Abstract
Behavioral differences between native and introduced species may contribute to the invasiveness of certain species. This includes differences at the species level, consistent variation among individuals (“personality”) and within-individual variation (e.g., behavioral plasticity). Here, we investigated swimming activity of individuals from four different amphipod species occurring in the river Rhine system, three of which were native or naturalized (>100 years present) while one is a recent invader (Dikerogammarus villosus, <25 years present). At the species level, D. villosus did not show higher average swimming activity than the three non-invasive species. However, the non-invasive species, on average, changed their behavior predictably over the course of the experiment (“average behavioral plasticity”), while D. villosus did not exhibit any consistent change in activity. At the individual level, D. villosus exhibited greater among- and within-individual variation in activity levels than all non-invasive species. The non-invasive species further showed significant individual differences in plasticity, that is, individuals of these species differed consistently in how they changed their activity over time. The high within-individual variation in D. villosus translated into a lack of consistent individual differences in plasticity in this species. We hypothesize that the highly variable and unpredictable patterns of individual activity variation in D. villosus might help this successful invader to cope with new environmental conditions encountered in the river Rhine system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are a common feature in a globalizing world (Davis 2009; Lockwood et al. 2013). However, to reach the stage of “full invasiveness” (Blackburn et al. 2011), a species has to pass successfully through several stages, including transport and introduction outside their natural distribution range, population establishment and further spread (reviewed in Chapple et al. 2012). At each stage, variation in physiological, morphological, life history, behavioral and other traits may hamper or promote invasion success (Kolar and Lodge 2001; Hayes and Barry 2008; Canestrelli et al. 2015). Hence, dispersing individuals likely represent a non-random sample of the initial population, selected on the basis of interindividual variation in multiple traits (Hayes and Barry 2008; Chapple et al. 2012; Canestrelli et al. 2015). Behavior may be especially relevant in determining how successful individuals of a given population are at invading a novel environment (Chapple et al. 2012; Sih et al. 2012; Wolf and Weissing 2012; Carere and Gherardi 2013; Canestrelli et al. 2015). Empirical studies for a range of taxa have documented differences between dispersers and residents (reviewed in Cote et al. 2010a; Canestrelli et al. 2015); for instance, dispersers tend to be, on average, more aggressive (Duckworth and Badyaev 2007) and less sociable than residents (Cote et al. 2010b).
Given the recent upsurge of research on animal personality (e.g., Magurran 1986; Sih et al. 2004, 2012; Réale et al. 2007), the question arises as to whether native and invading populations/species differ systematically in other components of behavioral variation beside mean differences between population/species (e.g., Carvalho et al. 2013; Canestrelli et al. 2015). Animal personality research emphasizes that individuals tend to differ consistently in their behavioral tendencies (among-individual variation). Among-individual behavioral variation within a subset of dispersing individuals may influence invasion success (Wright et al. 2010; Phillips and Suarez 2012), and two conflicting predictions can be formulated: (1) First, greater among-individual variation may be expected in successful invaders due to insurance effects (a.k.a. “portfolio effect,” see Schindler et al. 2010; Anderson et al. 2013), whereby the presence of different behavioral types buffers a population against harsh or novel environmental conditions encountered in the novel distribution range (see similar argumentations in Fogarty et al. 2011; Wolf and Weissing 2012). (2) Alternatively, invasive species/populations may exhibit lower among-individual variation in behavior if the population recently underwent a genetic bottleneck (Tsutsui et al. 2000, Canestrelli et al. 2015), or as a consequence of directional selection (Cote et al. 2010a).
Animal personality research also highlights the need to investigate components of within-individual variation (Fogarty et al. 2011; Dingemanse and Wolf 2013). Within-individual variation in behavior refers to any behavioral changes an individual makes over time (in behavioral experiments: with repeated testing) or in different situations (Dingemanse et al. 2010). In some cases, this variation can be considered noise in the behavioral signal, but unpredictable and changing behavior can be advantageous, for example, when avoiding predators (Dingemanse et al. 2010). A particular form of within-individual variation is behavioral plasticity, which can be defined as predictable changes in behavior over an environmental gradient (Dingemanse et al. 2010). Well-known examples are habituation (where individuals consistently decrease a given behavior over time) and sensitization (where individuals increase a given behavior; Brown 2001). High levels of within-individual variation allow individuals to quickly change their behavior in response to a novel environment (Gross et al. 2010), and so invasive populations are predicted to show greater within-individual behavioral variation than native populations (Chapple et al. 2012).
