Abstract
When exotic plant species share pollinators with native species, competition for pollination may lower the reproductive success of natives by reducing the frequency and/or quality of visits they receive. Exotic species often become numerically dominant in plant communities, and the relative abundance of these potential competitors for pollination may be an important determinant of their effects on the pollination and reproductive success of co-occurring native species. Our study experimentally tests whether the presence and abundance of an invasive exotic, Lythrum salicaria L. (Lythraceae), influences reproductive success of a co-flowering native species, Mimulus ringens L. (Phrymaceae). We also examine the mechanisms of competition for pollination and how they may be altered by changes in competitor abundance. We found that the presence of Lythrum salicaria lowered mean seed number in Mimulus ringens fruits. This effect was most pronounced when the invasive competitor was highly abundant, decreasing the number of seeds per fruit by 40% in 2006 and 33% in 2007. Reductions in the number of seeds per fruit were likely due to reduced visit quality resulting from Mimulus pollen loss when bees foraged on neighboring Lythrum plants. This study suggests that visit quality to natives may be influenced by the presence and abundance of invasive flowering plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When exotic species invade flowering plant communities, competition for pollination may lower the reproductive success of co-occurring native species (Chittka and Schurkens 2001; Ghazoul 2004; Morales and Aizen 2006; Bjerknes et al. 2007). These competitive effects generally occur through two nonexclusive mechanisms (Waser 1978). First, pollinator preference for an invasive species may reduce the frequency of pollinator probes to native plants, lowering conspecific pollen deposition (Waser 1983; Campbell 1985; Brown et al. 2002; Kandori et al. 2009). Second, invasive species may lower the quality of pollinator probes to native plants by interfering with conspecific pollen transfer. For example, pollen of native species may be deposited onto stigmas of the competitor, lowering seed set of the native species (Feinsinger and Tiebout 1991; Bell et al. 2005; Murcia and Feinsinger 1996; Larson et al. 2006; Morales and Traveset 2008). Reduced conspecific pollen deposition may also result from the loss of native species’ pollen when bees groom while foraging on an invasive species (Flanagan et al. 2009; Mitchell et al. 2009a).
The direction of interactions for pollination and the magnitude of competitive effects on pollination success of native species may depend on both the presence and relative abundance of invasive competitors (Bjerknes et al. 2007; Munoz and Cavieres 2008). Increases in the abundance of an invasive species may change the direction of interactions for pollination from facilitation to competition, or may increase the severity of competition that already occurs at equal species abundances (Munoz and Cavieres 2008; Morales and Traveset 2009). Earlier studies of competition for pollination often examined equal abundances of two competing species native to the same region, simulating sympatry of naturally co-occurring species and effectively comparing presence versus absence of a competitor (Waser 1978, 1983; Campbell 1985; Galen and Gregory 1989). More recent studies have demonstrated that competitive effects may vary with relative abundance, generally becoming more intense with increasing competitor abundance (Caruso 2002; Ghazoul 2006; Munoz and Cavieres 2008; Takakura et al. 2009; Morales and Traveset 2009). However, the effect of one species on another might be context-dependent, changing from positive to neutral to negative with changes in relative abundance (Rathcke 1983; Mitchell et al. 2009a). Invasive species commonly develop extensive and dense populations, and the relative and absolute abundances of these invasives are likely to influence the magnitude of competitive effects on natives (Bjerknes et al. 2007). For example, the presence of an invasive may increase local patch size, attracting more pollinators and potentially increasing pollinator visitation to natives (Rathcke 1983; Feldman et al. 2004; Totland et al. 2006; Munoz and Cavieres 2008; Molina-Montenegro et al. 2008). However, highly abundant invasive plants may monopolize pollinator visits—despite increases in local pollinator attraction—reducing the frequency of probes to less abundant native species (Rathcke 1983; Munoz and Cavieres 2008). Highly abundant invasive plants may also diminish visit quality to natives through increases in interspecific pollen transfer (Bjerknes et al. 2007; Morales and Traveset 2008). In particular, it has been hypothesized that an increase in the number of competitor flowers probed during interspecific pollinator transitions should exacerbate pollen losses when invasives are abundant (Morales and Traveset 2008). Reductions in visit quality may occur whether or not the frequency of pollinator probes to natives is affected (Waser 1983; Larson et al. 2006; Lopezaraiza-Mikel et al. 2007).
This study experimentally tests whether the presence and abundance of an invasive exotic, Lythrum salicaria L. (Lythraceae), influences reproductive success of a co-flowering native species, Mimulus ringens L. (Phrymaceae). In an earlier study, we found that bumblebee workers moving between Mimulus and Lythrum flowers deposited significantly less conspecific pollen onto Mimulus stigmas compared to foragers that only visited Mimulus (Flanagan et al. 2009). In the present study, we varied Lythrum abundance in competition with a constant number of Mimulus plants in order to address the following questions: (1) does the presence and abundance of Lythrum influence the quantity and quality of pollinator probes to Mimulus, and (2) does the presence and abundance of Lythrum influence Mimulus reproductive success?
