Abstract
Substrate choice, swimming activity and risk to predation by burbot (Lota lota) of the well established Gammarus roeselii and the invader Dikerogammarus villosus were studied in mixed and single-species aquarium experiments. We used stones, gravel and aquatic weeds (Elodea, Chara) as substrates. We hypothesized that both species have different substrate preferences and that substrate affects the predation risk. We also assumed that presence of D. villosus influences substrate preference and predation risk of G. roeselii since the invader is known to affect the behavior of other gammarids. Adults of D. villosus in single species experiments and juveniles in mixed and single species experiments were evenly distributed over the different substrates but adults in mixed species experiments were more likely to prefer stone substrate. In contrast, adults and juveniles of G. roeselii clearly preferred aquatic weeds independent of the presence/absence of the invader. Both species preferred substrates with fissured surface over substrates with smooth surface. Gammarus roeselii was observed swimming more often than D. villosus in the open water but its swimming activity was lower when its preferred substrate was present compared with its swimming activity if non-preferred substrates were present. Predation rate of burbot on D. villosus was comparatively low and independent of the substrate. Burbot consumed many more G. roeselii than D. villosus, both in mixed and single species experiments. But when the preferred substrate of G. roeselii (weeds) was used in the experiments, predation rate of burbot on G. roeselii was somewhat lower than that when non-preferred substrates were present. The results of the experiments support our hypothesis that the gammarids studied here have different substrate preferences and that presence of the preferred substrate can affect predation risk. However, there is no evidence that presence of D. villosus affected substrate choice or predation risk in G. roeselii. We consider that differences in use of spatial niches permit co-existence of G. roeselii and D. villosus in the wild when substrates are diverse. The fact that G. roeselii than D. villosus is more often observed swimming in the open water may explain its higher risk of being captured by fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades amphipods mainly from the Ponto-Caspian region have invaded European waters and have replaced native amphipods in many river reaches. Understanding the causes and mechanisms behind these replacement scenarios is important for the prediction of the impact of future invasions on the native fauna. Intraguild predation is often regarded as a major force in structuring freshwater gammarid communities (e.g., MacNeil et al. 1997; Dick et al. 2002). Dikerogammarus villosus (Sowinsky), e.g., a species which invaded Central Europe in the 1990s, is known to prey heavily on native and invasive gammarids (Dick and Platvoet 2000; Kinzler and Maier 2003). Although recent morphological investigations show that the species is not a highly specialized carnivore (Platvoet et al. 2006; Mayer et al. 2008, 2009, its predatory impact can be serious. In several reaches of European waters, the arrival of D. villosus has been accompanied by the disappearance of native gammarids (Dick and Platvoet 2000; Kley and Maier 2003, 2006) most likely because of asymmetric mutual predation.
Another factor that may influence the composition of gammarid communities is predation by fish. Gammarids can sense fish and are able to react by anti predator behaviors. For example, Wudkevich et al. (1997) showed that Gammarus lacustris (Sars) reduced its time in the open water in the presence of chemical stimuli from pike (Esox lucius L.). Dahl and Greenberg (1996) reported that Gammarus pulex (L.) responded to the presence of sculpins (Cottus gobio L.) by change of habitat use. Kinzler and Maier (2006) showed that D. villosus was less eaten by trout than native species and attributed this difference to the greater activity of the native species. Their findings on activity were supported by van Riel et al. (2007) who studied interference competition among native and invasive gammarids and found that G. roeselii and G. pulex were more frequently present in the open water in the presence of the invader D. villosus. Pennuto and Keppler (2008) observed differences in the response to fish predators between Echinogammarus ischnus (Stebbing) and Gammarus fasciatus (Say). Echinogammarus reduced its distance moved in presence of all fish species tested while Gammarus did not respond to all fish species. Kaldonski et al. (2008) showed that presence of preferred substrates reduced the risk of predation by fish. All these investigations show that gammarids have developed strategies to reduce their risk to fish predation. However, anti predator strategies and their magnitude may differ from species to species and susceptibility of gammarids to fish predation may depend on the type of fish, associated conspecifics and the environment. Complete displacement scenarios have been observed in degraded river systems where shore substrate structures are simple, i.e., where substrates are often dominated by cobbles, or boulder or both. If many spatial niches are present, i.e., if structural complexity is high, predatory interactions between different gammarid species and impact of fish predation should be less severe.
