Abstract
In this study we use a combination of techniques to assess the general risk of invasion by the aquatic invader Dikerogammarus villosus in Great Britain at multiple scales. First, bioclimatic models (Support Vector Machine algorithm) were used to identify regions showing the highest climatic match with the European range of the species, and that might be at highest risk of initial colonization if provided with propagules. The model showed that nearly 60% of Great Britain shows the minimum bioclimatic suitability for the species, particularly southern and eastern England. Afterwards, a Network Analysis was used to model the natural spatio-temporal spread of the killer shrimp in the Great Ouse River catchment (SE England), the first region invaded by this species. This model suggested that the northern part of the catchment, including two relevant Ramsar sites (Ouse Washes and Wicken fen) are at serious risk of being invaded in the short-term (<5 years). Taking into account the rapid spread of the killer shrimp in other European countries, its broad environmental tolerance, the high climate suitability of broad areas of Great Britain to the species, the current spread of other Ponto-Caspian species, and the high natural and artificial connectivity of the hydrological network, we conclude that D. villosus is very likely to continue its spread in Great Britain, dramatically affecting the native biodiversity. The multi-scale approach proposed in this study combines large-scale bioclimatic models with local-scale dispersal models, providing managers with a powerful spatial and temporal basis for informed decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species are recognised as one of the main drivers of biodiversity change at global scale (Sala et al. 2000). While invasive species affect all habitats, both terrestrial and aquatic, freshwater ecosystems are particularly vulnerable because of the high intrinsic dispersal ability of freshwater species when compared with terrestrial organisms, and the high level of human disturbance in aquatic ecosystems that attract biological invasions more than pristine systems (Gherardi 2007). In Great Britain, more than 100 freshwater non-native species have been reported (Keller et al. 2009) with an estimated direct cost of £26 million each year (Oreska and Aldridge 2011). Moreover, the number of invasive species increases at an accelerated rate, in direct relationship with population density and economic welfare (Keller et al. 2009). Consequently, the development of reliable tools to prevent and control the spread of freshwater invasive species is necessary to identify areas at high risk of invasion, to effectively manage invasive species and to conserve native populations.
Assuming the initial arrival of propagules, one of the main factors affecting the success of freshwater invasions is the match between the species’ ecological requirements and the environmental characteristics of the system being invaded (Gherardi 2007). Species distribution models are used to measure the climate suitability for an invasive species, by projecting a model of the known species distribution into a region of interest (Guisan and Thuiller 2005). For this reason, species distribution models are especially helpful to locate areas at continental or regional scale which are most climatically similar to the current range of the invasive species, and that are most susceptible to successful colonization in the event of an introduction (Peterson 2003).
Once an aquatic species is successfully introduced to a new area, its natural dispersal depends mainly on hydrological connectivity. River flow, dams, dykes, aquatic vegetation and human facilitation (waterway construction, water transfer, shipping or angling) are therefore important factors determining the dispersal of freshwater invasive species at local and regional scale that still need to be incorporated into prediction models. Geographic Information Systems (GIS) provide tools that allow modelling of the hydrological network considering all these different elements. For instance, the Network Analysis toolbox (©ESRI, ArcGIS 9.3) uses a network composed of nodes (confluences of the river network and outlet points) and lines (reaches connecting nodes) to model connectivity. Given a particular point of introduction to the network and a dispersal velocity, the model has the potential to calculate the spatio-temporal dispersal of an invasive species in the catchment. Network related analyses have been used increasingly in many disciplines including landscape ecology and conservation biology (see Urban et al. 2009 for a review). In the case of aquatic invaders, nearest neighbour analysis was used to describe the dispersal of zebra mussels over medium to large distances in Europe and North America (Kraft et al. 2002); graph theory measures were used to examine the spatial structure and demographic connectivity of salmonids (Schick and Lindley 2007); and nearest neighbour distance was used to analyse the dispersal of gastropods in South England (Niggebrugge et al. 2007). Species distribution models have also used GIS-derived hydrological indicators, such as flow accumulation (Kumar et al. 2009) and flow stability (Leathwick et al. 2008), to model invasive species. However, the ability of Network Analysis to predict and prevent the spread of aquatic species invasions based on hydrological connectivity still needs further evaluation. Such dispersal predictions are especially relevant because the impact of an invasive species depends directly on its geographic extent (Kraft et al. 2002). The implementation of the Network Analysis in combination with higher-scale bioclimatic models have the potential to provide a better understanding of the processes responsible for the dispersal of important pest species: information that can be used to more effectively support prevention and control efforts.
