Abstract
The behavioural trait axes of activity, exploration, boldness and sociability can help to understand the tendency of an invasive species to disperse, as may be expected at the fringe of an invasive population, or to socialise, as may be expected in well-established populations where densities of invaders are typically high. We compared behavioural traits between the gammarids Dikerogammarus villosus and Gammarus pulex from centre and fringe populations at Barton Broad, Norfolk, UK. Dikerogammarus villosus is invasive in Western Europe and has displaced many macroinvertebrate species, including the native G. pulex. Gammarus pulex is itself invasive in Northern Ireland and the Isle of Man, thus its displacement increases interest into what unique characteristics of D. villosus make it a dominantly successful invader. Dikerogammarus villosus was significantly less active, less explorative, and more social than G. pulex. We found no significant differences in the behaviours of D. villosus individuals from the central population and the invasive fringe. These patterns indicate active dispersal is likely important to the invasion success of G. pulex, while D. villosus might depend on passive movement. Our data suggest that behavioural factors determining invasive success within closely related taxa can differ considerably, and may lead to different patterns of invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Every biological function undertaken by an organism is composed of a set of behaviours (Reale et al. 2007). For this reason, the study of animal behaviour has long been recognised as integral to advancing our understanding of ecological dynamics at both the population and community level (Sih et al. 2012). Recently, the role of behavioural traits as related to invasion ecology has received increased attention (Chapple et al. 2012). Three major themes of this interest are (1) comparison of traits possessed by invasive and native species, (2) within an invasive species, comparison of traits possessed by individuals at the edge of the invasion front to those in the central population, and (3) how intraspecific trait variation impacts establishment success of an invasive species.
Ecologists have struggled to find consistent species-level characteristics that predict the establishment success of potential invaders, especially when extrapolating characteristics across taxonomic groups (Hayes and Barry 2008). As a result, propagule pressure, rather than any particular trait, is often considered a more consistent predictor of invasion success (Simberloff 2009). Inclusion of behavioural traits can improve models based on propagule pressure alone (Suarez et al. 2005; Sol et al. 2008), however, behavioural information is often lacking (Lester 2005). For invasive species where behaviour has been studied, differences in behavioural traits such as aggression and boldness may explain the success of non-natives in displacing their native congeners (Duckworth and Badyaev 2007; Kappes et al. 2012; Sanches et al. 2012). These two traits have, in turn, been positively correlated with other behaviours including high activity and exploration (e.g., Pintor et al. 2009; Cote et al. 2010).
After initial establishment, the next phase of the invasion process is dispersal (Chapple et al. 2012). There is evidence that individuals at the edge of an invasion front can display different physical and behavioural traits than individuals from the central, established population. In the well-known case of the cane toad invasion in Queensland, Australia, longer-legged toads are found at the invasion front, as they are able to move further in the same period of time than their shorter-legged conspecifics (Phillips et al. 2006). Behaviour traits especially tend to vary in newly established populations of species dependent on human-mediated dispersal pathways, where dispersed individuals exhibit behaviours such as high exploration that increase the likelihood of encountering a transport vector (Chapple et al. 2011).
The multi-step invasion process, which would seem to require that a successful invader possess a number of contradictory traits, is increasingly seen as a possible explanation for why it is so difficult to consistently predict the traits of a successful invasive species (Chapple et al. 2012). An asocial tendency, for example, is a trait that could contribute to dispersal success, but is seemingly incompatible with the high densities typically achieved by invasive species. This “problem of trade-offs” might be overcome through intraspecific trait variation, with species showing greater variation being able to remain established longer and spread more quickly (Fogarty et al. 2011; Forsman et al. 2012). Consistent differences in individual behaviour, one form of intraspecific trait variation, have now been documented in a variety of taxa including both vertebrates and invertebrates (Gosling 2001; Gherardi et al. 2012).