In our present study, we investigated behavioral differences at the species level, as well as patterns of among- and within-individual variation between four different amphipod species (2 natives, 1 naturalized and 1 invasive) occurring in the river Rhine system (Germany). Amphipods offer an excellent system to investigate how behavior influences invasion success (see Truhlar and Aldridge 2015), as there are several cases of invasive and native populations occurring syntopically or in close proximity, minimizing potentially confounding effects of ecological differences when comparing invasive and indigenous populations (Grabowski et al. 2007; Chen et al. 2012). One of the most successful amphipod invaders that entered the Rhine in 1994 is Dikerogammarus villosus Sowinsky, 1894 (Dick and Platvoet 2000; Bij de Vaate et al. 2002; Kinzler et al. 2009). Dikerogammarus villosus has the potential to affect several native species such as Gammarus fossarum, G. pulex and the well-established (naturalized) early invader G. roeseli (Dick and Platvoet 2000; Kinzler et al. 2009; Platvoet et al. 2009), which are the species we investigated in our present study.
A recent study investigated behavioral types of D. villosus and G. pulex in the UK, where both species are invasive, and G. pulex was found to be, on average, more active and more explorative than D. villosus (Truhlar and Aldridge 2015). Likewise, when tested in groups, more individuals that are moving were found in G. pulex compared to D. villosus (Maazouzi et al. 2011). In another study, the naturalized G. roeseli spent more time swimming freely than D. villosus and thus had an increased likelihood to fall victim to fish predation (Kley et al. 2009). However, neither have average activity levels been compared between invasive D. villosus and co-occurring native amphipods in Germany, nor have components of among- and within-individual variation been investigated in this framework.
Here, we compared the two native species G. fossarum and G. pulex, the naturalized G. roeseli and the invasive D. villosus from the Rhine system in Germany and repeatedly measured individual activity levels over the course of one week (5 days). This allowed us to quantify average behavioral differences between species, as well as among- and within-individual components of behavioral variation. We predicted that invasive D. villosus should exhibit (a) no higher overall levels of activity, but (b) greater among- and within-individual variation compared to the three other species (G. fossarum, G. pulex and G. roeseli). (c) Finally, we predicted that all species should exhibit habituation (e.g., decreases in activity with repeated testing; a form of behavioral plasticity), but again, the strongest among-individual variation in this plasticity should be seen in invasive D. villosus.
Materials and methods
Study system
The Rhine is one of the most dynamic and most heavily invaded freshwater ecosystems worldwide (Bij de Vaate et al. 2002; Leuven et al. 2009) due to man-made canals connecting Central and Eastern Europe freshwater biomes. Although canal construction started in the early eighteenth century, the Rhine-Main-Danube canal, which was completed in 1992, only recently opened invasion corridors that resulted in a massive influx of Ponto-Caspian amphipod species into Central and Western Europe (Bij de Vaate et al. 2002). They are key players in aquatic ecosystems, because their shredding activity of coarse particulate organic matter is crucial in many aquatic ecosystems, and detritus is an essential resource for maintaining diverse food webs (Wallace et al. 1997; Hunting et al. 2012; Jourdan et al. 2016). Invasive amphipods constitute up to 90 % of macroinvertebrates in terms of biomass and numerical abundance in the Rhine (Van Riel et al. 2006; Leuven et al. 2009), and so they can be considered ecosystem engineers (sensu Cassey et al. 2004) determining the functional diversity and food web structure (MacNeil et al. 2012).
Animal collection and maintenance
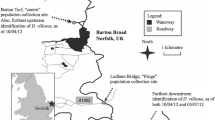
We collected amphipods between May 1, 2013 and August 28, 2013 with a “kick and sweep” technique (Barbour et al. 1999) at four nearby sites in the vicinity of Frankfurt am Main (Fig. 1) using a pond net with an opening of 25 × 25 cm, depth of 60 cm and mesh width of 500 µm (Bioform Entomology & Equipment, Germany). We identified species using the keys provided by Eggers and Martens (2001) in water-filled petri dishes under a stereomicroscope (Microscopes Nikon SMZ 1500 and Nikon AZ 100, Japan). Each sampling site was almost exclusively dominated (>90 % of collected individuals) by a single amphipod species (see Chen et al. 2012; Jourdan et al. 2016; Fig. 1). This eliminates potential effects of site-specific differences in community structures and species interactions on the amphipods’ behavior.