In this study we use a combination of techniques to assess the general risk of invasion of the aquatic invader Dikerogammarus villosus in Great Britain. D. villosus, commonly named the killer shrimp, is native to the Ponto Caspian region that in the last 15 years has invaded many countries in Europe thanks to a combination of natural and human-mediated dispersal (Pockl 2009). Currently, the killer shrimp is present in at least 17 European countries and is expected to continue its spread in Europe and eventually to invade North America (Ricciardi and Rasmussen 1998). The most recently invaded region in Europe is Great Britain, where it was first located in September 2010 at the River Great Ouse catchment in eastern England (Grafham reservoir and Diddington Brook, latitude 52.29°, longitude −0.31°) (MacNeil et al. 2010); and in November 2010 its presence was confirmed at Cardiff Bay (latitude 51.46°, longitude −3.16°) and Eglwys Nunydd Reservoir, Port Talbot (latitude 51.54°, longitude −3.73°) in South Wales. According to the Environment Agency the species is so far confined to these three locations (Madgwick and Aldridge 2011). The presence of this species is of particular importance in these areas because it is an omnivorous predator known to dramatically affect the native fauna of the ecosystems invaded, including fish stocks (Casellato et al. 2007); and for which there are no effective management or eradication options available (Madgwick and Aldridge 2011). For this reason, prevention and control of the spread of D. villosus are especially timely and relevant in Great Britain. First, bioclimatic models were used to identify regions that climatically matched the European range of the species, and that therefore might be at highest risk of colonization in the event of an introduction. This study assumes therefore that populations are more likely to successfully establish in climatically suitable than in unsuitable habitats. Once the species has successfully established a population, the possibility of dispersal through interconnected water-bodies can be critical to anticipate the spread of the species at local to regional scales. Consequently, a Network Analysis was used to model the spatio-temporal spread of D. villosus in the River Great Ouse catchment. This study aimed to evaluate the utility of a combination of techniques to provide quick, accurate and useful information to environmental managers and risk assessors at multiple scales.
Methods
Bioclimatic modelling at the scale of Great Britain
A total of 248 D. villosus occurrences in Europe were obtained from the Global Biodiversity Information Facility (GBIF, http://www.gbif.org/, last accessed 20 September 2010) and an extensive literature review on the ISI Web of Knowledge (see “Appendix 1” for a map of occurrences). Data on 19 bioclimatic variables and altitude was obtained from WorldClim (Hijmans et al. 2005) available at http://www.worldclim.org (last accessed 1 June 2010) with a 30 s resolution (approx. 1 km2). Bioclimatic variables are biologically meaningful factors derived from monthly temperature and rainfall values that represent annual trends, seasonality and extremes for species survival. These factors are known to constrain species distribution at a global scale, affecting directly their growth and reproduction and indirectly their habitat and interaction with other species. In the case of freshwater species, many studies have revealed strong correlations between species patterns and climatic variables, mostly temperature and the availability of water, and have used bioclimatic models to successfully predict the distribution of fishes (McNyset 2005; Chen et al. 2007; Leathwick et al. 2008), snails (Loo et al. 2007) and mussels (Drake and Bossenbroek 2004). Temperature is known to affect the fecundity, reproduction, egg size, and survival of gammarids (Sheader 1996; Piscart et al. 2003; Pockl et al. 2003; Wijnhoven et al. 2003), it particularly affects the reproductive period and growth of D. villosus (Devin et al. 2003), and its oxygen consumption (Bruijs et al. 2001). In addition, rainfall patterns determine the availability of water and the frequency of droughts and floods, which can have marked effects on freshwater organisms. Although these correlations are not necessarily causal and may reflect covarying environmental factors, they strongly suggest that climate plays a key role in the distribution of freshwater species (Jocque et al. 2010). For this study, only six bioclimatic variables showing a low inter-correlation (Pearson r < 0.80) and that were ecologically meaningful for D. villosus were used for modelling, including: maximum and minimum annual temperatures, temperature seasonality, annual precipitation, precipitation in the driest month and precipitation seasonality (Table 1).
The free software openModeller (http://openmodeller.sourceforge.net, de Souza Muñoz et al. 2011) was used to create bioclimatic models for D. villosus. Models were built using a Support Vector Machines (SVM) algorithm using the software default options. Two-class SVMs are a type of non-probabilistic algorithm that use a kernel function to fit a hyperplane in the feature space that maximizes the separation between presence and absence points (Schölkopf et al. 2000). SVMs are not based on statistical distributions and contrary to other algorithms do not assume that data are independent, although performance will be affected by how well the observations represent the range of environmental variables (Drake et al. 2006). This is because autocorrelated data can produce biased predictions and also because models assume the species is in equilibrium with the environment; therefore the observations used for calibration should comprehensibly represent the range of conditions suitable for the species. To compensate for the autocorrelation of data, a filter was applied to include spatially unique observations (single occurrence per pixel of 1 km2), although some bias might still be expected. Invasive species that are still spreading like the killer shrimp violate the equilibrium assumption, increasing the uncertainty of species distribution models (Guisan and Thuiller 2005). For this reason, authors have strongly suggested that the native range of the species be included in the calibration and to refine the models if the species is reported in an area outside its current climatic range (Beaumont et al. 2009). In this study, we have included all known occurrence localities of D. villosus, both in the native and invaded ranges.
For input, our SVMs used the dataset of 248 occurrences in Europe and the set of six bioclimatic factors that might limit the species’ capabilities to survive. Data were split into two sets: 80% of the data was used for modelling and the remaining 20% to test the accuracy of the predictions. Because no absence data was available, pseudo-absences were generated from the Europe-wide background. To assess model performance we used the Area Under the ROC Curve (AUC) (Hanley and McNeil 1982), which represents the probability that a random occurrence locality will be classified as more suitable than a random pseudo-absence. A model that performs no better than random will have an AUC of 0.5 whereas a model with perfect discrimination will score 1. Although AUC is commonly used to assess model accuracy, it has been shown to provide high scores for very poor models (Phillips and Elith 2010). Consequently, complementary criteria were used to evaluate the model as suggested by Rodda et al. (2011): the ability of the model to capture the native range of the species, the minimum suitability score of localities were the species is known to be present (minimum training presence), and the eco-plausibility of the resulting map considering the biology and ecology of the species.