Dikerogammarus villosus (Sowinsky 1894), an amphipod native to the Ponto–Caspian basin of Eastern Europe (Ricciardi and Rasmussen 1998), is a highly invasive species in Western Europe. It has spread rapidly, aided by the opening of canal systems such as that linking the Main and Danube Rivers (Mayer et al. 2008). The species was first recorded in Great Britain in 2010 at Grafham Water (MacNeil et al. 2010), and subsequently was recorded in two sites in South Wales during 2011 (Madgwick and Aldridge 2011). Due to the high climate suitability of much of Great Britain to the species and the high connectivity of the hydrological network, the spread of D. villosus is expected to continue in this region (Gallardo et al. 2012). The presence of D. villosus is likely to impact the nutrient and energy dynamics at an invaded site through multiple pathways, including altering the seasonal availability of particulate organic matter (Truhlar et al. 2013) and predating on the existing macroinvertebrate community assemblage (Dick and Platvoet 2000). The high densities reached at Grafham Water have already resulted in changes to the commercial trout fishery in the reservoir. Most notably, Brown Trout (Salmo trutta, [Linnaeus 1758]) and Rainbow Trout [Oncorhynchus mykiss, (Walbaum 1792)] have shifted to dominate the rocky margins of the reservoir where the D. villosus population is greatest; reports from anglers indicate that trout guts are full of the shrimp (Madgwick and Aldridge 2011).
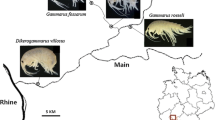
In March 2012, D. villosus populations were discovered at Barton Broad, Norfolk, United Kingdom, with the species locally highly abundant (Kelly, Broads Authority). Subsequent routine macroinvertebrate monitoring across the invaded catchment means that the invasion front within rivers connected to Barton Broad is clearly identifiable, and thus allows for comparisons between individuals in the fringe population and those in the established population.
Gammarus pulex (Linnaeus 1758) is a gammarid species native to Great Britain and Western Europe. Its distribution in Western Europe has already been impacted deleteriously by the presence of D. villosus (Bollache et al. 2004; Boets et al. 2010; Piscart et al. 2010). Gammarus pulex is a successful invader as well; it has displaced the native gammarid Gammarus duebeni celticus (Stock and Pinkster 1970) in many streams in Northern Ireland and the Isle of Man (MacNeil et al. 2004; Dick 2008). The ability of D. villosus to disrupt populations of another known invader like G. pulex has further increased interest in determining what characteristics of the species make it so markedly, and seemingly universally, successful.
We aimed to measure the behavioural trait axes of activity, exploration, boldness, and sociability for G. pulex and D. villosus. At a recently invaded location (Barton Broad) we quantified behavioural traits of the two species at the edge of the invasion front, and D. villosus from the central population. We made the following three predictions: (1) on average, D. villosus would exhibit higher levels of activity, exploration, boldness than G. pulex. We also expected D. villosus, as a species, to be slightly more asocial than G. pulex, due primarily to skewing from individuals in the invasion front (see next prediction); (2) D. villosus individuals from the invasion front would be more active, explorative, and bold than individuals from the central population, as well as less social; and (3) individual D. villosus and G. pulex would show repeatable behaviours as well as correlations between behaviours, indicative of distinct animal personalities.
Methods
Experimental animals
Dikerogammarus villosus and G. pulex individuals were collected in July 2012 from Barton Broad at Barton Turf (lat: 52°44′ 55″N; long: 1°29′27″W), the centre of the D. villosus distribution in Barton Broad, and from the River Ant at Ludham Bridge (lat: 52°41′58″N; long: 1°30′33″W), the furthest downstream location at which D. villosus had been identified within this invaded catchment (Fig. 1). Individuals were collected by scraping nets along dock sides, ropes, and boat hulls, all common habitats for the two species.
All amphipods were maintained individually in the lab to avoid predation. They were held in 200 mL aquaria of aerated, dechlorinated tap water in a 16L:8D light:dark schedule at a constant temperature of 15 °C. Except for a 24 h starvation period to standardize satiety prior to each round of behavioural testing, amphipods were fed ad libitum throughout the experiment with a mixture of freeze dried bloodworms (Interpet, Dorking, England) and dried willow leaves [Salix alba (Linnaeus 1753)].
Behavioural trials, overview
Four behaviours were measured over the course of the experiment: activity, exploration, boldness, and sociability. Sixty visibly non-parasitized, non-gravid D. villosus from each collection site and a combined total of 60 visibly non-parasitized, non-gravid G. pulex from both collection sites were selected. An additional ten D. villosus and ten G. pulex were randomly selected to serve as conspecifics for the sociability test, and maintained in two single-species aquaria, each containing 2 L of aged tap water. Half of the experimental animals were randomly selected to first undergo the activity trial followed immediately by the exploration and boldness trials. Simultaneously, the other half of the experimental animals underwent the sociability trial. Animals were then returned to their home aquaria for 2 days before undergoing a second round of experimentation, during which they completed the behavioural trials they were not involved in for the first round. The entire process was repeated 1 week later to measure behaviour repeatability for each individual. To balance for possible effects of the time of day on behaviour, the order of individual testing was shuffled such that no amphipod was tested within 3 h of the time at which it was tested the week before.