Materials and methods
Study species
Mimulus ringens L. (Phrymaceae) is a wetland perennial herb native to central and eastern North America (Grant 1924). Populations tend to be small, often fewer than 50 individuals. At our field site in southeastern Wisconsin, USA, Mimulus flowers from mid-July through mid-September. The large blue zygomorphic flowers open at 5 a.m. and last for half a day (Mitchell et al. 2004). Daily floral display size ranges from 1 to 22 flowers (Karron et al. 2009).
Flowers are pollinated by bumblebee workers as they contact the stigma and anthers with their tongues and faces (Karron et al. 1995a, b; Mitchell et al. 2004). A single Bombus probe typically deposits 3,000–7,000 pollen grains (Flanagan et al. 2009) onto the bilobed stigma. Nearly all flowers produce seed capsules (Karron et al. 2004; Mitchell et al. 2005). Each flower has approximately 6,000 ovules, and the number of seeds per fruit following a single probe is typically 1,600–2,300 seeds (Karron et al. 2006). Seed number for flowers receiving three probes is significantly (43.6%) higher than seeds per fruit for flowers receiving a single probe, suggesting that stigmas often fail to receive a saturating load of pollen during an initial probe (Karron et al. 2006).
Mimulus ringens flowers are self-compatible and have a mixed-mating system (Karron et al. 1995a). Outcrossing rates vary widely within and among populations, and are influenced by population density (Karron et al. 1995a, b), floral display size (Karron et al. 2004), floral morphology (Karron et al. 1997), and the presence of competitors for pollination (Bell et al. 2005). Karron et al. (2009) also documented striking variation in selfing rate among adjacent flowers which reflects increased deposition of geitonogamous (among-flower, within-display) self pollen as bumble bees probe consecutive flowers on a floral display.
Lythrum salicaria L. (Lythraceae) is a wetland perennial herb native to Eurasia and invasive in North America (Hager and McCoy 1998; Blossey et al. 2001; Farnsworth and Ellis 2001). In the species’ invasive range, it frequently grows in large, dense stands (Thompson et al. 1987; Mal et al. 1992; Farnsworth and Ellis 2001). Individual Lythrum plants may have up to 50 spikes of densely packed, showy magenta flowers that are very attractive to pollinators (Mal et al. 1992). Lythrum salicaria is tristylous, producing three floral morphs that differ in the relative positions of stigmas and anthers (Darwin 1877; Mal et al. 1992; Agren 1996). Lythrum flowers from July through October, overlapping substantially with the phenology of Mimulus. The introduced range of Lythrum overlaps broadly with the range of Mimulus and they occasionally co-occur. In areas of sympatry, bumblebee pollinators move freely between the two species.
Experimental arrays
To address the influence of Lythrum presence and abundance on Mimulus reproductive success, we constructed two-dimensional arrays using potted plants of each species. These plants were grown in 35.6-cm diameter pots at the University of Wisconsin-Milwaukee Field Station (Saukville, WI, USA). Competition treatments were established by placing pots of each species into a square grid with 1-m spacing between adjacent pot centers. This design enabled us to sequentially expose all competition treatments to the same local pollinator assemblage. Plants not used in an array were stored in a pollinator-free screenhouse.
In each array, we positioned 15 Mimulus plants in a checkerboard arrangement (Fig. 1). In the control treatment, no Lythrum plants were present (Fig. 1a). To quantify interactions for pollination when Mimulus and Lythrum were at an equal relative abundance, we added 15 Lythrum plants to the checkerboard design (Fig. 1b). To quantify interactions for pollination when Lythrum was at a high relative abundance, we established a second competition treatment consisting of 15 Mimulus plants and 41 Lythrum plants by surrounding 30 plants configured as in the equal abundance treatment with a ring of additional Lythrum plants (Fig. 1c). This design keeps the spacing of Mimulus plants constant but simulates a small patch of Mimulus embedded in a larger matrix of Lythrum. All three Lythrum style morphs were represented equally in each competitive array. We trimmed each vigorously-growing Mimulus plant to eight flowers to reflect the size of a typical Mimulus floral display in nature (Karron et al. 2009). These eight flowers were spread evenly across the plant, mimicking displays on unmanipulated plants. We trimmed the Mimulus displays at 5 a.m., before pollinators became active. We did not manipulate Lythrum displays, which already reflected display sizes typical of nearby populations (Flanagan et al. 2009).
Diagrammatic representation of experimental design used to quantify interactions for pollination. a Monospecific arrays of Mimulus were compared to arrays where b Mimulus and Lythrum were at an equal relative abundance and c where Lythrum outnumbered Mimulus by approximately 3:1. M Mimulus, L Lythrum. Plot sizes for a and b were 5 m × 5 m; for c 7 m × 7 m. There is 1 m spacing between adjacent plant centers in all plots
We established each competition treatment or control on a separate day. A set of two different competition treatments and one control comprised a block. Because we established all three treatments in the same location, we allowed 1 day between observations so that pollinators could acclimate to each newly-established array. We used a fresh set of Mimulus plants for each block. At the end of each block, we returned Mimulus plants to the screenhouse to protect them from further pollinator visitation and pests while fruits ripened. Therefore, except for a brief window when Mimulus plants were in the experimental array, all the plants used in this experiment were subject to very similar environmental conditions. The use of potted plants also gave us a high degree of control over the nutrient and moisture status of plants used in the experiment, allowing us to minimize variation in plant resource status. On the day after each array was established, we quantified pollinator visitation and then tagged flowers to measure seeds per fruit. Each block lasted 6–8 days, depending on weather conditions. Pollinator visitation rates and the resulting seeds per Mimulus fruit were measured for two blocks during August 2006 and three blocks during August 2007.
