production is to some extent independent of cross-pollination, and thus less affected by changed pollinator visitation. How much the plant relied on self-pollination is indicated by comparing the different flower treatments. The reduced seed set of emasculated flowers in 2006 compared to untreated flowers implies that selfing occurred, and that seed set was controlled by pollen limitation in the absence of autogamous pollination. However, this effect was not visible in 2007 as seed production for emasculated and untreated flowers were similar, indicating that all flowers were equally pollinated. On the other hand, when considering the difference in seed production in 2007 for untreated and hand-pollinated flowers, plant fitness was reduced by self-pollination compared to out-crossing. Thus, there was some pollen limitation in M. guttatus but the increased pollinator visitation and potential out-cross pollination did not lead to higher seed production. However, our study considered only seed quantity and not seed quality which could also be affected, particularly in self-compatible species (Bell et al. 2005).
For all treatments seed set was significantly higher in 2007 than in 2006. There were no differences in the handling and treatment of plants between the 2 years, and thus the variation is most likely due to lower temperatures and more rain in 2007.
Heterospecific pollen deposition
Competition for pollination through interspecific pollen transfer may have detrimental effects on seed production (e.g. Waser 1978; Galen and Gregory 1989; Caruso and Alfaro 2000). Since deposition of both con- and heterospecific pollen results in closure of the stigmatic lobes of M. guttatus, we hypothesized that hand-pollination with H. mantegazzianum pollen would result in lower seed production. This was confirmed in the garden experiment suggesting that M. guttatus plants were pollen-limited after application of heterospecific pollen.
The more frequent pollinator visits to co-flowering plants close to the invasive may increase deposition of heterospecific pollen (Waser 1978; Rathcke 1983). Thus, decreased reproductive output of M. guttatus was expected. Since seed set was unaffected by slightly increased pollinator visitation, it seems unlikely that the study plants were affected by this mechanism of competition. This might be because of the limited overlap in pollinator assemblages; the main visitors to M. guttatus only comprised 30% of the pollinators observed on H. mantegazzianum.
Conclusions
The invasive H. mantegazzianum is attractive to a high number of pollinators with large foraging areas, and pollinator communities were of greater diversity than expected for abandoned moist grassland. Thus, the high abundance of H. mantegazzianum may contribute to sustain local pollinator populations. We found a facilitative interaction between the invasive plant and the adjacent M. guttatus for pollinator visitation. This positive effect, however, led not to increased seed set in M. guttatus.
We adopted a ‘single species approach’, focusing on only two plants species at a small spatial scale. An important further step is to broaden the focus to the landscape level with entire communities of co-flowering plants. A recent study of a flowering plant community invaded by Impatiens glandulifera found evidence of facilitation for visits to native plants (Lopezaraiza-Mikel et al. 2007). However, because pollinators apparently were attracted to I. glandulifera from further distances, Lopezaraiza-Mikel et al. (2007) argued that competition for pollination could still occur at larger spatial scales among landscape units with alien presence and absence. This mechanism of attraction may also be important in our study, and given the annual variability among pollination systems, there is a need for further research to address the impact of invasive species on plant-pollinator interactions on greater spatial and temporal scales.
The degree to which an invasive plant is integrated in the native plant-pollinator network should be considered within management plans for this species (Zavaleta et al. 2001). Our study adds some important results about the functional role played by H. mantegazzianum in abandoned grasslands as the invasive may counteract the general decline in pollinators, albeit at same time reducing the abundance of native flowering species. New studies are now needed on how pollinators and communities of co-flowering native plants respond to the removal of invasive alien species. Such studies should be included in future eradication programmes.