After calibration, models were projected onto Europe to obtain a D. villosus suitability map. Although SVM are non-probabilistic algorithms, they can produce class probabilities by an improved implementation of Platt’s a posteriori probabilities (Platt 1999). This is equivalent to fitting a logistic regression model to the estimated decision values. In this case, the resulting logistic map describes the bioclimatic suitability of Europe for D. villosus in a 0–1 scale. Subsequently, we focused on Great Britain for closer examination. It has been suggested that species distribution models may fail when predicting within areas with novel climatic conditions outside the range of the calibration of the model, depending on the way the model extrapolates outside the range of calibration (Elith and Graham 2009). In our case, we did not expect problems related to the extrapolation of models in Great Britain as its climate is very similar to the North of Europe, where the killer shrimp is already present. Finally, the threshold maximizing the sensitivity (i.e. number of presences correctly predicted) and specificity (i.e. number of absences correctly predicted) of the model was used to calculate the percentage of Great Britain’s territory showing the minimum climatic conditions necessary for the species. These values refer therefore to the total surface of Great Britain and are provided as a general indicator of the potential threat posed by D. villosus on the island.
Network analysis at the scale of catchment
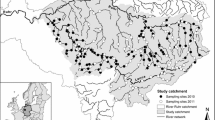
The River Great Ouse catchment, where D. villosus was first detected in September 2010, was selected to test the Network Analysis (Fig. 1). With a length of 230 km, a basin area of 3,400 km2 and an average discharge of 11.8 m3 s−1, the Great Ouse is the fourth-longest river in Great Britain. Three important Ramsar sites (Wetlands of International Relevance according to the International Convention on Wetlands: http://www.ramsar.org) are located in this catchment: Ouse Washes, Wicken and Chippenham Fens (Fig. 1). According to the Ramsar Information Sheets for these sites (http://www.jncc.gov.uk/page-1390, last accessed 6 December 2010), the Ouse Washes are a seasonally flooded washland habitat managed in a traditional agricultural manner. They support aquatic species of international relevance including the ray-finned fish Cobitis taenia, the dragonfly Libellula fulva and the rifle beetle Oulimnius major. The invertebrate fauna of Chippenham fen is also very rich, partly due to its transitional position between Fenland and Breckland. The species list includes many rare invertebrates characteristic of ancient fenland sites in Britain, for instance the diptera Parochthiphila spectabilis and Thaumatomyia sp. and the beetle Gyrophaena pseudonana. Wicken Fen hosts a high diversity of terrestrial and aquatic plants, including over 400 insect species that are listed nationally endangered, rare or scarce in the UK’s Red Data Books (http://www.wicken.org.uk).
Catchment boundaries and the hydrological network were downloaded as a vector file from the DivaGis Spatial Data Portal (http://www.diva-gis.org/Data, last accessed September 2010). This hydrological network was checked for digitalization errors, hydrologic connectivity and flow direction in ArcMap 9.3.1 (©ESRI GIS). We afterwards created a network dataset using the Network Analyst extension. The spatio-temporal modelling of dispersal within this network depends mainly on the distance between introduction and destination points and the dispersal velocity of the species. This is therefore a simplification of the natural conditions of the basin, considering that the flow is homogeneous and no influence of roughness, barriers to flow or water quality. The first observation of the killer shrimp in the River Great Ouse (at Grafham reservoir) was used as the introduction point. As destination points, we selected all the outlets and junctions in the hydrological network as well as the three Ramsar sites.
Regarding the dispersal velocity, D. villosus has been reported to spread downstream at a speed between 40 and 461 km year−1, with a mean of 112 km year−1 in the River Rhine (Leuven et al. 2009). For the River Great Ouse we took as reference the mean value (High speed scenario 100 km year−1) instead of the maximum velocity reported because of the lower size and flow of the River Great Ouse in comparison with the Rhine; with a lower flow a lower dispersal velocity might be expected. Furthermore, D. villosus has been observed to disperse at a rate not higher than 20 km year−1 in Flanders (P. Boets, personal communication), which we considered a better reference for the River Great Ouse and therefore was selected as second reference value (Low speed scenario 20 km year−1). Since no studies on the dispersal of this species exist in other environments, we included a third scenario (Intermediate speed scenario 60 km year−1) and compared the three outputs. Gammarids often move upstream against the water current, although the importance of such dispersal is not clear. Hancock and Hughes (1999) documented very restricted movement upstream by adult shrimps only while juvenile and larvae moved downstream. In contrast, Elliott (2003) found gammarids to move predominantly upstream (up to 2 km per year). Because reported upstream dispersal is either almost negligible or very high, we chose a conservative range of velocities of 2, 6 and 10 km year−1, which corresponds to 10% of the downstream dispersal in each modelled scenario. Though the selection of dispersal velocities is partially arbitrary, we believe a range of scenarios can effectively encompass the potential spread of the species in the catchment, which may depend on various abiotic (flow rate, thermal regime, water chemistry, barriers to dispersal) and biotic (initial density, spawning and growing seasons of the species) factors. The output maps show the estimated number of years the species will potentially need to arrive at all the destination points. Reaches expected to be invaded in <5 years were highlighted to show areas under a highest risk of short-term invasion.