Pollinator visitation patterns
On each observation day, we identified pollinators to species and recorded pollinator visitation rates during 10-min intervals scheduled every 30 min between 5 a.m. (prior to the arrival of the first bee) and 1 p.m. (after all Mimulus stigmas had closed). Because multiple bees frequently foraged simultaneously in the plot, we divided the central 30 positions in each array (e.g., 15 Mimulus plants and, if present, 15 Lythrum plants) into five sections, each with three Mimulus and three (or zero) Lythrum plants. During each 10-min window, we recorded the number of probes to Mimulus flowers and Lythrum flowers on plants in one section. This allowed us to record all bee probes to the six (or three) plants during the observation period. We were able to observe and record all probes using this method, even when multiple bees were present. We rotated among the five sections randomly throughout the day. In the high Lythrum abundance treatments, we collected visitation rate data for the inner 30 plants only. We used these data to estimate probes per Mimulus flower per hour over the 8-h window of observation.
For all analyses, we used JMP version 5.1.2 (SAS Institute 2004). To test the effects of Lythrum presence and abundance on the rate of visitation to Mimulus, we used ANOVA (general linear models procedure) with a single measurement of probes per flower per hour for each 8-h observation day as the unit of replication (n = 15). In the model, competition treatment and year were fixed effects, and block nested within year was a random effect. We treated year as a fixed factor because 2 years are not sufficient to allow a confident estimate of levels of variation among all possible years (Newman et al. 1997). To characterize among-year variation in the composition of pollinator species probing Mimulus or Lythrum during the 10-min visitation intervals, we used G tests of independence.
To determine whether Lythrum presence and abundance influenced pollinator arrivals to arrays, in 2007 we censused the number of bumblebees foraging on Mimulus and Lythrum plants in the arrays. We counted all bees visiting plants of either species in the entire array once every 30 min from 5 a.m. until 1 p.m. We then tested for treatment and block effects on the mean number of bees visiting each array using a two-way ANOVA.
Patterns of pollinator foraging between species
In mixed-species experimental arrays, we quantified two additional aspects of pollinator foraging behavior: the extent to which bees discriminated among species, preferentially visiting one species over another, and the extent to which bees moved between species in a single foraging bout. Immediately following each visitation rate census, we recorded the complete floral visitation sequence for each pollinator foraging in an array during a 20-min period. We noted the species and location of each plant visited. To test for pollinator preference, we compared the frequency of visits to plants of each species in the array to expected values which were calculated based on the availability of plants of each species. We tested for significant departure from these expected values with a G test (Sokal and Rohlf 1995; Husband and Barrett 1992). In this analysis, we used blocks as a replicated measure. Therefore, we present a G statistic for the pooled frequencies across blocks of each treatment (G P) and for heterogeneity among blocks of each treatment (G H).
We also used G tests to evaluate the tendency of foragers to make heterospecific (Mimulus-to-Lythrum or Lythrum-to-Mimulus) versus conspecific (Mimulus-to-Mimulus or Lythrum-to-Lythrum) transitions in mixed species arrays. In this analysis, we used blocks as a replicated measure, and expected values for plant-to-plant transitions were calculated based on the frequency of visits pollinators made to each species (Ippolito et al. 2004). Therefore, deviation from expected values should be independent from patterns of pollinator preference (Husband and Barrett 1992; Dafni et al. 2005).
Pollinator foraging data were also used to quantify the number of Lythrum flowers probed consecutively during each Mimulus-to-Lythrum-to-Mimulus transition in mixed species arrays. We used ANOVA (general linear models procedure) to test the effects of competition treatment on the number of Lythrum flowers probed per interspecific (Mimulus-to-Lythrum-to-Mimulus) transition. In the model, competition treatment and year were both fixed effects. The random effect of block nested within year was not significant (see “Results”) and so block effects were pooled in the final model. In this analysis, individual Mimulus-to-Lythrum-to-Mimulus pollinator transitions (n = 120) served as the unit of replication.
Number of seeds per Mimulus fruit
To quantify the effects of competition treatment on Mimulus reproductive success, we tied labeled plastic tags to the pedicels of two randomly-selected flowers on all 15 Mimulus plants in each array. We tagged a total of 450 flowers (2 flowers × 15 plants × 3 competition treatments × 5 blocks). We harvested the fruits upon ripening (~30 days) and counted all seeds in each fruit using a dissecting microscope.
We used ANOVA (general linear models procedure) to test the effects of Lythrum presence and abundance on the number of seeds per Mimulus fruit. In the model, competition treatment and year were both fixed effects. If our data are analyzed with year as a random effect, the only change is that the differences among years become insignificant. The random effect of block nested within year was not significant (see “Results”) and so block effects were pooled in the final model. In this analysis, a single measurement of mean seeds for each treatment day was the unit of replication (n = 15).