Sampling sites that were dominated by one of our four study species, invasive Dikerogammarus villosus (49°59′55.49″N, 8°23′8.35″E) from the city of Rüsselsheim, and indigenous Gammarus roeseli (50°10′33.68″N, 8°41′36.33″E), Gammarus pulex (50°10′7.46″N, 8°37′15.98″E) and Gammarus fossarum (50°12′58.11″N, 8°31′52.65″E) from the city of Frankfurt/Main, Germany
We transported animals in aerated, water-filled coolers to the animal care facilities at the University of Frankfurt/Main and kept them in 20-L tanks containing ADaM (Aachener Daphnien Medium; Klüttgen et al. 1994). Temperature was maintained at 10 °C throughout, and light/dark periods were adjusted to 12:12 h per day. We provided food in the form of leaf litter taken from the collection sites. Animals were maintained in these holding tanks for 5–7 days before the experiment started.
Experimental setup
To enable individual identification throughout the testing phase, amphipods were transferred into individual perforated conical Falcon tubes. Tubes were fixed within a filter sponge in 80-L tanks containing ADaM (max. 25 tubes per tank) in a way that two-third of the tube was submerged. Water quality and food provisioning was identical to their holding tanks. After a 3-day acclimation period in the tubes, we measured each individual’s activity in an open field test. We transferred individual test subjects into a glass petri dish (20 cm in diameter) filled to 3 cm height with ADaM, allowed them to acclimate for 5 min and then recorded their activity for 1 min using a webcam (Microsoft LifeCam VX-2000). A 2 × 2 cm grid on the bottom of the petri dish allowed us to count the number of squares crossed as a measure of activity. All recordings took place in a climate chamber maintaining a temperature of 10 °C throughout and minimizing disturbances of the focal animal. We measured each individual’s activity on five consecutive days and afterward sacrificed and stored amphipods individually in 70 % ethanol to determine body size and sex under a stereomicroscope. Sample sizes were N = 49 (D. villosus), N = 75 (G. roeseli), N = 107 (G. fossarum) and N = 45 (G. pulex).
Data analysis
Activity scores were square root transformed to normalize the distribution and stabilize variance; transformed scores were then centered on their grand mean to ease interpretation across species. To test our first prediction about average activity differences between all four species, we used linear mixed models (LMMs) with activity as the response variable and species as our fixed effect of interest. We also included sex, trial and body size as additional fixed effects and individual ID as a random effect to account for the multiple observations per individual.
Our second research question investigated the patterns of consistent individual variation in activity among the four species. To address this, we ran LMMs on each species separately. We included sex, size and trial as fixed effects and individual ID as a random effect. This allowed us to compare the total behavioral variance not accounted for by the fixed effects, as well as the among- and within-individual variance components across species (see Dingemanse et al. 2012). We also used these variance estimates to calculate a repeatability estimate. The repeatability of a behavior is defined as the proportion of the behavioral variance attributable to differences among individuals (Nakagawa and Schielzeth 2010). A significant repeatability estimate is interpreted as evidence of consistent individual differences. Significant differences in variance components and repeatability between the four species can be assumed when 95 % CI of the estimates do not overlap.
Our third and final research question was whether there was evidence for differences in individual behavioral plasticity over time (trials) between the four species. To test this, we fit separate LMMs for each species in which we included a random intercept and slope (across trials) for each individual and also included a covariance term between intercept and slope.