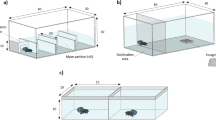
Activity was measured in the focal animal’s home aquarium. This is in accordance with the suggestion by Reale et al. (2007) that, to avoid confounding activity with exploration and boldness, activity levels be measured in non-novel environments. Trials to measure all other behaviours took place in two 20 × 32 cm aquaria, one for exploration and boldness and one for sociability. An opaque barrier separated the aquaria so that test animals could not see each other, and an opaque blind surrounded the entire set up so that test animals could not see the experimenter. A camera suspended above the two aquaria recorded all trials, and behaviours were quantified at a later time through viewing of the recordings. All behaviours were recorded under constant fluorescent lighting.
Behavioural trials, activity
Animals were observed in their home aquaria. The time they spent actively swimming was recorded on a stopwatch. Activity was quantified as the number of seconds, to the nearest second, during which the animal was actively swimming over a 60 s period.
Behavioural trials, exploration and boldness
The exploration/boldness aquarium was marked on the bottom with a 10 by 16 grid of 2-cm2 and contained a magnetic stir bar, centred 6 cm above one of the short ends. At the other short end was an opaque holding container of diameter 4 cm (Fig. 2). Prior to each trial, the aquarium water was emptied and a fresh 1 cm depth of aged tap water was added, thus removing any chemical cues that might have been left by previous test animals. Each trial began with an acclimation period, during which the focal animal was confined to a holding container. After 5 min, the container was raised via a pulley system and the focal animal was allowed to freely move about the aquarium for 10 min. The magnetic stir bar was at this point switched on and left running for 2 min, after which the trial ended.
The exploration and boldness aquarium set up. Each grid box measured 2 × 2 cm; the entire aquarium was 20 × 32 cm. The circle on the right represents the opaque holding container (4 cm diameter) used to acclimate test animals for 5 min prior to each trial. The oval on the left represents the magnetic stir bar (4 cm length), which was activated in the last 2 min of each trial. The rectangle around the stir bar was drawn on the bottom of the aquarium (8 × 8 cm); while the magnetic stir bar was activated, time spent in this rectangle by the test animal, as shown above, was counted toward the boldness measure
Exploration was quantified as the number of boxes within the 10 × 16 box grid occupied by the individual over a 1-min period, beginning thirty-seconds after the holding container was lifted. The first thirty-seconds after removal of the holding container were disregarded because individuals might react differently to the unexpected movement. As suggested by Reale et al. (2007), exploration was measured in a novel aquarium to capture an individual’s reaction to a new habitat.
Boldness was quantified as the time to enter a 8 × 8 cm box containing the spinning magnetic stir-bar (Fig. 2). In the event that the individual was located within the box when the stir bar began spinning, boldness was defined as the time for the individual to re-enter the box after leaving it for the first time. If individuals did not approach the box within 2 min, their score was recorded as 120 s (Chapple et al. 2011). Again following the suggestion of Reale et al. (2007), boldness refers to an individual’s reaction to a risky, not a novel, situation. To achieve this distinction, we conducted the boldness trial immediately following the exploration trial, in the same aquarium. Given the previous 10 min to explore the surroundings, including the stir bar, the situation would not be novel to the individual, however, the subsequent sudden movement of the stir bar could be perceived as a potential threat.
Behavioural trials, sociability
The sociability aquarium was divided into three compartments, separated by mesh cloth (approximately 0.03 × 0.03 mm square size), and filled with 1 cm depth of aged tap water. The two compartments at either end of the aquarium measured 5 cm in depth and spanned the entire 20 cm width of the aquarium. Both contained 10 cm of plastic pondweed (3 cm diameter), positioned adjacent to the mesh divider, and a piece of plastic tubing connected to an air pump for aeration. During trials, one end compartment contained the ten conspecifics of the same species as the trial individual, with the other end compartment functioning as a control (Fig. 3). The trial species were organized in blocks such that the conspecifics only had to be handled once per day. In between trials, the water in the aquarium was replaced and the artificial pondweed washed and then randomly returned to each compartment. To balance for any preference on the part of the focal individual for one side of the aquarium over the other, location of the conspecifics was randomised between ends.