We also used ANCOVA to examine the influence of increasing Lythrum abundance on seeds per Mimulus fruit. We scored competition treatment as a numerical covariate, corresponding to the relative abundance of Lythrum to Mimulus, and tested for its effects on Mimulus seeds per fruit. When Lythrum was absent, we scored Lythrum abundance as 0; when Lythrum and Mimulus plants were equally abundant, we scored Lythrum abundance as 1; and when Lythrum was nearly three times as abundant as Mimulus, we scored Lythrum abundance as 3. This model also incorporated the categorical variable year and the interaction of Lythrum abundance and year. As in the ANOVA described above, non-significant block effects were pooled and mean seed number per treatment day was the unit of replication. The non-significant interaction term was also dropped from the model in the final analysis.
Results
Variation in pollinator species composition
Bombus impatiens was the most prevalent visitor to both Mimulus and Lythrum flowers in 2006, accounting for 90% of 294 probes to Mimulus and 99% of 3,294 probes to Lythrum. The remaining 10% of probes to Mimulus and 1% of probes to Lythrum were by Bombusvagans. In 2007, Bombus impatiens was less prevalent, accounting for 46% of 292 probes to Mimulus and 63% of 1,923 probes to Lythrum. Bombus vagans also visited frequently during 2007, accounting for 54% of probes to Mimulus and 32% of probes to Lythrum. Two other species, B. griseocollis and Apis mellifera, also made occasional visits to Lythrum during 2007. Overall, the composition of Bombus species visiting Mimulus differed significantly between 2006 and 2007 (G = 146.50, df = 1, P < 0.001). Similarly, the composition of Bombus species visiting Lythrum differed significantly between years (G = 1,339.66, df = 2, P < 0.001).
In 2007, the presence and abundance of Lythrum significantly influenced the composition of pollinator species visiting arrays (G = 232.67, df = 2, P < 0.001). When only Mimulus was present, B. impatiens accounted for 61% of pollinator species visiting arrays and B. vagans accounted for 39%. When Mimulus and Lythrum were equally abundant, B. impatiens accounted for 45% of pollinator species visiting arrays, and B. vagans accounted for 54%. When Lythrum was at a high relative abundance, B. impatiens accounted for 80% of pollinator species visiting arrays, and B. vagans accounted for 20%.
Pollinator visitation rates and patterns of pollinator arrivals to arrays
There was a marginally non-significant effect of competition treatment on the rate of pollinator visitation to Mimulus (F 2,6 = 4.83, P = 0.06). In 2006, when no Lythrum was present, Mimulus received 3.77 ± 0.47 (mean ± 1 SE) visits per flower per hour. When Lythrum was present at an equal relative abundance, Mimulus received 1.72 ± 0.47 visits per flower per hour. When Lythrum was at a high relative abundance, Mimulus received 1.92 ± 0.47 visits per flower per hour. In 2007, when no Lythrum was present, Mimulus received 1.64 ± 0.38 visits per flower per hour. When Lythrum was present at an equal relative abundance, Mimulus received 1.05 ± 0.38 visits per flower per hour. When Lythrum was at a high relative abundance, Mimulus received 1.83 ± 0.38 visits per flower per hour. The rate of pollinator visitation per Mimulus flower did not vary significantly as a function of year, but did vary significantly among blocks (F 3,6 = 5.08, P = 0.04).
The total number of bees visiting arrays varied significantly among competition treatments (one-way ANOVA, F 2,59 = 5.42, P = 0.007). In 2007, we observed three times as many bees in arrays with equal frequencies of Mimulus and Lythrum as were present in arrays without Lythrum plants (Fig. 2). When Lythrum was at a high relative abundance, nearly four times as many bees visited the arrays. In an a posteriori test, pollinator arrivals to arrays were significantly lower in the absence of Lythrum, but did not differ significantly between the two Lythrum abundances (Tukey-Kramer HSD).
Patterns of pollinator preference and interspecific transitions within foraging bouts
In 2006, Lythrum plants received 64–87% of all pollinator visits in mixed-species arrays, significantly more than the expected number of visits (Table 1). Because visits by B. vagans were infrequent in 2006, we pooled pollinator species for this analysis. In 2007, Lythrum plants again received a large proportion (64–75%) of B. impatiens visits. When Mimulus and Lythrum were equally abundant, there was a significant excess of visits to Lythrum by B. impatiens, but not by B. vagans (Table 2). However, when Lythrum was at high relative abundance, there was a significant excess of visits to Mimulus plants by both Bombus species (Table 2). Visitation to Lythrum by B. vagans was highly variable (Table 2), and this variability contributed to significant heterogeneity among blocks.
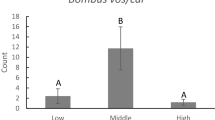
Interspecific transitions were common in the mixed species arrays during both years, making up 40–56% of all interplant transitions. In some cases, there was a significant excess of interspecific transitions relative to other transition types, but there was no consistent pattern relative to Lythrum abundance (Table 3). When foragers sequentially probed Mimulus, then Lythrum, then Mimulus, the number of intervening Lythrum flowers probed increased significantly with increasing Lythrum abundance (F 1,116 = 7.15, P < 0.05). In both years, foragers that made such interspecific transitions probed nearly twice as many intervening Lythrum flowers when Lythrum was at high relative abundance (Fig. 3).