Results
Risk model at the scale of Great Britain
The bioclimatic model performed for D. villosus showed a high accuracy score (AUC = 0.97). The native range of the species around the Black sea and the lower section of the Danube River was correctly captured by the model (Fig. 2a). The minimum training presence (minimum score of real occurrence points) of the model was relatively low: 11.0%. However, only ten points located at the east margin of the species distribution (Ukraine and Poland) showed suitability scores between 11.0 and 22.0%; if we exclude these points, the minimum training presence was of 31%. The Europe map showed in Fig. 2a showed highest suitability for the killer shrimp at the middle and lower sections of the Danube, north of mainland Europe, and south-east England, all regions where the species is already present. According to the model, the suitability for the killer shrimp in Europe increases at an altitude lower than 500 m; maximum temperatures between 20 and 30°C; minimum temperatures between −5 and 5°C; and annual precipitation lower than 1,000 mm. These values are in accordance with the observed bioclimatic ranges of the species as reported in Table 1. The response of D. villosus to these factors can be further consulted in “Appendix 2”.
Bioclimatic suitability for Dikerogammarus villosus in Europe (a) and Great Britain (b). Support Vector Machine (SVM) model based on the species presence in Europe (248 occurrence points) and seven environmental predictors (see Table 1)
The predicted suitability of Great Britain for the killer shrimp as presented in Fig. 2b, shows highest scores in the south and east of England (East and West Midlands, East Anglia, South-West and South-East). The threshold maximizing the sensitivity and specificity of the model (established at 26.6%) was used to transform the logistic map into a binary presence/absence map. The area where the species is predicted to be present accounts for 59.2% of the Great Britain territory. Furthermore, 44.77% of the territory showed climate suitability higher than 50%. The River Great Ouse catchment, where the killer shrimp was first located, showed a mean climatic suitability for D. villosus of approximately 70% (73% at Grafham reservoir) (Fig. 2b). While Wales had a mean climatic suitability lower than that of East Anglia, it is notable that its southern margin, where two new populations were located in November 2010, showed relatively higher suitability than the rest of Wales: 54% at Eglwys Nunydd Reservoir and 24% at Cardiff Bay.
Dispersal model at high-risk catchments
The Network Analysis provided the estimated time that it will take the species to arrive to the different reaches that compose the Great Ouse hydrological network (Fig. 3). Three scenarios were modelled using increasing dispersal velocities. The models were consistent and showed that the north-east part of the catchment might be at risk of being invaded in the next 5 years. In the low speed scenario (20 km year−1 downstream, 2 km year−1 upstream), the estimated dates of arrival ranged from 1.6 to 1.7 years in the case of the closest reaches, to 4.5 years to reach the estuary, and more than 60 years at the farthest sites upstream. Ouse Washes were reached in 1.8 years according to this model. At the intermediate speed scenario (60 km year−1 downstream, 6 km year−1 upstream) these velocities were reduced and new sites upstream of the introduction points were predicted to be invaded in <5 years. For instance the killer shrimp was expected to arrive to Ouse Washes in <1 year, and to reach the estuary in 1.5 years. Wicken fen was expected to be invaded in three years under this particular scenario. Finally, the high speed scenario (100 km year−1 downstream, 10 km year−1 upstream) included all reaches downstream the introduction point and more sites upstream, including the three Ramsar sites. Amongst them, Ouse Washes were predicted to be invaded in <5 years under all three scenarios as it is directly downstream the invaded sites; while Wicken and Chippenham fens were at risk in only the intermediate and high speed scenarios respectively.
Dispersal of Dikerogammarus villosus in the River Great Ouse catchment modelled with Network Analysis. Three scenarios (a, b, c) with increasing dispersal velocities are shown. Black thick lines represent the reaches showing a high invasion risk in the next 5 years. 1 Ouse Washes, 2 Wicken fen, 3 Chippenham fen
Discussion
Risk model at the scale of Great Britain
The bioclimatic model performed with data on the European range of D. villosus showed the climatic suitability of Great Britain for this species, based on the European native and invaded ranges of the species. According to this model, approximately a 60% of Great Britain shows the minimum climatic suitability for the establishment of the killer shrimp (Fig. 2b). However these values refer to the total surface of the country and are likely to differ if we consider aquatic ecosystems only. Those areas where the match between the climatic characteristics of the species’ current European range and that of Great Britain is particularly high could be successfully invaded provided the availability of propagules and that other factors (especially water chemistry, availability of hard substrate and presence of predators) are not limiting. In England, the extensive midland canal system, constructed predominantly in the eighteenth and nineteenth centuries, provides widespread and interconnected hard substrates along which the killer shrimp can spread and reach the waterbodies most climatically suitable. Moreover, the areas where climate suitability for the killer shrimp is especially high already support a well-established and abundant population of another Ponto-Caspian species, the zebra mussel (Dreissena polymorpha) (Aldridge 2010), which is known to facilitate the dispersal of the killer shrimp. These two species have co-evolved over a long period of time, being commonly found in the same assemblages (Casellato et al. 2007). Zebra mussel populations may help the colonization of the killer shrimp by providing habitat complexity through the production of byssus threads and shells, and food material through biodeposition (Gergs and Rothhaupt 2008). Currently the zebra mussel is present in much of England, and localised parts of Wales and the south of Scotland; and it is experiencing an increase in abundance and spread in relation to increasing water quality and waterway connectivity (Aldridge et al. 2004). Species distribution models calibrated in early stages of invasions, like in the case of the killer shrimp, tend to underestimate the potential spread of species highly constrained by dispersal and colonization processes (Václavík and Meentemeyer 2011). The similarity between the suitability map for the killer shrimp (Fig. 2b) and the current spread of the zebra mussel in England suggests that this is unlikely to be the case. Nevertheless, models should be refined to include new data if the species continue its spread in UK or other areas (Beaumont et al. 2009).