For all analyses, we used LMMs with Gaussian error distribution and Markov Chain Monte Carlo estimation, using MCMCglmm (Hadfield 2010) in R v3.0 (R_Core_Team, 2013). MCMC estimation offers a particularly powerful method for partitioning variance among random effects. MCMC also returns 95 % credibility intervals for all model effects, allowing for easy comparison of effects. For fixed effects, if the 95 % CI of an effect does not overlap zero, we interpret this as evidence for a significant effect of that factor. We additionally tested for the significance of each effect by comparing the deviance information criteria (DIC) of a model including the factor to a model without the factor. If including the effect reduces the DIC value by greater than 2, we considered this support that the effect improved the model. This was especially important to test the significance of the random effects, as variance estimates, by definition, are constrained to be non-negative. For all models, we used non-informative proper priors and 500,000 iterations, while discarding 1000 iterations as burn-in and sampling every 100 iterations. We ensured model convergence and proper mixing by running five independent chains for each model and visually inspecting the resulting autocorrelation and posterior distributions of model effects.
Results
Invasive species are not more active than non-invasive species
As predicted, the invasive D. villosus did not exhibit higher average activity levels compared to the three non-invasive species (Table 1). We found that across all four species, individuals decreased their activity across the five trials (but see single-species analyses below). We also found that larger individuals tended to be more active (the 95 % CI of “size” did not overlap zero), even though this effect was weak, as evidenced by the marginal decrease in DIC and small effect size.
Greater behavioral variation in invasive compared to non-invasive species
We found strong support for the presence of consistent individual differences (i.e., repeatability) in activity levels in all species, which accounted for at least one-third of the total behavioral variation (Table 2). In support of our second prediction, both the among- and within-individual variance components were significantly larger in the invasive D. villosus compared to any of the three non-invasive species (95 % CIs for both variance components did not overlap, Table 2), indicating that individuals of the invasive species were more different from each other and more variable in their behavior over repeated testing (Fig. 2). Although total behavioral variance was greater in D. villosus, a similar proportion of this variance was due to individual differences (i.e., repeatability) as in the non-invasive species, as 95 % CIs of the repeatability estimates all overlapped.
Population-level average activity levels (top, centered around grand mean), individual reaction norm slopes between the five repeated measures (middle, centered around grand mean) and frequency distribution of the reaction norm slopes (bottom) of invasive and native/naturalized amphipod species in the Rhine system, Dikerogammarus villosus representing the invasive species
Native and naturalized, but not invasive species exhibit individual variation in plasticity
We found that individuals in the three non-invasive species, on average, changed their behavior in the same way (average behavioral plasticity) over the course of the experiment (effect of “trial” in Table 2). However, in direct contrast to our prediction, not all three non-invasive species showed the same direction of effect: G. roeseli and G. fossarum both decreased activity with repeated trials (i.e., habituation), whereas G. pulex increased activity (i.e., sensitization). In line with our prediction, the invasive D. villosus did not exhibit any consistent effect of trial (Table 2). This does not indicate that this species is invariable in its behavior over time; rather, the lack of a significant trial effect appears to be due to the high levels of within-individual variation in activity in this species. There was no evidence for differences between the sexes or an effect of body size in any species.
We predicted that the invasive species should show stronger among-individual variation in habituation effects (behavioral plasticity) over repeated testing compared to the non-invasive species. Indeed, in the three non-invasive species, there was considerable support for the inclusion of an “individual × trial” interaction term (individual slopes), indicating the presence of individual variation in behavioral plasticity in those three species (Table 3; Fig. 2). Not unexpectedly, we also found support for negative covariance between individual slopes and intercepts in the three non-invasive species, suggesting that individuals with higher initial activity levels showed the greatest decrease in activity with repeated testing. Interestingly, we did not find support that including either an individual slope or covariance term improved the model for the invasive D. villosus (see Table 3). Again, this finding was likely driven by the high levels of within-individual variation in behavior: There was no predictable pattern in how individuals of D. villosus would behave with repeated testing.