Effects of Lythrum presence and abundance on Mimulus reproductive success
The number of seeds per Mimulus fruit varied both among competition treatments and among years (Fig. 4, two-way ANOVA, adjusted R 2 = 0.56). Both the effect of competition treatment (F 2,9 = 4.42, P < 0.05) and the effect of year (F 1,9 = 13.79, P < 0.005) were significant. There was no significant interaction between competition treatment and year (F 2,9 = 0.11, P > 0.90). In 2006, Mimulus seed number per fruit was 32% lower in arrays with an equal frequency of Lythrum and Mimulus than in arrays without Lythrum. When Lythrum was at a high relative abundance, Mimulus seed number was further reduced (40% lower than in arrays without Lythrum). In 2007, Mimulus seed number per fruit was 17% lower in arrays with an equal frequency of Lythrum and Mimulus than in arrays without Lythrum. When Lythrum was at a high relative abundance, Mimulus seed number was further reduced (33% lower than in arrays without Lythrum; Fig. 4). In an a posteriori test, Mimulus seeds per fruit were found to differ significantly between the arrays with a high relative abundance of Lythrum and arrays without Lythrum (P < 0.05), but the other comparisons were not significant (Fig. 4, Tukey-Kramer HSD). There is a strong directional trend to these data, with increasing Lythrum abundance significantly reducing Mimulus seed number (ANCOVA, adjusted R 2 = 0.60, F 1,11 = 7.93, P < 0.05). There were also significant differences among years, but no significant interactions between competition treatment and year.
Discussion
Competition for pollination with invasive exotic Lythrum salicaria lowered mean seed number in Mimulus ringens fruits. This reduction was most pronounced when invasive Lythrum was highly abundant, decreasing the number of seeds per fruit by 40% in 2006 and 33% in 2007. This finding is consistent with the results of a recent meta-analysis (Morales and Traveset 2009) which suggested that an increase in the abundance of invasives may exacerbate the competitive effects that occur at equal species abundances. Here, we explore the mechanisms responsible for this reduction in seeds per fruit and discuss how changes in the relative abundance of invasive competitors may influence the reproduction of native species.
Patterns of pollinator visitation
Exotic invasive species may lower the pollination success of co-occurring native plants if pollinators exhibit preference for the invasive and visit it more frequently than the native species. Through such competition for pollinator preference, the invasive may lower conspecific pollen transfer and seed production of the native species (Waser 1983). Although Lythrum is highly attractive to pollinators (Brown et al. 2002), and received an overall higher number of pollinator visits in all contexts, some bees did not exhibit preference for Lythrum. For example, in both years, there was a marginally significant trend toward increased visitation to Mimulus when Lythrum was highly abundant, compared to arrays in which Lythrum was present at an equal relative abundance. A positive effect of high Lythrum abundance on Mimulus visitation rate may have been even more likely in 2007, since the rate of visitation to Mimulus when Lythrum was abundant was higher than visitation to Mimulus in arrays with no Lythrum present.
These patterns of pollinator preference and visitation to Mimulus may have been influenced by the observed tripling in the number of pollinators recruited to arrays containing Lythrum (Fig. 2). Showy invasive species such as Lythrum salicaria may act as “magnet species” that increase the overall attractiveness of the patch, recruiting pollinators to a floral stand they would not otherwise forage in (Thomson 1978; Molina-Montenegro et al. 2008). Due to increased pollinator arrivals to arrays, invasives may increase pollinator visitation rates and reproductive success of co-occurring natives if the pollinators do not show perfect preference for the invasive, but rather forage on both species (Rathcke 1983; Johnson et al. 2003; Moeller 2004; Feldman et al. 2004; Ghazoul 2006; Totland et al. 2006; Munoz and Cavieres 2008; Bjerknes et al. 2007). In our study, increased pollinator arrivals to mixed-species arrays may have offset any reduction in Mimulus visitation due to pollinator preference for Lythrum. Such a scenario may have been especially likely in 2007, when the visitation rate to Mimulus increased with a higher relative abundance of Lythrum. Our results suggest that the effects of invasives on visitation rates to native species may largely depend on interactions between pollinator preference and changes in patterns of pollinator arrivals to arrays (Bjerknes et al. 2007; Lopezaraiza-Mikel et al. 2007; Munoz and Cavieres 2008; Mitchell et al. 2009a).
Effects of visit quality on pollen deposition and seed production
Pollinator movement between native and invasive species can strongly influence patterns of pollen transfer, and may lower the reproductive success of native species, even if visitation rate is not affected (Waser 1983). Interspecific transitions were very common in all mixed species arrays during both years, making up 40–56% of all interplant transitions. Although there was no consistent pattern with respect to Lythrum abundance, the high proportion of interspecific pollinator movement in the mixed-species arrays suggests that there is strong potential for interspecific pollen transfer to lower reproductive success of the native species.