D. villosus was first recorded in the north-western part of Lake Constance in 2002 (Mürle et al. 2004). In the following 2 years the species had spread over the entire shore of the Upper Lake and has now reached the Lower Lake (ANEBO Website). Before the invasion of D. villosus into Lake Constance, G. roeselii (Gervais) was the dominant species—a species of Balkan origin that had invaded Central Europe already in the 19th century; it is often named native to Central Europe or “well established” (e.g., Grabowski et al. 2007). Although presence of D. villosus can have negative effects on the established gammarid G. roeselii in Lake Constance (Mörtl et al. 2004), co-existence of the two seems likely by segregation of spatial niches. Lake Constance shores offer more complex substrates than the degraded shores of European waterways. Dikerogammarus villosus is often found in stone substrates (Kley and Maier 2005; van Riel et al. 2006, 2007; Hesselschwerdt et al. 2008; MacNeil et al. 2008) or substrates with Dreissena or Corbicula bivalves (Devin et al. 2003; van Overdijk et al. 2003; Kley and Maier 2005; Lods-Crozet and Reymond 2006; Kobak and Zytkowicz 2007). Substrate choice depends on the invaders size/age with smaller individuals preferring substrates with smaller particle size (Devin et al. 2003). In contrast to the upper mentioned studies, Guthruf-Seiler and Guthruf (unpublished monitoring report) found the highest densitiy of D. villosus in beds of Myriophyllum spicatum (L.) in the river Aare (Switzerland). Substrate choice of Gammarus roeselii is less known. In laboratory experiments with Dreissena shells, G. roeselii showed a preference for shells with biodeposited material and for shells with biodeposited material with chironomids whereas D. villosus showed only a preference for shells with biodeposited material with chironomids (Gergs and Rothhaupt 2008). In enclosure experiments conducted in Lake Constance, G. roeselii barely discriminated between stones, stones with Dreissena, Chara sp., shells of Corbicula, leaves and sand. In the river Ouche, Kaldonski et al. (2008) observed G. roeselii to be more frequent in the vegetation than in hard substrate. Thus, substrate choice of both gammarids may vary depending on ambient conditions and between populations.
In this paper, we tested substrate preference of D. villosus and G. roeselii in mixed and single species combinations in laboratory experiments, as well as the risk of both gammaridean species to predation by burbot (Lota lota, L.) in different substrates. Burbot, an important fish in the shore area of Lake Constance, is known to predate upon amphipods (Bailey 1972; Ryder and Pesendorfer 1992; Baumgärtner et al. 2003; Eckmann et al. 2008). Based on observations in the Danube River, where D. villosus was found under stones whereas G. roeselii preferred tree roots or grass bundles hanging into the water, we hypothesized that substrate preference differs considerably between the two species. We further hypothesized that presence of D. villosus can affect substrate choice of G. roeselii and that the risk of being eaten by burbot differs between the two gammarid species and depends on the substrate.
Methods
Maintenance
Gammarids were collected using a pond net (mesh size 250–400 μm) at the shore of Lake Constance near Langenargen (E 9°31′57′′; N 47°36′07′′). If necessary (low numbers of G. roeselii), additional samples were taken from a river near Ulm (E 10°2′; N 48°25′). Upon capture, gammarids were immediately transported to the laboratory, sorted by species and kept at natural densities (250–350 ind. l−1) in plastic containers (50 × 40 × 40 cm) in a climate-controlled room at a temperature of 18°C and a 14:10 h light:dark cycle. Light was provided by Osram cool white lamps. All containers contained aged tap water and were equipped with substrates (pebbles, stones). Air stones provided a smooth water movement and sufficient oxygen. Leaves of alder and ash and chironomid larvae served as food for gammarids during their maintenance. Mortality of gammarids was low under these conditions.
Substrate preference
Experiments were conducted in aquaria (100 × 40 × 40 cm) provided with three equally sized areas of different substrates. One part contained gravel (grain size 2–3 cm; height 4 cm), one 10–12 large stones (ca. 15 × 15 × 3 cm) and one about 20 stems of aquatic weeds (Elodea canadensis, Michx.). Water height above the hard substrates was 25–30 cm. Two mixed-species series were run with Chara sp.; since the results were the same as for Elodea they were pooled. Substrates were cleaned (stones scraped and macrophytes washed) before they were used in an experiment. Thus attached benthos and periphyton communities were removed to exclude possible effects of food on substrate choice. Temperature and light conditions were kept the same as for the maintenance of the animals.
In the first mixed species trials, 40 adult individuals each of D. villosus and G. roeselii (size > 15 mm) were introduced randomly into one aquarium and their distribution over the three different substrates was monitored. A set of experiments were run for each 24, 48 and 72 h. After the experimental time, substrates (different parts of the aquarium) were separated from each other by the introduction of glass plates; then substrates were removed and gammarids present in each substrate were counted. Pre-experiments indicated that gammarids immediately occupied substrates and that the number of substrate changes decreased to about 30% after 1 h. We pooled the data for the three experimental sets since results did not significantly differ. In the single species trials with adults and the mixed and single species trials with juveniles, size of aquaria and substrates were nearly the same as in the mixed species ones with adults, but experiments were run for 24 h only and substrates were searched for gammarids after this 24 h exposure time only. Size of juveniles was 8–10 mm. Nineteen mixed species trials and 20 single species trials (10 with G. roeselii and 10 with D. villosus) were run with adults; and seven mixed species trials and 19 single species trials (ten with G. roeselii and nine with D. villosus) were run with juveniles.
Activity of prey organisms can be an important determinant of the predation risk. Active prey is more conspicuous than an inactive one and therefore easily captured by a predator. Activity of adult gammarids was investigated in mixed species trials with adults. Before substrates were searched for gammarids, number of gammarids swimming in the open water (defined as active ones) was noted during a 5 min observation period. Activity was monitored in 16 trials such that we had 48 observations for each gammarid (16 for each substrate).