Discussion
In congruence with our first prediction and previous research (Truhlar and Aldridge 2015), the invasive amphipod D. villosus did not show higher mean activity levels compared to the three non-invasive species. This contrasts with the results of studies on other invasive species (e.g., Cote et al. 2010b; Canestrelli et al. 2015). We argue that higher activity rates increase the likelihood of invasions only in actively dispersing species like Western mosquitofish (Gambusia affinis; Cote et al. 2010b), while D. villosus depends on passive drift (van Riel et al. 2011), or ships function as dispersal vectors (Leuven et al. 2009; see also Chen et al. 2012 for discussion). However, we found D. villosus to exhibit greater among-individual and within-individual variation in activity levels (prediction 2). The high within-individual variation in this species was striking and, in essence, swamped out any signal of consistent changes over the repeated testing (average- or individual-level plasticity). In contrast, within-individual behavioral variation was more structured in the three other species, in which individuals exhibited predictable changes in activity with repeated testing. Not all of the three species changed their behavior in the same way over time though, indicating that activity habituation may not be a universal characteristic of more established (naturalized) and native populations. The dramatic differences in the patterns of behavioral variation between native (or naturalized) and invasive species suggest that differences in within-species behavioral variation could indeed play a role in determining the invasion success of a species. This result is in congruence with the “insurance hypothesis” (see Wolf and Weissing 2012), which assumes invading species to benefit from a higher behavioral variability (see “Discussion” section below).
Modeling frameworks suggest that individual differences in plasticity may arise when the benefits of showing a plastic response depend on the frequency of individuals that show either plastic or non-plastic responses (i.e., negative frequency-dependent selection; Wolf et al. 2008; Dubois et al. 2010). Such a mechanism could explain the observed composition of reaction norms in the native or naturalized species, in which we found consistent individual differences in behavioral plasticity. The pattern for the invasive D. villosus, however, seems to follow a different logic with no detectable plasticity differences among individuals due to high and non-predictable within-individual variation in behavior. There are several (not necessarily mutually exclusive) potential explanations for this finding:
-
1.
First, some authors propose a predatory lifestyle for D. villosus (Dick and Platvoet 2000). Individuals may frequently move between microhabitats in search of prey, and selection could favor those individuals that are highly flexible in their responses to ecological gradients (Dingemanse and Wolf 2013). Moreover, predatory behavior might include alternating sit-and-wait and active search phases (Dick and Platvoet 2000), leading to highly variable activity patterns. However, native species are also known to exhibit variation in foraging behaviors, including both leaf-shredding and predatory foraging like cannibalism (Dick and Platvoet 1996), and so it seems unlikely that differences in foraging behavior are a major explanation here.
-
2.
Recent invaders could face relaxed natural selection pressures (Hayes and Barry 2008; Davis 2009; Blackburn et al. 2011), e.g., due to neophobia of their potential predators (Greenberg 1990). Thus, even otherwise maladapted individuals may survive during the initial stages of a biological invasion, leading to higher behavioral variation in invasive populations. Likewise, it was shown that the Ponto-Caspian racer goby (Babka gymnotrachelus) preferred G. fossarum over D. villosus as prey in Poland where B. gymnotrachelus is invasive (Błońska et al. 2015). Using immobilized amphipods, the authors demonstrated that this is not due to differences in behavior but to a harder exoskeleton of D. villosus. If D. villosus is indeed less palatable, it might be able to express more variable swimming behavior while still being protected from predation. High predation rates on D. villosus by the highly abundant invasive piscine predator Neogobius melanostamus (Emde et al. 2014), however, render this scenario unlikely. Also, native fish species like European eel (Anguilla anguilla) and perch (Perca fluviatilis) are known to readily include D. villosus in their diets when available (Eckmann et al. 2008).
-
3.
Piscine predation could rather play an active role in generating the greater and more unpredictable behavioral variation found in D. villosus. The now very common invasive round goby (N. melanostomus) was found to predominantly feed on D. villosus in the Rhine, ignoring the locally much more abundant amphipod Echinogammarus trichiatus that invaded the Rhine even more recently (Emde et al. 2014). The observed unpredictable activity levels in the invasive D. villosus—with alternating periods of high activity and resting/hiding—may in fact be an antipredator response, precluding learning about their prey’s activity patterns by N. melanostomus.
-
4.
Truhlar and Aldridge (2015) proposed differences in parasite loads as one factor that might affect individual differences in amphipod behavior. For example, parasites are known to affect photophobic behavior in G. pulex (Bethel and Holmes 1973), with infected individuals showing a weaker photonegative response (see also Perrot-Minnot et al. 2012). Furthermore, the influence of some parasites on their host’s behavior seems to be species-specific (Bauer et al. 2000). However, measuring parasitization rates was beyond the scope of our current study, and we strongly advise inclusion of those measurements in future studies.