According to the bioclimatic model, Grafham reservoir, the first introduction point located in England showed climate suitability as high as 73%, which reinforces the ability of bioclimatic models to locate areas of likely colonization at large scales (Peterson 2003). The two location points in South Wales showed lower suitability (24 and 54%) although still above the minimum training presence of the model (11%). This may reflect a poor predictive capacity of a model based on climatic conditions only, even though it generally fulfils the four criteria used for evaluation (high AUC, native range inclusion, minimum training presence, and eco-plausibility). Both Welsh locations are very popular fisheries with considerable boating activities and both support large zebra mussel populations (Madgwick and Aldridge 2011). These factors may be enough to explain why the killer shrimp has successfully colonized them instead of other areas with similar or even more favourable climatic conditions. Therefore, although bioclimatic factors are known to limit the distribution of species at large scales, accurate predictions at the local scale should consider other habitat-related factors as well as socio-economic factors such as the presence of ports, reservoirs, aquaculture facilities and fisheries. In the case of the killer shrimp, intense ship traffic, hard artificial substrate, a high oxygen saturation and low conductivity have been identified as important suitability factors (Boets et al. 2010). In addition, we advocate the intense human exploitation of lakes and rivers and the presence of zebra mussels as potential relevant indicators. Ideally, distribution models would include these factors as predictors, which might be possible at local to regional scales, though increasingly difficult at large scales such as the European scale used in this study. For instance, water quality data is provided by the European Environment Agency, but the interpolation at large scale of this information is difficult and probably not reliable. Substrate composition may be mapped with great accuracy at local scales, but this information is not available at the large scales used in this study. Information about the human use of aquatic ecosystems is scattered amongst administrations and is difficult to integrate into GIS layers. Nevertheless, authors are making remarkable advances in the development of new predictors to model the distribution of aquatic species. For instance Leathwick et al. (2008) derived a number of predictors at catchment scale describing the river and stream network to model the distribution of fishes in New Zealand; Drake and Bossenbroek (2004) used geology as a proxy of water chemistry to predict the distribution of zebra mussel in United States; and several physiographic and hydrological features were used to model the distribution of fishes in Kansas (McNyset 2005). Apart from habitat suitability, biotic interaction also needs to be considered, as it can explain why species are not present in sites that are climatically suitable (Peterson 2003). In the case of the killer shrimp, no limiting predators have been reported though its positive interaction with the zebra mussel might be a significant factor explaining its spread (Gergs and Rothhaupt 2008). Finally, limited dispersal across unsuitable patches separating otherwise suitable areas may also explain why species do not occupy their whole potential range (Peterson 2003). In the case of the killer shrimp, the total percentages of Great Britain suitable for the species given above refer to the total territory and not particular waterbodies, which might be separated by unsuitable habitat. The colonization of climatically suitable habitats will depend therefore on the ability of the species to disperse through interconnected waterbodies and the role played by vectors (e.g. animals, anglers and boaters). As already noted, the extensive waterway system in England provides the means to colonize numerous otherwise isolated waterbodies, and the widespread distribution of the zebra mussel suggests that aquatic invaders may be able to overcome the limitations imposed by unsuitable land patches. This highlights the importance of a rapid implementation of prevention measures to minimize the human-related spread of aquatic invaders between catchments.
Concluding, considering (1) the climate suitability of Great Britain for D. villosus and the rapid spread of the species in countries with similar characteristics (for instance The Netherlands, France and Belgium), (2) its broad environmental and habitat tolerance, (3) the current spread of the zebra mussel, and (4) its close association with the sport and recreational exploitation of rivers and lakes, we conclude that D. villosus is very likely to continue its spread in Great Britain with dramatic consequences on the native aquatic biodiversity.