In an other series of experiments we tested whether the surface of substrates affected substrate choice of gammarids. Substrates of similar size (10 × 20 × 3 cm) but with different surface (one stone with fissures, one with smooth surface and a piece of wood with fissures) were used in the experiments. In one set of experiments, two stones with different surfaces were placed into an aquarium (distance between the stones was ca.5 cm) and water was added to a height of 10 cm. Then 30–40 individuals of either D. villosus or G. roeselii were introduced and allowed to spread out over the substrates. After 2 h the number of gammarids under the two stones as well as those in the open water was noted. Again, the fraction in the open water was defined as active ones. In another set of experiments, the piece of wood was tested versus the stone with smooth surface. Experimental conditions and procedure were the same as in the stone versus stone experiments. Thirty experiments (10 with G. roeselii and 20 with D. villosus) were run with fissured stones versus smooth stones and 25 (10 with G. roeselii and 15 with D. villosus) with smooth stones versus fissured wood.
Experiments with fish predators
The experiments with fish were conducted in 100 × 35 × 35 cm (ca.120 l) aquaria. Temperature and light were as in the substrate preference experiments. The aquaria were stocked with either ten stones (size 10 × 10 × 3 cm), aquatic weeds (20 stems of Elodea) or a mixture of both. Substrates/weeds were thoroughly cleaned before they were used in an experiment to remove adhesive animals. In the mixed prey experiments, 30 adult individuals of which D. villosus and G. roeselii were taken from the stock cultures and introduced into each aquarium. The final density of gammarids of 180 ind. m−2 was within the range of densities observed in the field. We selected the largest individuals of G. roeselii and smaller adults of D. villosus (size range 10–15 mm) so that test animals were in about same size. After an acclimatization time of 15 min, which proved to be sufficient for gammarids to occupy substrates (e.g., MacNeil et al. 2000; this study), two fish (burbot; length 9–11 cm), which had been starved for 48 h, were introduced into the aquaria and allowed to feed on gammarids for 1 h. Burbot was obtained from the Limnological Institute at Lake Constance. Earlier experiments showed that between 10 and 50% of gammarids had been consumed after that time; longer exposure times proved to be unfavorable (Kinzler and Maier 2006). At the end of an experiment the fish was removed and the aquarium, in particular the substrate, was carefully searched for remaining gammarids. In the single-prey experiments 60 adult individuals of the same species (either D. villosus or G. roeselii) were introduced into the aquaria. Experimental conditions were the same as in the mixed-prey experiments. Twenty-nine mixed species and 53 single species, 24 with G. roeselii and 29 with D. villosus, were run.
Although we tried to use new specimens in each experiment, some gammarids were used more than one time in a type of experiment since we had not enough specimens to use each gammarid only once. However, in different experimental types we used different sets of gammarids, but only the ones that appeared healthy.
Statistics
Non-parametric paired tests (Friedman followed by Wilcoxon) were used to test for differences in substrate preference and for differences in activity between species. In the experiments with fish we used a paired test (Wilcoxon) to test for differences in predation risk between gammarids at a certain substrate. Then a Kruskal–Wallis ANOVA (followed by U-test, Mann–Whitney) was employed to test for differences in predation risk at different substrates. We selected non-parametric tests because, in some experiments (e.g., substrate preference of mixed species juveniles), we had only a few replicates and not all data sets were normally distributed.
Results
Substrate preference, activity
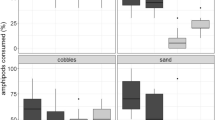
In the mixed species trials, adult G. roeselii preferred the plant substrate while adult D. villosus were most frequent in stone substrate (Fig. 1). The Friedman test showed significant differences in substrate choice both for adult G. roeselii and adult D. villosus (G. roeselii: χ 2 = 21.2, P < 0.0001, n = 19; D. villosus: χ 2 = 25,6, P < 0.0001, n = 19). The Wilcoxon test revealed significant differences in use of plant versus hard substrate in adult G. roeselii (Table 1). Adult D. villosus showed a preference for stones versus gravel and plants; plant and gravel substrates did not significantly differ (Table 1). In the single species trials again a much higher proportion of adult G. roeselii was found in plant compared with hard substrate whereas adult D. villosus was evenly distributed over all three substrates (Fig. 1). The Friedman test showed significant differences in the substrate choice for adult G. roeselii (χ 2 = 15.45, P < 0.0005, n = 10) but not for adult D. villosus (χ 2 = 0.95, P = 0.62 ns, n = 10). The Wilcoxon test revealed significant differences in use of plants versus hard substrate by adult G. roeselii but no difference in use between hard substrates (Table 1).
In mixed-species experiments (Fig. 2), juveniles of G. roeselii preferred the plants (Friedman: χ 2 = 10.5, P < 0.006, n = 7; Table 1) while those of D. villosus were evenly distributed over the substrates (Friedman: χ 2 = 5.2, P = 0.073 ns, n = 7;). In the single-species experiments (Fig. 2), juveniles of G. roeselii also preferred the plants over stony substrates (Friedman: χ 2 = 20.0, P < 0.0001, n = 10; Table 1). Again, juveniles of D. villosus were evenly distributed over the substrates (Friedman: χ 2 = 2.39, P = 0.30 ns, n = 9).