Interestingly, average activity levels changed predictably over time in the native and naturalized species, albeit not into the same direction. Even though we are lacking a compelling explanation for the observed differences, predictability seems to be a feature of populations that have been established for long enough in a certain habitat. This view is underpinned by the study of Truhlar and Aldridge (2015) who found no repeatable behavior in G. pulex and D. villosus collected in Great Britain, where both species are invasive. Possibly, colonization of new habitats leads to an initial loss of predictable individual differences in behavior. Future studies should pursue this topic further and should also ask whether ecological or life history features (Bollache et al. 2006; Grabowski et al. 2007) might explain the direction of the observed average behavioral plasticity.
In conclusion, our results indicate pronounced differences in patterns of individual behavioral variation between non-native and invasive amphipod species from river Rhine and support hypotheses that predict invading species to benefit from high levels of among- and between-individual variation (e.g., “insurance hypothesis” sensu Wolf and Weissing 2012). As our current study presents data from only one population per species, we encourage sampling populations along the entire distribution range of D. villosus, that is, native and invasive populations, including invasive populations that are at different stages of the invasion process [see Truhlar and Aldridge (2015) for a similar approach on G. pulex and D. villosus in Great Britain]. Such an approach will allow determining whether differences between non-invasive and invasive species as reported here might be washed out over time through natural selection.
References
Anderson SC, Cooper AB, Dulvy NK (2013) Ecological prophets: quantifying metapopulation portfolio effects. Methods Ecol Evol 4:971–981
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, 2nd edn. U.S. Environmental Protection Agency, Washington
Bauer A, Trouvé S, Grégoire A, Lc Bollache, Cézilly F (2000) Differential influence of Pomphorhynchus laevis (Acanthocephala) on the behaviour of native and invader gammarid species. Int J Parasitol 30:1453–1457
Bethel WM, Holmes JC (1973) Altered evasive behaviour and responses to light in amphipods harboring acanthocephalan cystacanths. J Parasitol 59:945–956
Bij de Vaate A, Jazdzewski K, Ketelaars HAM, Gollasch S, Van der Velde G (2002) Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59:1159–1174. doi:10.1139/f02-098
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, Wilson JRU, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339. doi:10.1016/j.tree.2011.03.023
Błońska D, Grabowska J, Kobak J, Jermacz Ł, Bącela-Spychalska K (2015) Feeding preferences of an invasive Ponto-Caspian goby for native and non-native gammarid prey. Freshw Biol 60:2187–2195. doi:10.1111/fwb.12647
Bollache L, Kaldonski N, Troussard J-P, Lagrue C, Rigaud T (2006) Spines and behaviour as defences against fish predators in an invasive freshwater amphipod. Anim Behav 72:627–633. doi:10.1016/j.anbehav.2005.11.020
Brown C (2001) Familiarity with the test environment improves escape responses in the crimson spotted rainbowfish, Melanotaenia duboulayi. Anim Cogn 4:109–113. doi:10.1007/s100710100105
Canestrelli D, Bisconti R, Carere C (2015) Bolder takes all? The behavioral dimension of biogeography. Trends Ecol Evol 31:35–43. doi:10.1016/j.tree.2015.11.004
Carere C, Gherardi F (2013) Animal personalities matter for biological invasions. Trends Ecol Evol 28:5–6. doi:10.1016/j.tree.2012.10.006
Carvalho CF, Leitão AV, Funghi C, Batalha HR, Reis S, Mota PG, Lopes RJ, Cardoso GC (2013) Personality traits are related to ecology across a biological invasion. Behav Ecol 24:1081–1091. doi:10.1093/beheco/art034
Cassey P, Blackburn TM, Jones KE, Lockwood JL (2004) Mistakes in the analysis of exotic species establishment: source pool designation and correlates of introduction success among parrots (Aves: Psittaciformes) of the world. J Biogeogr 31:277–284. doi:10.1046/j.0305-0270.2003.00979.x