The setup for the aquarium used to test sociability. The entire aquarium was 20 × 32 cm. The opaque holding container (4 cm diameter) that held each test animal for an initial 5 min acclimatization period is depicted by the circle at the top centre. The bold line on each side represents the mesh cloth used to separate the three compartments of the aquarium. End compartments were 5 cm deep. During the trials, conspecifics were kept in one of the end compartments, as represented by the 10 amphipods here. The lighter lines represent the 2 cm zones marked in the aquarium; time spent by the focal amphipod in the 2 cm zone closest to the compartment with conspecifics, as demonstrated above, was counted toward the sociability measure. Artificial pondweed (10 cm length, 3 cm diameter) was placed in both end compartments. The small circles in the bottom corners represent the location of the tubing used for aeration
Each trial began with a 5 min acclimation period where the focal individual was held in an opaque container in the centre compartment. After this period, the container was raised with a pulley and the amphipod was allowed to move freely around the centre compartment for 10 min.
Sociability was quantified as the amount of time, to the nearest second, spent within 2 cm of the partition in the test aquarium containing conspecifics.
Statistical analyses
The data for all behavioural metrics were non-normal and could not be transformed to approximate a normal distribution. The non-parametric Kruskal–Wallis test was therefore used to examine behavioural medians by population, namely G. pulex, fringe-population D. villosus collected from Barton Turf, and core-population D. villosus collected from Ludham Bridge. When the results of the Kruskal–Wallis test were significant, the non-parametric Mann–Whitney U test was used for post hoc pairwise comparisons (Dytham 2011). Prior to analysis, the paired behavioural measurements taken from each individual were averaged to avoid pseudoreplication.
To determine whether D. villosus and G. pulex showed repeatable behaviours, Spearman’s rank correlations were calculated between measurements taken the first and second weeks. To determine whether certain behaviours were correlated, Spearman’s rank correlations were calculated between all four behaviour metrics using week one measurements. Week one measurements were used for the latter analysis with the assumption that they would represent the least-altered behaviours from what would be observed in the field.
All statistical analyses were performed in R version 2.14.0 (R Development Core Team 2011).
Results
The distributions of each behavioural metric, averaged between week one and two, for the three populations measured are summarized in Fig. 4. The averaged metrics had distributions comparable to the original distributions of measurements from week one and two, and were thus determined to be representative summary variables. The Kruskal–Wallis test indicated significant differences by species group for the behavioural metrics of activity (H = 24.050, df = 2, p < 0.001), exploration (H = 12.552, df = 2, p = 0.002), and sociability (H = 14.421, df = 2, p = 0.001), but not boldness (H = 0.550, df = 2, p = 0.760). After post hoc Mann–Whitney U tests, it was determined that the G. pulex population was significantly more active and marginally less social than the D. villosus populations collected from both Barton Turf and Ludham Bridge (Table 1). Furthermore, the G. pulex population was significantly more exploratory and less social than the D. villosus population from Ludham Bridge, only (Table 1). Furthermore, we note that Kruskal–Wallis tests indicated no significant difference between males and females of a given species on any behavioural trait.
Spearman’s rank correlations between each of the four behavioural metrics measured on week one and two of the experiment in each of the three study populations are shown in Table 2. None of the behavioural metrics were significantly repeatable within individuals from each of the three study populations. Spearman’s rank correlations between each of the four behavioural metrics, calculated using the measurements from week one, are shown in Table 3. There were no significant correlations between behaviours for individuals from any of the populations.
Discussion
Contrary to our expectations, the D. villosus populations tended to be less active, more social and less explorative than the G. pulex population, and were equally as bold. We found no differences in the behaviours of the D. villosus populations from the centre of the species distribution (Barton Turf) and the invasive fringe (Ludham Bridge).