Dispersal model at high-risk catchments
The three dispersal models performed for D. villosus in the River Great Ouse catchment consistently suggested that the northern part of the catchment is under a serious risk of invasion in the next 5 years (Fig. 3). This area showed the highest climate suitability for the species according to our bioclimatic model (Fig. 2b). Dispersal models predict the distribution of the species in the catchment according to the hydrological connectivity of the river network and the species’ natural ability to spread. Downstream natural dispersal in the case of the killer shrimp is likely to be achieved through drift (Bilton et al. 2001; van Riel et al. 2006). In this sense, a strong relationship between drift and the density of shrimps in the benthos has been noted in Gammarus pulex (Elliott 2002) which suggests that the higher the density of the invasive killer shrimp, the higher the probability of downstream drift. In this regard, high densities (ca. 400 individuals m−2) of D. villosus have been observed throughout Grafham reservoir, especially in the margins (MacNeil et al. 2010); as well as the two Welsh locations (up to 4,000 individuals m−2, S. Ormerod unpublished data). The natural spread of the species is therefore not constant and depends on a variety of biotic (density, presence of predatory fish and invertebrates) and abiotic (water current, temperature, oxygen, seasonality) factors; hence the selection of a range of velocities is more appropriate to evaluate its potential spread rather than focusing on a single scenario. As noted above, limited dispersal through unsuitable habitats may hamper the ability of the species to spread as might be predicted by the dispersal models used in this study. However, as highlighted by Gherardi (2007) the high inherent dispersal ability of aquatic species often allows them to swim or drift through unsuitable areas, making aquatic habitats especially vulnerable to biological invasions. Consequently, while aquatic species would need external vectors to invade new catchments at a continental scale, unsuitable patches at the catchment scale may only slow down their dispersal. By providing scenarios assuming increasing dispersal velocities, our study provides a spatio-temporal framework to anticipate the spread of the species at local scale. Nevertheless, further research on the ability to disperse of the killer shrimp would greatly improve the predictive capacity of the models presented here. In addition to natural spread, dispersal through human activities has received considerable attention in the last years (Bilton et al. 2001), as it is especially relevant to invasive species. In the case of the killer shrimp, spread can be facilitated by human activities such as fishing, angling or boating (Leuven et al. 2009). Surfaces such as waders, boats and equipment are vulnerable to fouling and could transport the species between water bodies (MacNeil et al. 2010). The species could also be transferred with movements of fish stocks or foraging water birds. Accounting for such human introduction of freshwater species is not trivial though it can be achieved through gravity models based on interviews to calculate the probability of anglers’ movements between lakes (e.g. Timar and Phaneuf 2009).
The area under a high risk of invasion according to dispersal models includes Ouse Washes, a Ramsar site of international relevance that hosts relict Fenland fauna including the dragonfly Libellula fulva and the rifle beetle Oulimnius major (Ramsar Information Sheet, available at http://www.jncc.gov.uk). The killer shrimp is known to inhabit preferably hard artificial substratum (Boets et al. 2010), which in the River Great Ouse catchment is available in the downstream boulder-dominated reaches. Ouse Washes are commonly used for recreation, sport fishing, transport and navigation; hence the propagule pressure in this protected area will be probably high. Consequently, taking into account the intensive human use of this wetland, its high climate suitability for the killer shrimp, the presence of artificial canals which are the preferred habitat for the species, and its direct hydrologic connection with invaded sites upstream, we can consider the Ouse Washes to be under serious risk of invasion in the short term. Additionally, zebra mussel populations have been observed in high densities at the main river channel between Bedford and King’s Lynn (Aldridge 2005).
Wicken fen is also predicted to be invaded by the killer shrimp under the intermediate and high dispersal scenarios. Wicken fen is connected to the main hydrological network through artificial canals (lodes) used for boating and fishing, which are highly vulnerable to act as introduction vectors. Moreover, according to the National Biodiversity Network’s Gateway (http://data.nbn.org.uk/), the zebra mussel is present in both Ouse Washes and Wicken fen, which might further facilitate the establishment of the killer shrimp. On the contrary, Chippenham fen is not connected to the main hydrologic network and does not provide suitable hard substrate for the shrimp, so we can consider this Ramsar site to be at low risk of invasion. According to its impact in other European countries, D. villosus is likely to threaten the native fauna through competition and predation, especially with other native shrimps such as Gammarus pulex, but also mayflies, damselflies, leeches, chironomids, cladocera, isopods, snails and fishes (Devin et al. 2001; Dick et al. 2002; Boets et al. 2010). Furthermore, D. villosus may affect also the native fisheries as it has been reported to prey on fish eggs and larvae (Platvoet et al. 2009), and experimental analyses have shown its preference for whitefish eggs and chironomid larvae over other prey (Casellato et al. 2007). Finally, several canals connect the Great Ouse catchment with the nearby River Nene catchment through its northern side, so the risk of invasion of this catchment might also be high. Certainly, construction and interlinking of waterways has played a major role in facilitating spread of invasive species across Europe (Leuven et al. 2009) as well as Great Britain (Aldridge et al. 2004), hence major efforts should be devoted to control the inter-catchment spread of D. villosus through the network of artificial canals.
Network Analysis focuses on the control of invasive species and provides a time frame of invasion especially useful for conservation and management purposes. As most environmental managers have basic notions of GIS, Network Analysis can be easily implemented into normal risk assessment protocols. In this study, we tested the potential of Network Analysis to locate areas at high risk of invasion at different time-scales, though the model offers other utilities for invasive species prevention than those explored here. For instance, different introduction and destination points can be simulated to identify strategic points of invasion that may result in a rapid or slow dispersal of the species. Popular fishing lakes or ports with intensive ship traffic can be identified as potential introduction points, while destination points of interest might include protected wetlands or human infrastructures negatively affected by an eventual invasion. Better estimates of downstream and upstream movement in a particular catchment can be estimated once the species starts dispersing, which would notably increase the reliability of Network Analysis models. Nonetheless, because dispersal is not constant and depends on numerous biotic and abiotic factors, various dispersal velocities can still be compared thereby covering a range of possible invasion scenarios. Furthermore, as organisms have different movement capacities, habitat selection and competitive abilities, the introduction of other habitat suitability factors in the model such as river discharge, substrate, water quality, barriers to flow (e.g. dams, embankments) or biotic interaction may produce even more reliable models (Wiens 2002).