Activity of adult G. roeselii in the mixed species trials depended on the substrate (Friedman: χ 2 = 7.1, P < 0.03, n = 16); it was lower in the plant substrate compared with stones (Fig. 3; Table 2). Activity of adult D. villosus did not depend on the substrate (Friedman: χ 2 = 0.4, P = 0.82 ns, n = 16) and overall activity of D. villosus was significantly lower than overall activity of G. roeselii (Wilcoxon: Z = −5.4, P < 0.0001, n = 48).
In experiments with different substrate surfaces, both Gammarus species preferred the fissured substrate over that with smooth surface but activity in adults of G. roeselii and D. villosus differed (Fig. 4) The Friedman test showed significant differences between the three measured parameters in both species (smooth stones versus fissured stones versus outside: G. roeselii: χ 2 = 15.3, P < 0.0005, n = 10; D. villosus: χ 2 = 29.8, P < 0.0001, n = 20; smooth stones versus fissured wood versus outside: G. roeselii: χ 2 = 12.9, P < 0.002, n = 10; D. villosus: χ 2 = 19.7, P < 0.0001, n = 15). In G. roeselii, a higher or almost the same proportion of individuals was outside the substrates as in the fissured substrates (Fig. 4; Table 3) while in D. villosus much fewer individuals were observed outside the substrates than in the fissured substrate (Table 3).
Substrate surface choice and activity of adults of the established gammarid Gammarus roeselii (left column) and the invasive Dikerogammarus villosus (right column) in single species aquarium experiments with a combination of fissured stone and stone with smooth surface or a combination of fissured wood and stone with smooth surface
Fish predation
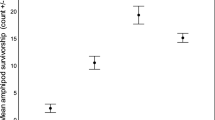
In the mixed-prey experiments, burbot ate many more G. roeselii than D. villosus (Wilcoxon: Z = −4.7, P < 0.0001, n = 29). On average 12–19 specimens of G. roeselii were consumed by the fish while only 4–6 specimens of D. villosus were consumed during the one hour exposure period (Fig. 5). Consumption rate on D. villosus was independent of the substrate (Kruskal–Wallis ANOVA: H = 3.13, P = 0.21 ns, n = 29) whereas that on G. roeselii depended on the substrate (Kruskal–Wallis ANOVA: H = 14.6, P < 0.0007, n = 29). Burbot consumed least individuals of G. roeselii when plants were used as substrate; consumption rate of the fish did not differ between the experiments with stones and stones/plants (Table 4).
In the single prey experiments similar results were observed as in mixed prey experiments (Fig. 5). Burbot ate many more G. roeselii than D. villosus (Wilcoxon: Z = −4.3, P < 0.0001, n = 24). Consumption rate on D. villosus was independent of the substrate (Kruskal–Wallis ANOVA: H = 4.7, P = 0.096 ns, n = 29). Consumption rate on G. roeselii depended on the substrate (Kruskal–Wallis ANOVA: H = 15.70; P < 0.0004, n = 24). Burbot consumed fewer individuals when plants were used as substrate than when stones were used and the same number of individuals when stones/plants were used compared with plants (Table 4).
The mean percentage of G. roeselii over all substrates relative to the initial density consumed by burbot in mixed species trials was 40.1 ± SD 18.3 and in the single species trials 49.3 ± SD 10.1. The mean percentage of D. villosus consumed by burbot in mixed and single species trials was 15.2 ± SD 7.7 and 16.1 ± SD 5.4, respectively. There was only a difference in predation rate of burbot on G. roeselii between mixed and single species trials when the stones/plant substrate was compared and there was no difference in percentage of D. villosus consumed by burbot between mixed and single species trials at all substrates (Table 5).
Discussion
Substrate choice and activity patterns of gammarids can be influenced by a number of factors such as age of the gammarid and time of day (Elliott 2005), structure of substrates and physical and chemical parameters in the environment (Wijnhoven et al. 2003; Palmer and Ricciardi 2004; Franken et al. 2006; McGrath et al. 2007), availability of food (van Dolah 1978; Dahl and Greenberg 1996; De Lange et al. 2005; McGrath et al. 2007), presence/absence of parasites in gammarids (Mazzi and Bakker 2003; Kaldonski et al. 2007), presence/absence/density of predators or predator odors and age of predators (Mathis and Hoback 1997; Dahl and Greenberg 1996; Wudkevich et al. 1997; Max Neil et al. 1999; Sudo and Azeta 2001; Baumgärtner et al. 2002, 2003; Pennuto and Keppler 2008) and presence/absence of conspecifics (van Riel et al. 2007; Piscart et al. 2007).
We conducted the substrate-choice experiments in the absence of predators, parasites and food (except macrophytes) and both test species faced the same physical and chemical conditions. Under these conditions D. villosus and G. roeselii show different substrate preferences and activities and substrate preference and activity differed between species independent of whether the two species were present together or singly. Our results further suggest that substrate preference is more pronounced in G. roeselii than in D. villosus. Adult G. roeselii always preferred the plant substrate whereas adult D. villosus in single species trials and juveniles in single and mixed species trials were evenly distributed over all substrates tested. That D. villosus did not necessarily prefer stones or gravel was unexpected because this species generally occurs in hard substrates.