On average, D. villosus populations from both sites were less active than the G. pulex population. This is consistent with measurements made by Maazouzi et al. (2011), which revealed lower oxygen consumption rates and less swimming activity by D. villosus than G. pulex when individuals were kept in non-stressful conditions. While D. villosus is considered an aggressive predator (Dick and Platvoet 2000), it has been hypothesized that D. villosus pursues a “sit-and-wait” predation technique (Platvoet et al. 2009; Maazouzi et al. 2011), rather than actively seeking its prey. Recent experiments comparing the maximum feeding rate of D. villosus and G. pulex individuals on Asellus aquaticus (Linnaeus 1758) and Daphnia magna (Straus 1820) support this hypothesis. When no substrate was available, D. villosus had a higher maximum feeding rate than G. pulex on both A. aquaticus and D. magna; with the addition of substrate, D. villosus had a lower maximum feeding rate than G. pulex on A. aquaticus, but still had the higher maximum feeding rate on D. magna (Dodd et al. 2013). The substrate potentially provided refugia for both predators and the benthic A. aquaticus, but, as a pelagic species, D. magna likely would not seek refuge in the substrate. The likelihood of encounter with a predator utilizing a “sit-and-wait” technique would therefore decrease for A. aquaticus, and remain nearly the same for D. magna, providing a possible explanation of the lowered and unchanged feeding rate of D. villosus on these two species, respectively, in the presence of substrate. Meanwhile, the maximum feeding rate of G. pulex on A. aquaticus did not lower, possibly due to G. pulex utilising an active, searching predation technique. Further research is needed to elucidate these predator–prey interactions (Dodd et al. 2013). The lower activity levels of D. villosus might provide the species a further advantage over G. pulex in environments with high predation pressure. For example, in a study comparing fish predation on D. villosus and Gammarus roeseli (Gervais 1835), G. roeseli was observed free swimming more frequently and also experienced higher rates of predation (Kley et al. 2009). Thus, a low activity behavioural trait can have a twofold benefit for D. villosus: less energy expended searching for prey and less risk of capture. Furthermore, in a study with mosquitofish, it has been shown that the presence of predators can decrease the strength of personality-dependent dispersal (Cote et al. 2013). This may, in part, explain the lack of different behaviour between D. villosus individuals collected from Barton Turf and Ludham Bridge.
The Ludham Bridge and Barton Turf D. villosus populations were significantly and marginally more sociable than the G. pulex population, respectively. Social tolerance in a species can increase individual fitness through improved survival and reproductive success (Reale et al. 2007). For example, at high densities G. pulex has been reported to commit more intraspecific predation (i.e. cannibalism) than D. villosus (Kinzler et al. 2009). At high predation pressure, cannibalism can become a contributing factor in the elimination of a gammarid species (Dick et al. 1993). Therefore, it is possible that the greater social tolerance of D. villosus may be a behavioural trait that confers a competitive advantage for the continued establishment of D. villosus over that of native taxa such as G. pulex.
The D. villosus population at Ludham Bridge was, on average, significantly less explorative than the G. pulex population. A low exploration rate offers the same benefits as low activity, in the form of reduced mortality risk (Boon et al. 2008). However, it would also result in decreased ability to locate food in novel environments (van Overveld and Matthysen 2010).
In other studies of the behavioural traits linked to dispersal distance in invasive species, the species considered dispersed through active means. For example, Cote et al. (2010) studied swimming distance in mosquitofish (Gambusia spp.), and reported that individuals which dispersed further were more asocial. Similarly, newly established populations of the invasive Western bluebird, which must distribute through actively flying, tend to be more aggressive than longer-established populations (Duckworth and Badyaev 2007). The characteristic traits observed in D. villosus, namely low activity, low exploration, and high sociability compared to G. pulex, may suggest that rather than being an active disperser, the dispersal of D. villosus is driven primarily through passive means, such as drifting or human-mediated transport while attached to boating equipment (Nesemann et al. 1995). This is emphasized by the lack a significant relationship between any of the behavioural traits and the relative location (i.e. centre vs. fringe) of the D. villosus population measured.
In comparing the behaviour traits of D. villosus and G. pulex, it is important to also note that G. pulex can be a highly invasive species outside its native range. In Northern Ireland and the Isle of Man, the native gammarid G. celticus has been completely and rapidly replaced by G. pulex in the lower stretches of many rivers (MacNeil et al. 2004; Dick 2008). Gammarus pulex is often introduced intentionally to a new region, after which it follows an invasion pattern of rapid establishment in the lower reaches of streams followed by gradual dispersal upstream (Dick 2008). The behavioural traits of relatively high activity, high exploration, and low sociability we observed for G. pulex are consistent with the behavioural traits we would predict for an invader reliant on active dispersal. It is thus evident that species with different sets of traits, as in the case of D. villosus and G. pulex, can be successful invaders in different environments. To improve our conclusions as to what traits contribute to the displacement of G. pulex by D. villosus, we would require information on the traits expressed by the species displaced by G. pulex.