References
Aigner PA (2004) Ecological and genetic effects on demographic processes: pollination, clonality and seed production in Dithyrea maritime. Biol Conserv 116:27–34
Amiet F (1996) Insecta Helvetica Fauna 12, Hymenoptera Apidae, 1. Teil, Allgemeiner Teil, Gattungsschüssel, die Gattungen Apis, Bombus und Psithyrus. Schweizerischen Entomologischen Gesellschaft, Neuchatel, Switzerland
Appanah S (1993) Mass flowering of dipterocarp forests in the aseasonal tropics. J Biosci 18:457–474
Bell JM, Karron JD, Mitchell RJ (2005) Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology 86:762–771
Bergman P (1996) Early flowers of Bartsia alpina (Scrophulariaceae) and the visitation of bumblebees. Acta Bot Neerl 45:355–366
Bertsch A, Schweer H, Titze A, Tanaka H (2005) Male labial gland secretions and mitochondrial DNA markers support species status of Bombus cryptarum and B. magnus (Hymenoptera, Apidae). Insect Soc 52:45–54
Bjerknes AL, Totland Ø, Hegland SJ, Nielsen A (2007) Do alien plant invasions really affect pollination success in native plant species? Biol Conserv 138:1–12
Brown BJ, Mitchell RJ (2001) Competition for pollination: effects of pollen of an invasive plant on seed set of a native congener. Oecologia 129:43–49
Brown BJ, Mitchell RJ, Graham SA (2002) Competition for pollination between an invasive species (Purple loosestrife) and a native congener. Ecology 83:2328–2336
Caruso CM, Alfaro M (2000) Interspecific pollen transfer as a mechanism of competition: effect of Castilleja linariefolia pollen on seed set of Ipomopsis aggregate. Can J Bot 78:600–606
Chittka L, Schürkens S (2001) Successful invasion of a floral market—An exotic Asian plant has moved in on Europe’s river-banks by bribing pollinators. Nature 411:653
De Waal LC, Child LE, Wade PM, Brock JH (1994) Ecology and management of invasive riverside plants. Wiley, Chichester
Dole JA (1990) Role of corolla abscission in delayed self-pollination of Mimulus guttatus (Scrophulariaceae). Am J Bot 77:1505–1507
Dole JA (1992) Reproductive assurance mechanisms in three taxa of the Mimulus guttatus complex (Scrophulariaceae). Am J Bot 79:650–659
Drake JA, Mooney HA, di Castri F, Groves RH, Kruger FJ, Rejmánek M, Williamson M (1989) Biological invasions. A global perspective. Wiley, Chichester
Dramstad WE (1996) Do bumblebees (Hymenoptera: Apidae) really forage close to their nests? J Insect Behav 9:163–182
Ellenberg H (1988) Vegetation ecology of Central Europe. Cambridge University Press, Cambridge
Galen C, Gregory T (1989) Interspecific pollen transfer as a mechanism of competition: consequences of foreign pollen contamination for seed set in the alpine wildflower, Polemonium viscosum. Oecologia 81:120–123
Ghazoul J (2004) Alien abduction: disruption of native plant-pollinator interactions by invasive species. Biotropica 36:156–164
Grabas GP, Laverty TM (1999) The effect of purple loosestrife (Lythrum salicaria L; Lythraceae) on pollination and reproductive success of sympatric co-flowering wetland plants. Ecoscience 6:230–242
Grace J, Nelson M (1981) Insects and their pollen loads at a hybrid Heracleum site. New Phytol 87:413–423
Grime JP (2001) Plant strategies, vegetation processes, and ecosystem. Wiley, Chichester
Hammer K, Holm S (1970) Danske humlebier og snyltehumler. Natur og Museum, Naturhistorisk Museum, Århus, Denmark
Hansen K (ed) (1999) Dansk feltflora. Gyldendal, Copenhagen, Denmark
Harder LD, Williams NM, Jordan CY, Nelson WA (2001) The effects of floral design and display on pollinator economics and pollen dispersal. In: Chittka L, Thomson JD (eds) Cognitive ecology of pollination. Cambridge University Press, Cambridge, pp 297–317
Heinrich B (1975) Energetics of pollination. Ann Rev Ecol Syst 6:139–170
Krupnick GA, Weis AE, Campbell DR (1999) The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology 80:125–134
Larson DL, Royer RA, Royer MR (2006) Insect visitation and pollen deposition in an invaded prairie plant community. Biol Conserv 130:148–159
Lobo JA, Quesada M, Stoner KE, Fuchs EJ, Herrerias-Diego Y, Rojas J, Saborio G (2003) Factors affecting phenological patterns of bombacaceous trees in seasonal forests in Costa Rica and Mexico. Am J Bot 90:1054–1063
Lodge DM (1993) Biological invasions—lessons for ecology. Trends Ecol Evol 8:133–37
Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J (2007) The impact of an alien plant on a native plant-pollinator network: an experimental approach. Ecol Lett 10:539–550
Macnair MR, Macnair VE, Martin BE (1989) Adaptive speciation in Mimulus: an ecological comparison of M. cupriphilus with its presumed progenitor, M. guttatus. New Phytol 112:269–279
Moragues E, Traveset A (2005) Effect of Carpobrotus spp on the pollination success of native plant species of the Balearic Islands. Biol Conserv 122:611–619
Nielsen C, Ravn HP, Nentwig W, Wade M (2005) The giant hogweed best practice manual. Guidelines for the management and control of an invasive weed in Europe. Forest & Landscape, Hørsholm, Denmark
Ohashi K, Yahara T (2001) Behavioural responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. In: Chittka L, Thomson JD (eds) Cognitive ecology of pollination. Cambridge University Press, Cambridge, pp 274–296
Olesen JM, Jordano P (2002) Geographic patterns in plant-pollinator mutualistic networks. Ecology 83:2416–2424
Olesen JM, Eskildsen LI, Venkatasamy S (2002) Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Div Distrib 8:181–192
Osborne JL, Williams IH (2001) Site constancy of bumblebees in an experimentally patchy habitat. Agr Ecosyst Environ 83:129–141
Osborne JL, Clark SJ, Morris RJ, Williams IH, Riley JR, Smith AD, Reynolds DR, Edwards AS (1999) A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J Appl Ecol 36:519–533
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. The New Naturalist, Harper & Collins Publishers, London, UK
Prŷs-Jones OE, Corbet SA (1991) Bumblebees Naturalists’ Handbooks 6. Richmond Publishing Co Ltd., Slough
Rathcke B (1983) Competition and facilitation among plants for pollinators. In: Real L (ed) Pollination biology. Academic Press, New York, pp 305–329
Rathcke B (1988) Interaction for pollination among co-flowering shrubs. Ecology 69:446–457
Ritland K (1990) Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution 44:1230–1241
Ritland C, Ritland K (1989) Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae). Am J Bot 76:1731–1739
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell Science, Oxford
Teräs I (1985) Food plants and flower visits of bumblebees (Bombus: Hymenoptera, Apidae) in southern Finland. Acta Zool Fenn 179:1–120
Thomson JD (1981) Field measures of flower constancy in bumblebees. Am Midl Nat 105:377–380
Totland Ø, Nielsen A, Bjerknes AL, Ohlson M (2006) Effects of an exotic plant and habitat disturbance on pollinator visitation and reproduction in a boreal forest herb. Am J Bot 93:868–873
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Truscott A-M, Soulsby C, Palmer SCF, Newell L, Hulme PE (2006) The dispersal characteristics of the invasive plant Mimulus guttatus and the ecological significance of increased occurence of high-flow events. J Ecol 94:1080–1091
Vickery RK (1978) Remarkable waxing, waning, and wandering of populations of Mimulus guttatus: an unexpected example of global warming. Great Basin Nat 59:112–126
Waser NM (1978) Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology 59:934–944
Waser NM, Real LA (1979) Effective mutualism between sequentially flowering plant species. Nature 281:670–672
Weidema IR (2000) Introduced species in the Nordic countries. Nordic Council of Ministers, Copenhagen, Denmark
Westrich P (1989) Die Wildbienen Baden-Württembergs. Ulmer, Stuttgart, Germany
Willems JH, Lahtinen ML (1997) Impact of pollination and resource limitation on seed production in a border population of Spiranthes spiralis (Orchidaceae). Acta Bot Neerl 46:365–375
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16:454–459
Acknowledgements
We thank Mads Nielsen for field work assistance and Mai-Britt Sauer for help in the greenhouse. Thanks to Henning Bang Madsen, University of Copenhagen, for verification of bumblebee species and identification of solitary bees. The study was supported through a PhD fellowship of the University of Copenhagen to CN, and a study grant by the Paul + Maria Kremer-Stiftung to CH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nielsen, C., Heimes, C. & Kollmann, J. Little evidence for negative effects of an invasive alien plant on pollinator services. Biol Invasions 10, 1353–1363 (2008). https://doi.org/10.1007/s10530-007-9210-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-007-9210-1