Conclusions
In recent years, a combination of techniques integrating large-scale species distribution predictions and local-scale mechanistic models, also called “hybrid models”, has emerged as an optimal way of overcoming the limitations inherent to both techniques. Accordingly, the multi-scale approach proposed in this study combines large-scale bioclimatic models with local-scale dispersal models. This approach focuses on the prevention and control of invasive species and can effectively inform on species management. Ultimately, despite the inherent degree of uncertainty in the spread of invasive species, the increasing mechanistic understanding of their biology, ecology and dispersal will support an increasingly effective prevention and control.
References
Aldridge DC (2005) The zebra mussel, Dreissena polymorpha, in the Anglian region: results of river and water treatment works surveys. Cambridge Environmental Consultants, Cambridge, p 31
Aldridge DC (2010) Dreissena polymorpha in Great Britain: history of spread, impacts and control. In: van der Velde G, Rajagopal S, bij de Vaate A (eds) The Zebra Mussel in Europe. Backhuys Publishers, Leiden, pp 79–91
Aldridge DC, Elliott P, Moggridge GD (2004) The recent and rapid spread of the zebra mussel (Dreissena polymorpha) in Great Britain. Biol Conserv 119:253–261
Beaumont LJ, Gallagher RV, Thuiller W et al (2009) Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15:409–420
Bilton DT, Freeland JR, Okamura B (2001) Dispersal in freshwater invertebrates. Annu Rev Ecol Syst 32:159–181
Boets P, Lock K, Messiaen M et al (2010) Combining data-driven methods and lab studies to analyse the ecology of Dikerogammarus villosus. Ecol Inform 5:133–139
Bruijs MCM, Kelleher B, van der Velde G et al (2001) Oxygen consumption, temperature and salinity tolerance of the invasive amphipod Dikerogammarus villosus: indicators of further dispersal via ballast water transport. Arch Hydrobiol 152:633–646
Casellato S, Visentin A, La Piana G (2007) The predatory impact of Dikerogammarus villosus on fish. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution and threats. Springer, Dordrecht, pp 495–506
Chen PF, Wiley EO, Mcnyset KM (2007) Ecological niche modeling as a predictive tool: silver and bighead carps in North America. Biol Invasions 9:43–51
de Souza Muñoz M, De Giovanni R, de Siqueira M, Sutton T, Brewer P, Pereira R, Canhos D, Canhos V (2011) openModeller: a generic approach to species’ potential distribution modelling. GeoInformatica 15:111–135
Devin S, Beisel JN, Bachmann V et al (2001) Dikerogammarus villosus (Amphipoda: Gammaridae): another invasive species newly established in the Moselle river and French hydrosystems. Ann De Limnol Int J Limnol 37:21–27
Devin S, Piscart C, Beisel JN et al (2003) Ecological traits of the amphipod invader Dikerogammarus villosus on a mesohabitat scale. Arch Hydrobiol 158:43–56
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084
Drake JM, Bossenbroek JM (2004) The potential distribution of zebra mussels in the United States. Bioscience 54:931–941
Drake JM, Randin C, Guisan A (2006) Modelling ecological niches with support vector machines. J Appl Ecol 43:424–432
Elith J, Graham CH (2009) Do they? how do they? why do they differ? On finding reasons for differing performances of species distribution models. Ecography 32:66–77
Elliott JM (2002) The drift distances and time spent in the drift by freshwater shrimps, Gammarus pulex, in a small stony stream, and their implications for the interpretation of downstream dispersal. Freshw Biol 47:1403–1417
Elliott JM (2003) A comparative study of the dispersal of 10 species of stream invertebrates. Freshw Biol 48:1652–1668
Gergs R, Rothhaupt KO (2008) Feeding rates, assimilation efficiencies and growth of two amphipod species on biodeposited material from zebra mussels. Freshw Biol 53:2494–2503
Gherardi F (2007) Biological invasions in inland waters: an overview. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution, and threats. Springer, Netherlands, pp 3–25
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009
Hancock MA, Hughes JM (1999) Direct measures of instream movement in a freshwater shrimp using a genetic marker. Hydrobiologia 416:23–32
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1:29–36
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Jocque M, Field R, Brendonck L et al (2010) Climatic control of dispersal-ecological specialization trade-offs: a metacommunity process at the heart of the latitudinal diversity gradient? Global Ecol Biogeogr 19:244–252
Keller RP, Ermgassen P, Aldridge DC (2009) Vectors and timing of freshwater invasions in Great Britain. Conserv Biol 23:1526–1534
Kraft CE, Sullivan PJ, Karatayev AY et al (2002) Landscape patterns of an aquatic invader: assessing dispersal extent from spatial distributions. Ecol Appl 12:749–759
Kumar S, Spaulding SA, Stohlgren TJ et al (2009) Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front Ecol Environ 7:415–420