The lack of consistency between week one and two measurements of all behavioural metrics, except for exploration in the Barton Turf population of D. villosus, provide no evidence that D. villosus and G. pulex individuals possess distinct personalities. Furthermore, this result means we cannot know whether the observed behaviours of the fringe population were intrinsic to those individuals or temporary behavioural changes associated with the dispersal process. We suggest more investigation is needed to determine conclusively whether or not amphipod individuals exhibit consistent behaviours and encourage such investigations, especially in the case of the invasive D. villosus. It has recently been shown that founder groups of crickets that include a greater variety of colour morphs, which are known to be associated with differing behaviours, have greater establishment success (Forsman et al. 2012). This is particularly intriguing considering the number of invasive species that are known to exhibit high levels of colour variation, including D. villosus (Devin et al. 2004; Forsman et al. 2012). Further, it has long been acknowledged that parasite infection can change host behaviour (e.g. Holmes and Bethel 1972); there is some evidence that the repeatability of behaviours in amphipods may increase when individuals are infected with parasites (Coats et al. 2010), and that the individuals collected during sampling might be biased by parasites altering behaviours such as phototaxis in their hosts (Fisher et al. 2014). While individuals were visually inspected for indications of parasitism in this study, this consideration should receive closer attention in any future behaviour work with D. villosus as the populations at different invasion sites, or even within an invasion site, could have very different parasite loads. For example, it is known that UK populations of D. villosus lack certain microsporidia and viral pathogens that commonly affect European populations (Bojko et al. 2013). The enemy release hypothesis suggests that such reduced control of invasive species from parasites and other natural enemies in their introduced range, as compared to their native range, may explain their rapid establishment and proliferation (e.g. Keane and Crawley 2002).
This study represents an initial investigation into the comparative behaviour of two successful aquatic invaders and there remain many opportunities to improve on our knowledge of this topic. One factor not considered by this study is the photophobic nature of amphipods that often results in greater levels of activity at night (Lagrue et al. 2011). Gammarus pulex has been found to maintain relatively constant drift rates (Lagrue et al. 2011), while D. villosus shows a diurnal drift pattern, with more individuals drifting at night (van Riel et al. 2011). The drift patterns of the two species have never been directly compared, so it is not known whether these differences are significant. As at least some dispersal of both species occurs at night, it would be beneficial to conduct the same behavioural trait measurements under infrared light to simulate night conditions. It is possible, for example, that D. villosus individuals may demonstrate higher activity levels at night than G. pulex individuals, which could result in higher overall activity levels for the species during a 24-h period. Sex is another factor that could play an important role in individual behaviour. We observed no significant difference in any behavioural trait between males and females of a given species, which is in line with other studies of crustacean behaviour (Briffa and Dallaway 2007; Brodin and Drotz 2014). However, significant behavioural differences between sexes have been found in non-crustacean species (e.g., Cote et al. 2010), and so further investigation may be warranted. Finally, we note that future studies may wish to consider individuals from closer to the true “fringe” population, which, in this case, would have been closer to the A149 bridge (Fig. 1). For this study, the collection of individuals from this location was limited by access to the waterway and by the number of individuals required for the experiment.
Our study emphasizes that a variety of factors can be important in affecting the invasiveness of a species. The invasive ‘success’ of G. pulex may be attributed to traits associated with active dispersal (high activity, high exploration, low sociability); however, this would need to be tested on invasive and native populations of G. pulex, as well as core and fringe populations in invaded regions, to be confirmed. In contrast, D. villosus is perhaps best described as a passive disperser with a high suitability for human-mediated transport. Dikerogammarus villosus has been observed attached to the hulls of ships (Nesemann et al. 1995), fishing nets (van Riel et al. 2011), waders, and rubber dinghies (Aldridge, pers. observ.). Nesemann et al. (1995) describe the behaviour of D. villosus on porous material, with the individual “creeping most deeply into the holes where it seems to be rooted, so that it is pulled out only by force.” We have not made, nor are we aware of, any similar observations for G. pulex. It is therefore likely that these characters, rather than any particular invasive behavioural traits, play a more important role in driving its recent invasion across Western Europe. Our results suggest that study of the intra- and inter-specific behaviours of amphipods, and especially those of Ponto–Caspian origin, may help us to better understand and predict the invasion potential, pathways and vectors of these important pests.