Frequent pollinator movements between a focal species and a competitor are likely to promote pollen loss, which may be magnified with increasing relative abundance of the competitor (Morales and Traveset 2008). Although the proportion of interspecific transitions did not vary with Lythrum abundance, the number of Lythrum flowers probed per interspecific transition did vary with Lythrum abundance, which could magnify Mimulus pollen loss. When Lythrum was abundant, inconstant foragers departing Mimulus sequentially probed nearly twice as many Lythrum flowers before returning to Mimulus (Fig. 3). Pollen loss may result from pollinator contact with the stigmas or other floral surfaces of competitor flowers (Campbell 1985; Feinsinger et al. 1988; Feinsinger and Tiebout 1991; Murcia and Feinsinger 1996; Morales and Traveset 2008) or from grooming or other transport-related losses that occur while the pollinator forages (Thomson 1986; Inouye et al. 1994; Harder and Wilson 1998; Flanagan et al. 2009; Mitchell et al. 2009a). Following these losses, when a pollinator returns to flowers of a focal species, fewer conspecific pollen grains may remain available for deposition onto stigmas of the focal species, potentially leading to a reduction in Mimulus reproductive success (Flanagan et al. 2009).
The reproductive success of native species may also be lowered if pollinators deposit pollen grains of the invasive onto the stigma of the native species, blocking or clogging the stigmatic surface or interfering with the successful germination of conspecific pollen (Waser 1978; Waser and Fugate 1986; Galen and Gregory 1989; Caruso and Alfaro 2000; Brown and Mitchell 2001). However, it is also possible that the presence of heterospecific grains will have little influence on seed number (Campbell and Motten 1985; Murcia and Feinsinger 1996; Jakobsson et al. 2008). Pollen of the two plant species is partitioned on the pollinator’s body, such that little contact is made between Lythrum pollen carried by the bee and Mimulus stigmas. Additionally, in an earlier study (Flanagan et al. 2009), we showed that very few Lythrum grains were deposited on Mimulus stigmas by bees carrying Lythrum pollen, and that the presence of Lythrum pollen on Mimulus stigmas did not influence seed number. Therefore, inbound interspecific pollen transfer is unlikely to be responsible for the observed reductions in Mimulus reproductive success. Rather, we think reductions in Mimulus seed set are due to pollen loss during interspecific visits to Lythrum. In particular, we think these losses may be due to pollinator grooming and passive transport-related loss during interspecific transitions by bumblebees (Flanagan et al. 2009). This result is in contrast to Brown and Mitchell’s (2001) finding that deposition of Lythrum salicaria grains on stigmas of native Lythrum alatum lowered L. alatum seed set.
Seed number in Mimulus fruits varied markedly among years. This finding may reflect changes in the composition of bumblebee species probing Mimulus in 2006 and 2007 and differences in their preference for Lythrum versus Mimulus. Although the presence of Lythrum lowered Mimulus seed number in both years, the striking among-year variation in seeds per fruit emphasizes the importance of studying competition for pollination in multiple years (Larson et al. 2006).
A number of factors relating to plant size and vigor, resource limitation, or other abiotic or biotic environmental conditions could also contribute to the observed among-year variation in seed number (Klinkhamer and DeJong 1987; DiFazio et al. 1998; Griffin and Barrett 2002; Shimono and Washitani 2007). In particular, one weakness of our study is the absence of a supplemental hand-pollination treatment (Mitchell et al. 2009b). However, the use of potted plants allowed us to have a high level of control over the resource status of plants used in this experiment (see “Materials and methods”). Therefore, we feel confident that only the competitive background experienced by plants varied, and that differences in seed set among treatment groups were due to differences in pollination, rather than to differences in resource status.
Conclusion
Invasive species often have large populations, and their abundance relative to co-occurring natives may influence the outcome of competition for pollination. We found that the presence of invasive Lythrum salicaria increased the number of pollinators visiting experimental arrays, but did not lower the visitation rate to Mimulus ringens. However, the presence of Lythrum lowered mean seed number in Mimulus fruits, with reductions most pronounced when the invasive was highly abundant. We attribute much of this reduction in seed number to pollen loss when bees carrying Mimulus pollen probed intervening Lythrum flowers. Although the proportion of interspecific transitions made by pollinators was not influenced by Lythrum abundance, an increase in Lythrum abundance led to a doubling in the number of intervening Lythrum flowers probed, potentially magnifying pollen loss. This study emphasizes the importance of studying competition for pollination across a range of competitor abundances, and demonstrates that visit quality, not just visit number, may be an important determinant of the effects of exotic, invasive species on the reproductive success of native species.