Leathwick JR, Elith J, Chadderton WL et al (2008) Dispersal, disturbance and the contrasting biogeographies of New Zealand’s diadromous and non-diadromous fish species. J Biogeogr 35:1481–1497
Leuven R, van der Velde G, Baijens I et al (2009) The River Rhine: a global highway for dispersal of aquatic invasive species. Biol Invasions 11:1989–2008
Loo SE, Mac Nally R, Lake PS (2007) Forecasting New Zealand mudsnail invasion range: model comparisons using native and invaded ranges. Ecol Appl 17:181–189
MacNeil C, Platvoet D, Dick JTA et al (2010) The Ponto-Caspian ‘killer shrimp’, Dikerogammarus villosus (Sowinsky, 1894), invades the British Isles. Aquat Invasions 5:441–445
Madgwick G, Aldridge DC (2011) Killer shrimps in Britain: hype or horror? British Wildlife Publishing, Dorset, UK, pp 408–412
McNyset KM (2005) Use of ecological niche modelling to predict distributions of freshwater fish species in Kansas. Ecol Freshw Fish 14:243–255
Niggebrugge K, Durance I, Watson AM et al (2007) Applying landscape ecology to conservation biology: spatially explicit analysis reveals dispersal limits on threatened wetland gastropods. Biol Conserv 139:286–296
Oreska M, Aldridge D (2011) Estimating the financial costs of freshwater invasive species in Great Britain: a standardized approach to invasive species costing. Biol Invasions 13:305–319
Peterson AT (2003) Predicting the geography of species’ invasions via ecological niche modeling. Q Rev Biol 78:419–433
Phillips SJ, Elith J (2010) POC plots: calibrating species distribution models with presence-only data. Ecology 91:2476–2484
Piscart C, Devin S, Beisel JN et al (2003) Growth-related life-history traits of an invasive gammarid species: evaluation with a Laird-Gompertz model. Can J Zool Revue Canadienne De Zoologie 81:2006–2014
Platt JC (1999) Probabilistic outputs for Support Vector Machines and comparison to regularized likelihood methods. In: Scholkopf B, Burges CJC, Smola AJ (eds) Advances in Kernel methods—support vector learning. MIT Press, Cambridge, pp 185–208
Platvoet D, Dick JTA, MacNeil C et al (2009) Invader-invader interactions in relation to environmental heterogeneity leads to zonation of two invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus. Biol Invasions 11:2085–2093
Pockl M (2009) Success of the invasive Ponto-Caspian amphipod Dikerogammarus villosus by life history traits and reproductive capacity. Biol Invasions 11:2021–2041
Pockl M, Webb BW, Sutcliffe DW (2003) Life history and reproductive capacity of Gammarus fossarum and G. roeseli (Crustacea: Amphipoda) under naturally fluctuating water temperatures: a simulation study. Freshw Biol 48:53–66
Ricciardi A, Rasmussen JB (1998) Predicting the identity and impact of future biological invaders: a priority for aquatic resource management. Can J Fish Aquat Sci 55:1759–1765
Rodda GH, Jarnevich CS, Reed RN (2011) Challenges in identifying sites climatically matched to the native ranges of animal invaders. Plos One 6(2):e14670. doi:10.1371/journal.pone.0014670
Sala OE, Chapin FS, Armesto JJ et al (2000) Biodiversity—global biodiversity scenarios for the year 2100. Science 287:1770–1774
Schick RS, Lindley ST (2007) Directed connectivity among fish populations in a riverine network. J Appl Ecol 44:1116–1126
Schölkopf B, Smola AJ, Williamson RC et al (2000) New support vector algorithms. Neural Comput 12:1207–1245
Sheader M (1996) Factors influencing egg size in the gammarid amphipod Gammarus insensibilis. Mar Biol 124:519–526
Timar L, Phaneuf DJ (2009) Modeling the human-induced spread of an aquatic invasive: the case of the zebra mussel. Ecol Econ 68:3060–3071
Urban DL, Minor ES, Treml EA et al (2009) Graph models of habitat mosaics. Ecol Lett 12:260–273
Václavík T, Meentemeyer RK (2011) Equilibrium or not? Modelling potential distribution of invasive species in different stages of invasion. Divers Distrib. doi:10.1111/j.1472-4642.2011.00854.x
van Riel MC, van der Velde G, Rajagopal S et al (2006) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58
Wiens JA (2002) Riverine landscapes: taking landscape ecology into the water. Freshw Biol 47:501–515
Wijnhoven S, van Riel MC, van der Velde G (2003) Exotic and indigenous freshwater gammarid species: physiological tolerance to water temperature in relation to ionic content of the water. Aquat Ecol 37:151–158
Acknowledgments
The research leading to these results has received funding from the European Commission (FP7/2007-2013, Marie Curie IEF program) under grant agreement no 251785. The author would like to thank C. McLaughlan for her help with the English editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallardo, B., Errea, M.P. & Aldridge, D.C. Application of bioclimatic models coupled with network analysis for risk assessment of the killer shrimp, Dikerogammarus villosus, in Great Britain. Biol Invasions 14, 1265–1278 (2012). https://doi.org/10.1007/s10530-011-0154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0154-0