One factor—in addition to those mentioned above—associated with morphology involved in food collection may also affect substrate choice and activity of gammarids. In some earlier works, Ponyi (1956, 1961) showed that differences in feeding modes exist between freshwater gammarids. He distinguished between “filter-feeding” gammarids (D. villosus) and “chewing” ones (G. roeselii). Platvoet et al. (2006) showed that D. villosus is able to collect suspended algae with the help of long setae on the antennae and gnathopods. Recently, Mayer et al. (2009) who compared mouthpart morphology of G. roeselii and D. villosus found the appendages involved in food acquisition to differ, in particular in the molar surfaces of the mandibles and the endites of the maxillulae, which are more suited for grinding plant material prior to ingestion and for scraping off adherent food in G. roeselii than in D. villosus. Furthermore, setae of antennae and gnathopods are shorter and more sparse in G. roeselii compared with D. villosus, implying that filter-feeding plays a minor role in food acquisition in G. roeselii. If filter-feeding is an important mode of food collection in D. villosus, this could explain its low activity since low activity or even sessile mode of life is common in filter-feeding animals. The ability of G. roeselii to feed on plant material and adherent food growing on it and grind them may contribute to its preference for macrophytes. That G. roeselii is able to feed on fresh leaf litter has already been reported by Pöckl (1995).
That the two gammarids studied preferred fissured substrate over substrate with smooth surface is expected. Fissured substrate offers more refuge, more possibilities for attachment and may also host more prey organisms than substrate with smooth surface. The fact that G. roeselii was present more frequently outside the substrate than D. villosus may partly be an effect of the substrates used in the “surface” experiments. Since we used no plant substrate there, this could have triggered search behavior in G. roeselii. This suggestion is supported by our third experiment where G. roeselii was least active in the presence of plants. On the other hand, this study as well as those of Krisp (2004) and Kinzler and Maier (2006) suggest that G. roeselii is generally more active than D. villosus.
Invasion of a new gammarid species can influence substrate choice of native and/or established gammarids. Van Riel et al. (2007) showed that in the presence of D. villosus, G. pulex shifted toward smaller stones and increased its activity while there was no change in the behavior of D. villosus. Also Krisp (2004) observed that G. pulex who generally preferred (similar to D. villosus) stone substrate in his experiments shifted to plant substrate in the presence of D. villosus. That we did not observe shifts in substrate choice in G. roeselii in the presence of D. villosus may be because differences in substrate preferences will prevent or reduce competition for substrates. We cannot exclude, however, that D. villosus may have accelerated the occupation of the preferred substrate in G. roeselii.
Fish predators can shape the structure of freshwater benthic communities (Newman and Waters 1984). Fishes generally prefer large and conspicuous prey (Dahl 1998; Mac Neil et al. 1999, 2000). Conspicuous color and large body mass increase the visibility of prey organisms. High activity results in frequent encounters between predator and prey. Gammarids seek to hide in refuges to reduce encounters with fish (Williams and Moore 1982; Hoyle and Holomuzki 1990; Wooster 1998). Dikerogammarus villosus is a large amphipod species and therefore an attractive prey for fish. However, hiding under stones and reduced activity may be an effective anti-predator strategy. The fact that G. roeselii is inclined to swim in the open water irrespective of if D. villosus is present or not has undoubtedly contributed to its comparatively high mortality observed in our experiments with burbot. We have no evidence that presence of the invader D. villosus increased the predation risk of the established G. roeselii. The percentage of G. roeselii consumed by burbot in mixed and single species experiments was quite similar.
Bollache et al. (2006) studied predation risk of Gammarus pulex and G. roeselii to fish predators and found that G. roeselii was more often rejected by predators than G. pulex. They attributed this to dorsal spines of G. roeselii, which are absent in G. pulex, thus interpreting the spines as morphological defence structures. Although spines can reduce predation risk, it is unlikely that they played an important role in our experiments. This because despite the presence of spines G. roeselii was more frequently eaten by burbot than D. villosus. Thus, differences in activity patterns (times spend in shelter) appears to be more important determinants of predation risks. The fact that G. roeselii is less frequently eaten by burbot in the presence of plant substrate compared with stone substrate may originate from its somewhat lower activity in its preferred substrate. Also Kaldonski et al. (2008) have shown that G. roeselii was less prone to predation by bullheads (Cottus gobio) in the presence of vegetation.
Pennuto and Keppler (2008) suggested that superiority in predator avoidance behavior in invasive gammarids when faced with fish may lead to increased predation on native gammarids. We also assume that effective anti-predator responses to a wide array of predators could be characteristic for invasive gammarids and could contribute to their success in their novel habitats.