References
Agren J (1996) Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicaria. Ecology 77:1779–1790
Bell JM, Karron JD, Mitchell RJ (2005) Interspecific competition for pollination lowers seed production and outcrossing rate in Mimulus ringens. Ecology 86:762–771
Bjerknes AL, Totland O, Hegland SJ, Neilsen A (2007) Do alien plant invasions really affect pollination success in native plant species? Biol Conserv 138:1–12
Blossey BL, Skinner O, Taylor J (2001) Impact and management of purple loosestrife (Lythrum salicaria) in North America. Biodivers Conserv 10:1787–1807
Brown BJ, Mitchell RJ (2001) Competition for pollination: effects of pollen of an invasive plant on seed set of a native congener. Oecologia 129:43–49
Brown BJ, Mitchell RJ, Graham SA (2002) Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83:2328–2336
Campbell DR (1985) Pollinator sharing and seed set of Stellaria pubera: competition for pollination. Ecology 66:544–563
Campbell DR, Motten AF (1985) The mechanism of competition for pollination between two forest herbs. Ecology 66:554–563
Caruso CM (2002) Influence of plant abundance on pollination and selection on floral traits of Ipomopsis aggregata. Ecology 83:241–254
Caruso CM, Alfaro M (2000) Interspecific pollen transfer as a mechanism of competition: effect of Castilleja linariaefolia pollen on seed set of Ipomopsis aggregata. Can J Bot 78:600–606
Chittka L, Schurkens S (2001) Successful invasion of a floral market. Nature 411:653
Dafni A, Kevan PG, Husband BC (eds) (2005) Practical pollination biology. Enviroquest, Cambridge
Darwin C (1877) The different forms of flowers on plants of the same species. Appleton, New York
DiFazio SP, Wilson MV, Vance NC (1998) Factors limiting seed production of Taxus brevifolia (Taxaceae) in western Oregon. Am J Bot 85:910–918
Farnsworth EJ, Ellis DR (2001) Is purple loosestrife (Lythrum salicaria) an invasive threat to freshwater wetlands? Conflicting evidence. Wetlands 21:199–209
Feinsinger P, Tiebout HM III (1991) Competition among plants sharing hummingbird pollinators: laboratory experiments on a mechanism. Ecology 72:1946–1952
Feinsinger P, Busby WH, Tiebout HM III (1988) Effects of indiscriminate foraging by tropical hummingbirds on pollination and plant reproductive success: experiments with two tropical treelets (Rubiaceae). Oecologia 76:471–474
Feldman TS, Morris WF, Wilson WG (2004) When can two plants facilitate each other’s pollination? Oikos 105:197–207
Flanagan RJ, Mitchell RJ, Knutowski D, Karron JD (2009) Interspecific pollinator movements reduce pollen deposition and seed production in Mimulus ringens (Phrymaceae). Am J Bot 96:809–815
Galen C, Gregory T (1989) Interspecific pollen transfer as a mechanism of competition: consequences of foreign pollen contamination for seed set in the alpine wildflower, Polemonium viscosum. Oecologia 81:120–123
Ghazoul J (2004) Alien abduction: disruption of native plant-pollinator interactions by invasive species. Biotropica 36:156–164
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304
Grant AL (1924) A monograph of the genus M. ringens. Ann M Bot Gard 11:99–389
Griffin SR, Barrett SCH (2002) Factors affecting low seed-ovule ratios in a spring woodland herb, Trillium grandiflorum (Melanthiaceae). Int J Plant Sci 163:581–590
Hager H, McCoy KD (1998) The implications of accepting untested hypotheses: a review of the effects of purple loosestrife (Lythrum salicaria) in North America. Biodivers Conserv 7:1069–1079
Harder LD, Wilson WG (1998) Theoretical consequences of heterogeneous transport conditions for pollen dispersal by animals. Ecology 79:2789–2807
Husband BC, Barrett SCH (1992) Pollinator visitation in populations of tristylous Eichhornia paniculata in northeastern Brazil. Oecologia 89:365–371
Inouye DW, Gill DE, Dudash MR, Fenster CB (1994) A model and lexicon for pollen fate. Am J Bot 81:1517–1530
Ippolito A, Fernandes GW, Holtsford TP (2004) Pollinator preferences for Nicotiana alata, N. forgetiana, and their F1 Hybrids. Evolution 58:2634–2644
Jakobsson A, Padron B, Traveset A (2008) Pollen transfer from invasive Carpobrotus spp. to natives—a study of pollinator behaviour and reproduction success. Biol Conserv 141:136–145
Johnson SD, Peter CI, Nilsson LA, Agren J (2003) Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84:2919–2927
Kandori I, Hirao T, Matsunaga S, Kurosaki T (2009) An invasive dandelion unilaterally reduces the reproduction of a native congener through competition for pollination. Oecologia 159:559–569
Karron JD, Thumser NN, Tucker R, Hessenauer AJ (1995a) The influence of population density on outcrossing rates in Mimulus ringens. Heredity 75:175–180
Karron JD, Tucker R, Thumser NN, Reinartz JA (1995b) Comparison of pollinator flight movements and gene dispersal patterns in Mimulus ringens. Heredity 75:612–617
Karron JD, Jackson RT, Thumser NN, Schlicht SL (1997) Outcrossing rates of individual Mimulus ringens genets are correlated with anther–stigma separation. Heredity 79:365–370
Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B (2004) The influence of floral display size on selfing rates in Mimulus ringens. Heredity 92:242–248