References
Boets P, Lock K, Messiaen M et al (2010) Combining data-driven methods and lab studies to analyse the ecology of Dikerogammarus villosus. Ecol Inform 5:133–139
Bojko J, Stebbing PD, Bateman KS et al (2013) Baseline histopathological survey of a recently invading island population of ‘killer shrimp’, Dikerogammarus villosus. Dis Aquat Org 106:241–253
Bollache L, Devin S, Wattier R et al (2004) Rapid range extension of the Ponto–Caspian amphipod Dikerogammarus villosus in France: potential consequences. Archiv Für Hydrobiol 160:57–66
Boon AK, Reale D, Boutin S (2008) Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117:1321–1328
Briffa M, Dallaway D (2007) Inter-sexual contests in the hermit crab Pagurus bernhardus: females fight harder but males win more encounters. Behav Ecol Sociobiol 61:1781–1787
Brodin T, Drotz MK (2014) Individual variation in dispersal associated behavioral traits of the invasive Chinese mitten crab (Eriocheir sinensis, H. Milne Edwards, 1854) during initial invasion of Lake Vänern, Sweden. Curr Zool 60:410–416
Chapple DG, Simmonds SM, Wong BB (2011) Know when to run, know when to hide: can behavioral differences explain the divergent invasion success of two sympatric lizards? Ecol Evol 1:278–289
Chapple DG, Simmonds SM, Wong BBM (2012) Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64
Coats J, Poulin R, Nakagawa S (2010) The consequences of parasitic infections for host behavioural correlations and repeatability. Behaviour 147:367–382
Cote J, Fogarty S, Weinersmith K et al (2010) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc R Soc B Biol Sci 277:1571–1579
Cote J, Fogarty S, Tymen B et al (2013) Personality-dependent dispersal cancelled under predation risk. Proc R Soc B Biol Sci 280:20132349
Devin S, Piscart C, Beisel JN et al (2004) Life history traits of the invader Dikerogammarus villosus (Crustacea: Amphipoda) in the Moselle River, France. Int Rev Hydrobiol 89:21–34
Dick JTA (2008) Role of behaviour in biological invasions and species distributions; lessons from interactions between the invasive Gammarus pulex and the native G. duebeni (Crustacea: Amphipoda). Contrib Zool 77:91–98
Dick JTA, Platvoet D (2000) Invading predatory crustacean Dikerogammarus villosus eliminates bath native and exotic species. Proc R Soc Lond Ser B Biol Sci 267:977–983
Dick JTA, Montgomery I, Elwood RW (1993) Replacement of the indigenous amphipod Gammaurs duebeni celticus by the introduced Gammarus pulex—differential cannibalism and mutual predation. J Anim Ecol 62:79–88
Dodd JA, Dick JA, Alexander ME et al (2013) Predicting the ecological impacts of a new freshwater invader: functional responses and prey selectivity of the ‘killer shrimp’, Dikerogammarus villosus, compared to the native Gammarus pulex. Freshw Biol. doi:10.1111/fwb.12268
Duckworth RA, Badyaev AV (2007) Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc Natl Acad Sci USA 104:15017–15022
Dytham C (2011) Choosing and using statistics: a biologist’s guide. Wiley, Chichester
Fisher JDL, Mushet DM, Stockwell CA (2014) Potential for parasite-induced biases in aquatic invertebrate population studies. Hydrobiologia 722:199–204
Fogarty S, Cote J, Sih A (2011) Social personality polymorphism and the spread of invasive species: a model. Am Nat 177:273–287
Forsman A, Wennersten L, Karlsson M et al (2012) Variation in founder groups promotes establishment success in the wild. Proc R Soc B Biol Sci 279:2800–2806
Gallardo B, Paz Errea M, Aldridge DC (2012) Application of bioclimatic models coupled with network analysis for risk assessment of the killer shrimp, Dikerogammarus villosus, in Great Britain. Biol Invasions 14:1265–1278
Gherardi F, Aquiloni L, Tricarico E (2012) Behavioral plasticity, behavioral syndromes and animal personality in crustacean decapods: an imperfect map is better than no map. Curr Zool 58:567–579
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506
Holmes JC, Bethel WM (1972) Modification of intermediate host behaviour by parasites. In: Canning EU, Wright CA (eds) Behavioural aspects of parasite transmission. Academic Press, London, pp 123–149
Kappes H, Stoll S, Haase P (2012) Differences in field behavior between native gastropods and the fast-spreading invader Arion lusitanicus auct. non MABILLE. Belg J Zool 142:49–58