The immigration of D. villosus into Lake Constance was accompanied by a decrease in numbers of G. roeselii (Mörtl et al. 2004; Eckmann et al. 2008). Meanwhile, 6 years after its immigration, D. villosus has become an important component of benthic food webs. But despite its predatory habit, D. villosus could not extirpate G. roeselii in Lake Constance until now. Most likely, differences in spatial niches and behaviors may permit co-existence of the two species. In places where sediments are diverse, i.e., composed of stones and macrophytes, both species may use different substrates and thus avoid competition and mutual predation. Co-existence of several gammarid species including G. roeselii and D. villosus has already been observed in a small brook near the Rhine River where sediments are composed of gravel, stones and different macrophytes (Kley and Maier 2005). In spite of its low activity and its hiding under stones, D. villosus has become an important food source for some littoral fish in Lake Constance (Eckmann et al. 2008). Possibly fish have adapted to these behaviors and have learned to capture this attractive prey.
Finally, we stress that we tested substrate choice using a limited number of substrates and predation risk with only one fish species of a certain age. Conditions in nature are much more diverse and complex. Grain size of hard substrates, interstitial pore space sizes and structure of plants, e.g., distance between leaves, may be important determinants of substrate choice. Elodea and Chara may be more suitable substrates for G. roeselii than other plants. Eckmann et al. (2008) analyzed stomach contents from 15 fish species sampled in Lake Constance and found that only four fish species consumed amphipods: burbot, European eel (Anguilla anguilla L., perch (Perca fluviatilis L.) and ruffe (Gymnocephalus cernuus L.). Thus, it is unlikely that the invasion of D. villosus into Lake Constance has a marked influence on the fish fauna. However, as D. villosus can be a predator, preying on various benthic species, it can affect fishes indirectly by reducing the available food. That D. villosus can impact on macro-invertebrate communities in particular in its preferred stone substrate has already been shown (Dick et al. 2002; van Riel et al. 2006).
References
Bailey MM (1972) Age, growth, reproduction, and food of the burbot, Lota lota (Linnaeus), in Southwestern Lake Superior. Trans Am Fish Soc 101:667–674. doi:10.1577/1548-8659(1972)101<667:AGRAFO>2.0.CO;2
Baumgärtner D, Jungbluth A-D, Koch U, von Elert E (2002) Effects of infochemicals on microhabitat choice by the freshwater amphipod Gammarus roeseli. Arch Hydrobiol 155:353–367
Baumgärtner D, Koch U, Rothhaupt K-O (2003) Alteration of kairomone-induced response of the freshwater amphipod Gammarus roeseli by sediment type. J Chem Ecol 29:1391–1401. doi:10.1023/A:1024213403537
Bollache L, Kaldonski N, Troussard J-P, Lagrue C, Tierry R (2006) Spines and behaviour as defences against fish predators in an invasive freshwater amphipod. Anim Behav 72:627–633. doi:10.1016/j.anbehav.2005.11.020
Dahl J (1998) Effects of a benthivorous and a drift feeding fish on a benthic stream assemblage. Oecologia 116:426–432. doi:10.1007/s004420050606
Dahl J, Greenberg L (1996) Effects of habitat structure on habitat use by Gammarus pulex in artificial streams. Freshw Biol 36:487–495. doi:10.1046/j.1365-2427.1996.00096.x
De Lange HJ, Lüring M, Van Den Borne B, Peeters THM (2005) Attraction of the amphipod Gammarus pulex to water-borne cues of food. Hydrobiologia 544:19–25. doi:10.1007/s10750-004-7896-y
Devin S, Piscart C, Beisel JN, Moreteau JC (2003) Ecological traits of the amphipod invader Dikerogammarus villosus on a mesohabitat scale. Arch Hydrobiol 158:43–56. doi:10.1127/0003-9136/2003/0158-0043
Dick JTA, Platvoet D (2000) Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. P R Soc Lond B Bio 267:977–983. doi:10.1098/rspb.2000.1099
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084. doi:10.1139/f02-074
Eckmann R, Mörtl M, Baumgärtner D, Berron C, Fischer P, Schleuter D, Weber A (2008) Consumption of amphipods by littoral fish after the replacement of native Gammarus roeseli by invasive Dikerogammarus villosus in Lake Constance. Aquat Invasions 3:184–188
Elliott JM (2005) Day-night changes in the spatial distribution and habitat preferences of freshwater shrimps, Gammarus pulex, in a stony stream. Freshw Biol 50:552–566. doi:10.1111/j.1365-2427.2005.01345.x
Franken RJM, Batten S, Beijer JAJ, Gardeniers JJP, Scheffer M, Peeters ETHM (2006) Effects of interstitial refugia and current velocity on growth of the amphipod Gammarus pulex Linnaeus. J N Am Benthol Soc 25:656–663. doi:10.1899/0887-3593(2006)25[656:EOIRAC]2.0.CO;2