Karron JD, Mitchell RJ, Bell JM (2006) Multiple pollinator visits to Mimulus ringens (Phrymaceae) flowers increase mate number and seed set within fruits. Am J Bot 93:1306–1312
Karron JD, Holmquist K, Flanagan RJ, Mitchell RJ (2009) Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Ann Bot 103:1379–1383
Klinkhamer PGL, DeJong TJ (1987) Plant size and seed production in the monocarpic perennial Cynoglossum officinale L. New Phytol 106:773–783
Larson DL, Royer RA, Royer MR (2006) Insect visitation and pollen deposition in an invaded prairie plant community. Biol Conserv 130:148–159
Lopezaraiza-Mikel ME, Hayes RB, Whalley R, Memmott J (2007) The impact of an alien plant on a native plant-pollinator network: an experimental approach. Ecol Lett 10:539–550
Mal TK, Lovett-Doust J, Lovett-Doust L, Mulligan GA (1992) The biology of Canadian weeds. 100. Lythrum salicaria. Can J Plant Sci 72:1305–1330
Mitchell RJ, Karron JD, Holmquist KG, Bell JM (2004) The influence of Mimulus ringens floral display size on pollinator visitation patterns. Funct Ecol 18:116–124
Mitchell RJ, Karron JD, Holmquist KG, Bell JM (2005) Patterns of multiple paternity in fruits of Mimulus ringens (Phrymaceae). Am J Bot 92:885–890
Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD (2009a) New frontiers in competition for pollination. Ann Bot 103:1403–1413
Mitchell RJ, Irwin RE, Flanagan RJ, Karron JD (2009b) Ecology and evolution of plant-pollinator interactions. Ann Bot 103:1355–1363
Moeller DA (2004) Facilitative interactions among plants via shared pollinators. Ecology 85:3289–3301
Molina-Montenegro MA, Badano EI, Cavieres LA (2008) Positive interactions among plant species for pollinator service: assessing the ‘magnet species’ concept with invasive species. Oikos 117:1833–1839
Morales CL, Aizen MA (2006) Invasive mutualisms and the structure of plant-pollinator interactions in the temperate forests of north-west Patagonia, Argentina. J Ecol 94:171–180
Morales CL, Traveset A (2008) Interspecific pollen transfer: magnitude, prevalence, and consequences for plant fitness. Crit Rev Plant Sci 27:221–238
Morales CL, Traveset A (2009) A meta-analysis of impacts of alien vs. native plants on pollinator visitation and reproductive success of co-flowering native plants. Ecol Lett 12:716–728
Munoz AA, Cavieres LA (2008) The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. J Ecol 96:459–467
Murcia C, Feinsinger P (1996) Interspecific pollen loss by hummingbirds visiting flower mixtures: effects of floral architecture. Ecology 77:550–560
Newman JA, Bergelson J J, Grafen A (1997) Blocking factors and hypothesis tests in ecology: is your statistics text wrong? Ecology 78:1312–1320
Rathcke B (1983) Competition and facilitation among plants for pollination. In: Real L (ed) Pollination biology. Academic, Orlando, pp 305–309
SAS Institute (2004) JMP version 5.1.2 statistical software. SAS Institute, Cary
Shimono A, Washitani I (2007) Factors affecting variation in seed production in the heterostylous herb Primula modesta. Plant Species Biol 22:65–76
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Takakura K, Nishida T, Matsumoto T, Nishida S (2009) Alien dandelion reduces the seed-set of a native congener through frequency-dependent and one-sided effects. Biol Invasions 11:973–981
Thompson DQ, Stuckey RL, Thompson EB (1987) Spread, impact and control of purple loosestrife in North American wetlands. US Fish and Wildlife Research, Washington DC
Thomson JD (1978) Effect of stand composition on insect visitation in two-species mixtures of Hieracium. Am Midl Nat 100:431–440
Thomson JD (1986) Pollen transport and deposition by bumble bees in Erythronium: influences of floral nectar and bee grooming. J Ecol 74:329–341
Totland O, Nielsen A, Bjerknes AJ, Ohlson M (2006) Effects of an exotic plant and habitat disturbance on pollinator visitation and reproduction in a boreal forest herb. Am J Bot 93:868–873
Waser NM (1978) Competition for pollination and sequential flowering in two Colorado wildflowers. Ecology 59:934–944
Waser NM (1983) Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold, New York, pp 277–293
Waser NM, Fugate ML (1986) Pollen precedence and stigma closure: a mechanism of competition for pollination between Delphinium nelsonii and Ipomopsis aggregata. Oecologia 70:573–577
Acknowledgments
The authors thank T. Schuck for assistance propagating Mimulus ringens and J. Reinartz, G. Meyer, and L. Nelson for help with field work. We thank S. Johnson, O. Totland, and an anonymous reviewer for helpful comments on an earlier draft of this manuscript. This research was supported by a grant from Applied Ecological Services to R. J. F. and grants from the National Science Foundation to J. D. K. (DEB 9816712) and R. J. M. (DEB 9903308). This experiment complies with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Johnson.
Rights and permissions
About this article
Cite this article
Flanagan, R.J., Mitchell, R.J. & Karron, J.D. Increased relative abundance of an invasive competitor for pollination, Lythrum salicaria, reduces seed number in Mimulus ringens . Oecologia 164, 445–454 (2010). https://doi.org/10.1007/s00442-010-1693-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1693-2