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kinzler W, Kley A, Mayer G et al (2009) Mutual predation between and cannibalism within several freshwater gammarids: Dikerogammarus villosus versus one native and three invasives. Aquat Ecol 43:457–464
Kley A, Kinzler W, Schank Y et al (2009) Influence of substrate preference and complexity on co-existence of two non-native gammarideans (Crustacea: Amphipoda). Aquat Ecol 43:1047–1059
Lagrue C, Kaldonski N, Motreuil S et al (2011) Interspecific differences in drift behaviour between the native Gammarus pulex and the exotic Gammarus roeseli and possible implications for the invader’s success. Biol Invasions 13:1409–1421
Lester PJ (2005) Determinants for the successful establishment of exotic ants in New Zealand. Divers Distrib 11:279–288
Maazouzi C, Piscart C, Legier F et al (2011) Ecophysiological responses to temperature of the “killer shrimp” Dikerogammarus villosus: is the invader really stronger than the native Gammarus pulex? Comp Biochem Physiol A Mol Integr Physiol 159:268–274
MacNeil C, Prenter J, Briffa M et al (2004) The replacement of a native freshwater amphipod by an invader: roles for environmental degradation and intraguild predation. Can J Fish Aquat Sci 61:1627–1635
MacNeil C, Platvoet D, Dick JTA et al (2010) The Ponto–Caspian ‘killer shrimp’, Dikerogammarus villosus (Sowinsky, 1894), invades the British Isles. Aquat Invasions 5:441–445
Madgwick G, Aldridge DC (2011) Killer shrimps in Britain: hype or horror? Br Wildl 22:408–412
Mayer G, Maier G, Maas A et al (2008) Mouthparts of the Ponto–Caspian invader Dikerogammarus villosus (Amphipoda : Pontogammaridae). J Crustac Biol 28:1–15
Nesemann H, Pockl M, Wittmann KJ (1995) Distribution of epigean malacostraca in the middle and upper Danube (Hungary, Austria, Germany). Misc Zool Hung 10:49–68
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803
Pintor LM, Sih A, Kerby JL (2009) Behavioral correlations provide a mechanism for explaining high invader densities and increased impacts on native prey. Ecology 90:581–587
Piscart C, Bergerot B, Laffaille P et al (2010) Are amphipod invaders a threat to regional biodiversity? Biol Invasions 12:853–863
Platvoet D, Dick JTA, MacNeil C et al (2009) Invader–invader interactions in relation to environmental heterogeneity leads to zonation of two invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus Sexton: amphipod pilot species project (AMPIS) report 6. Biol Invasions 11:2085–2093
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 1 Nov 2011
Reale D, Reader SM, Sol D et al (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Ricciardi A, Rasmussen JB (1998) Predicting the identity and impact of future biological invaders: a priority for aquatic resource management. Can J Fish Aquat Sci 55:1759–1765
Sanches FHC, Miyai CA, Costa TM et al (2012) Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. PLoS One 7:e29746. doi:10.1371/journal.pone.0029746
Sih A, Cote J, Evans M et al (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:1965–1974
Sol D, Bacher S, Reader SM et al (2008) Brain size predicts the success of mammal species introduced into novel environments. Am Nat 172:S63–S71
Suarez AV, Holway DA, Ward PS (2005) The role of opportunity in the unintentional introduction of nonnative ants. Proc Natl Acad Sci USA 102:17032–17035
Truhlar AM, Dodd JA, Aldridge DC (2013) Differential leaf-litter processing by native (Gammarus pulex) and invasive (Dikerogammarus villosus) freshwater crustaceans under environmental extremes. Mar Freshw Ecosyst, Aquatic Conservation. doi:10.1002/aqc.2375
van Overveld T, Matthysen E (2010) Personality predicts spatial responses to food manipulations in free-ranging great tits (Parus major). Biol Lett 6:187–190
Van Riel MC, Van der Velde G, De Vaate AB (2011) Dispersal of invasive species by drifting. Curr Zool 57:818–827
Acknowledgments
We thank B. Gallardo and two anonymous reviewers for their helpful comments on this manuscript. AMT gratefully acknowledges support from a Gates Cambridge scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Truhlar, A.M., Aldridge, D.C. Differences in behavioural traits between two potentially invasive amphipods, Dikerogammarus villosus and Gammarus pulex . Biol Invasions 17, 1569–1579 (2015). https://doi.org/10.1007/s10530-014-0816-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0816-9