Gergs R, Rothhaupt K-O (2008) Effects of zebra mussels on an native amphipod and the invasive Dikerogammarus villosus: the influence of biodeposition and structural complexity. J N Am Benthol Soc 27:541–548. doi:10.1899/07-151.1
Grabowski M, Jazdzewski K, Konopacka A (2007) Alien crustacea on polish waters–amphipoda. Aquat Invasions 2:25–38. doi:10.3391/ai.2007.2.1.3
Hesselschwerdt J, Necker J, Wantzen KM (2008) Gammarids in Lake Constance: habitat segregation between the invasive Dikerogammarus villosus and the indigenous Gammarus roeselii. Fundam Appl Limnol 173:177–186. doi:10.1127/1863-9135/2008/0173-0177
Hoyle JD, Holomuzki JR (1990) Effect of predatory fish presence on habitat use and diel movement of the stream amphipod Gammarus minus. Freshw Biol 24:509–517. doi:10.1111/j.1365-2427.1990.tb00728.x
Kaldonski N, Perrot-Minnot M-J, Cézilly F (2007) Differential influence of two acanthocephalan parasites on the antipredator behaviour of their common intermediate host. Anim Behav 74:1311–1317. doi:10.1016/j.anbehav.2007.02.027
Kaldonski N, Lagrue C, Motreuil S, Rigaud T, Bollache L (2008) Habitat segregation mediates predation by the benthic fish Cottus gobio on the exotic amphipod species Gammarus roeseli. Naturwissenschaften 95:839–844. doi:10.1007/s00114-008-0392-x
Kinzler W, Maier G (2003) Asymmetry in mutual predation: possible reason for the replacement of native gammarids by invasives. Arch Hydrobiol 157:473–481. doi:10.1127/0003-9136/2003/0157-0473
Kinzler W, Maier G (2006) Selective predation by fish: a further reason for the decline of native gammarids in the presence of invasives? J Limnol 65:27–34
Kley A, Maier G (2003) Life history characteristics of the invasive freshwater gammarids Dikerogammarus villosus and Echinogammarus ischnus in the river Main and the Main–Donau canal. Arch Hydrobiol 156:473–481. doi:10.1127/0003-9136/2003/0156-0457
Kley A, Maier G (2005) An example of niche partitioning between Dikerogammarus villosus and other invasive and native gammarids: a field study. J Limnol 64:85–88
Kley A, Maier G (2006) Reproductive characteristics of invasive gammarids in the Rhine-Main-Danube catchment, South Germany. Limnologica 36:79–90. doi:10.1016/j.limno.2006.01.002
Kobak J, Zytkowicz (2007) Preferences of invasive Ponto-Caspian and native gammarids for zebra mussel (Dreissena polymorpha, Bivalvia) shell habitat. Hydrobiologia 589:43–54. doi:10.1007/s10750-007-0716-4
Krisp H (2004) Substratpräferenz, Aktivität, Prädationsneigung und Wachstum von neozoischen und heimischen Gammaridenarten. Diploma Thesis in Biology, University of Ulm,. 67 pp
Lods-Crozet B, Reymond O (2006) Bathymetric expansion of an invasive gammarid (Dikerogammarus villosus, crustacea, amphipoda) in Lake Leman. J Limnol 65:141–144
Mac Neil C, Elwood RW, Dick JTA (1999) Predator-prey interactions between brown trout Salmo trutta and native and introduced amphipods; their implications for fish diets. Ecography 22:686–696. doi:10.1111/j.1600-0587.1999.tb00518.x
MacNeil C, Dick JTA, Elwood RW (1997) The trophic ecology of freshwater Gammarus (crustacea: amphipoda); problems and perspectives concerning the functional feeding group concept. Biol Rev Camb Philos Soc 72:349–364. doi:10.1017/S0006323196005038
MacNeil C, Elwood RW, Dick JTA (2000) Factors influencing the importance of Gammarus spp. (Crustacea: Amphipoda) in riverine salmonid diets. Arch Hydrobiol 149:87–107
MacNeil C, Platvoet D, Dick JTA (2008) Potential roles for differential body size and microhabitat complexity in mediating biotic interactions within invasive freshwater amphipod assemblages. Fundam Appl Limnol 172:175–182. doi:10.1127/1863-9135/2008/0172-0175
Mathis A, Hoback W (1997) The influence of chemical stimuli from predators on precopulatory pairing by the amphipod, Gammarus pseudolimnaeus. Ethology 103:33–40
Mayer G, Maier G, Maas A, Waloszek D (2008) Mouthparts of the Ponto-Caspian invader Dikerogammarus villosus (amphipoda: pontogammaridae). J Crustac Biol 28:1–15. doi:10.1651/07-2867R.1
Mayer G, Maier G, Maas A, Waloszek D (2009) Mouthpart morphology of Gammarus roeselii compared to a successful invader, Dikerogammarus villosus (Amphipoda). J Crustac Biol (in press)
Mazzi D, Bakker TCM (2003) A predator’s dilemma: prey choice and parasite susceptibility in three-spined sticklebacks. Parasitology 126:339–347. doi:10.1017/S0031182003003019
McGrath KE, Peeters ETHM, Beijer JAJ, Scheffer M (2007) Habitat-mediated cannibalism and microhabitat restriction in the stream invertebrate Gammarus pulex. Hydrobiologia 589:155–164. doi:10.1007/s10750-007-0731-5
Mörtl M, Mürle U, Ortlepp J, Rey P, Schleifhacken N, Werner S (2004) Dikerogammarus villosus (crustacea: amphipoda) und Corbicula fluminea (Bivalvia: Veneroidea) im Bodensee. In: Wirbellose Neozoen im Bodensee. LfU Baden-Württemberg, Institut für Seenforschung. City Satz GmbH, Herxheim, pp 15–30
Mürle U, Becker A, Rey P (2004) Dikerogammarus villosus (amphipoda), new in Lake Constance. Lauterbornia 49:77–79
Newman RM, Waters TH (1984) Size-selective predation on Gammarus pseudolimnaeus by trout and sculpins. Ecology 65:1535–1545. doi:10.2307/1939133
Palmer ME, Ricciardi A (2004) Physical factors affecting the relative abundance of native and invasive amphipods in the St Lawrence River. Can J Zool 82:1886–1893. doi:10.1139/z04-186
Pennuto C, Keppler D (2008) Short-term predator avoidance behaviours by invasive and native amphipods in the Great Lakes. Aquat Ecol 42:629–641. doi:10.1007/s10452-007-9139-6
Piscart C, Manach A, Copp GH, Marmonier P (2007) Distribution and microhabitats of native and non-native gammarids (amphipoda, crustacea) in Brittany, with particular reference to the endangered endemic sub-species Gammarus duebeni celticus. J Biogeogr 34:524–533. doi:10.1111/j.1365-2699.2006.01609.x
Platvoet D, Dick JTA, Konijnendijk N, van der Velde G (2006) Feeding of micro-algae in the invasive Ponto-Caspian amphipod Dikerogammarus villosus (Sowinsky, 1894). Aquat Ecol 40:237–245. doi:10.1007/s10452-005-9028-9
Pöckl M (1995) Laboratory studies on growth, feeding, moulting and mortality in the freshwater amphipods Gammarus fossarum and G. roeseli. Arch Hydrobiol 134:223–253
Ponyi E (1956) Ökologische, ernährungsbiologische und systematische Untersuchungen an verschiedenen Gammarus-Arten. Annu Rev Ecol Syst 20:297–330
Ponyi E (1961) Über Ernährung einiger Amphipoden (Crustacea) in Ungarn. Ann Inst Biol Tihany 28:117–123
Ryder RA, Pesendorfer J (1992) Food, growth, habitat, and community interactions of young-of-the year burbot, Lota lota L., in a precambrian Shield lake. Hydrobiologia 243–244:211–227
Sudo H, Azeta M (2001) The microhabitat and size of gammarid species selectively predated by young red sea bream Pagrus major. Fish Sci 67:389–400. doi:10.1046/j.1444-2906.2001.00274.x
Van Dolah RF (1978) Factors regulating the distribution and population dynamics of the amphipod Gammarus palustris in an intertidal salt mars community. Ecol Monogr 48:191–217. doi:10.2307/2937299
Van Overdijk CDA, Grigorovich IA, Mabee T, Ray WJ, Ciborowski JJH, MacIsaac HJ (2003) Microhabitat selection by the invasive amphipod Echinogammarus ischnus and native Gammarus fasciatus in laboratory experiments and in Lake Erie. Freshw Biol 48:567–578. doi:10.1046/j.1365-2427.2003.01041.x
Van Riel M, van der Velde G, Rajagopal S, Marguillier S, Dehairs F, bij de Vaate A (2006) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58. doi:10.1007/s10750-005-1904-8
Van Riel M, Healy EP, van der Velde G, bij de Vaate A (2007) Interference competition among native and invader amphipods. Acta Oecol 31:282–289. doi:10.1016/j.actao.2006.12.006
Wijnhoven S, van Riel MC, van der Velde G (2003) Exotic and indigenous freshwater gammarid species: physiological tolerance to water temperature in relation to ionic content of the water. Aquat Ecol 37:151–158. doi:10.1023/A:1023982200529
Williams DD, Moore (1982) The effect of environmental factors on the activity of Gammarus pseudolimnaeus (amphipoda). Hydrobiologia 96:137–147. doi:10.1007/BF02185429
Wooster DE (1998) Amphipod (Gammarus minus) reseponses to predators and predator impact on amphipod density. Oecologia 115:253–259. doi:10.1007/s004420050514
Wudkevich K, Wisenden BD, Chivers DP, Smith RJF (1997) Reactions of Gammarus lacustris to chemical stimuli from natural predators and injured conspecifics. J Chem Ecol 23:1163–1173. doi:10.1023/B:JOEC.0000006393.92013.36
Acknowledgments
This study is part of the project “ANEBO” (Aquatische Neozoen im Bodensee und seinen Zuflüssen); it is financially supported by the European Union within the scope of the Interreg III A programme. We are grateful for this support. We thank anonymous reviewers and R. Gulati for valuable critical comments on an earlier version of this manuscript. We also thank members of the staff of Reiner Eckmann (Konstanz) who provided burbots used in our experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kley, A., Kinzler, W., Schank, Y. et al. Influence of substrate preference and complexity on co-existence of two non-native gammarideans (Crustacea: Amphipoda). Aquat Ecol 43, 1047–1059 (2009). https://doi.org/10.1007/s10452-009-9242-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-009-9242-y