Chapple DG, Simmonds SM, Wong BBM (2012) Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64. doi:10.1016/j.tree.2011.09.010
Chen W, Bierbach D, Plath M, Streit B, Klaus S (2012) Distribution of amphipod communities in the Middle to Upper Rhine and five of its tributaries. BioInvasion Rec 1:263–271
Cote J, Clobert J, Brodin T, Fogarty S, Sih A (2010a) Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos Trans R Soc B 365:4065–4076. doi:10.1098/rstb.2010.0176
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010b) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc R Soc B 277:1571–1579. doi:10.1098/rspb.2009.2128
Davis MA (2009) Invasion biology. Oxford University Press, Oxford
Dick J, Platvoet D (1996) Intraguild predation and species exclusions in amphipods: the interaction of behaviour, physiology and environment. Freshw Biol 36:375–383. doi:10.1046/j.1365-2427.1996.00106.x
Dick JTA, Platvoet D (2000) Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc R Soc B 267:977–983. doi:10.1098/rspb.2000.1099
Dingemanse NJ, Wolf M (2013) Between-individual differences in behavioural plasticity within populations: causes and consequences. Anim Behav 85:1031–1039. doi:10.1016/j.anbehav.2012.12.032
Dingemanse NJ, Kazem AJN, Réale D, Wright J (2010) Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol 25:81–89. doi:10.1016/j.tree.2009.07.013
Dingemanse NJ, Bouwman KM, van de Pol M, van Overveld T, Patrick SC, Matthysen E, Quinn JL (2012) Variation in personality and behavioural plasticity across four populations of the great tit Parus major. J Anim Ecol 81:116–126. doi:10.1111/j.1365-2656.2011.01877.x
Dubois F, Morand-Ferron J, Giraldeau L-A (2010) Learning in a game context: strategy choice by some keeps learning from evolving in others. Proc R Soc B 277:3609–3616. doi:10.1098/rspb.2010.0857
Duckworth RA, Badyaev AV (2007) Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc Natl Acad Sci USA 104:15017–15022. doi:10.1073/pnas.0706174104
Eckmann R, Mörtl M, Baumgärtner D, Berron C, Fischer P, Schleuter D, Weber A (2008) Consumption of amphipods by littoral fish after the replacement of native Gammarus roeseli by invasive Dikerogammarus villosus in Lake Constance. Aquat Invasion 3:184–188. doi:10.3391/ai.2008.3.2.9
Eggers T, Martens A (2001) Bestimmungsschlüssel der Süßwasser-Amphipoda (Crustacea) Deutschlands. Lauterbornia 42:1–68
Emde S, Kochmann J, Kuhn T, Plath M, Klimpel S (2014) Getting what is served? Feeding ecology influencing parasite–host interactions in invasive round goby Neogobius melanostomus. PLoS ONE 9:e109971. doi:10.1371/journal.pone.0109971
Fogarty S, Cote J, Sih A (2011) Social personality polymorphism and the spread of invasive species: a model. Am Nat 177:273–287
Grabowski M, Bacela K, Konopacka A (2007) How to be an invasive gammarid (Amphipoda: Gammaroidea)—comparison of life history traits. Hydrobiologia 590:75–84. doi:10.1007/s10750-007-0759-6
Greenberg R (1990) Feeding neophobia and ecological plasticity: a test of the hypothesis with captive sparrows. Anim Behav 39:375–379. doi:10.1016/S0003-3472(05)80884-X
Gross K, Pasinelli G, Kunc HP (2010) Behavioral plasticity allows short-term adjustment to a novel environment. Am Nat 176:456–464
Hadfield JD (2010) MCMC methods for multi-response generalised linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22
Hayes K, Barry S (2008) Are there any consistent predictors of invasion success? Biol Invasion 10:483–506. doi:10.1007/s10530-007-9146-5
Hunting ER, Whatley MH, Geest HGvd, Mulder C, Kraak MHS, Anton MB, Admiraal W (2012) Invertebrate footprints on detritus processing, bacterial community structure, and spatiotemporal redox profiles. Freshwat Sci 31:724–732. doi:10.1899/11-134.1
Jourdan J, Westerwald B, Kiechle A, Chen W, Streit B, Klaus S, Oetken M, Plath M (2016) Pronounced species turnover, but no functional equivalence in leaf consumption by invasive amphipods in the river Rhine. Biol Invasion 18:763–774. doi:10.1007/s10530-015-1046-5
Kinzler W, Kley A, Mayer G, Waloszek D, Maier G (2009) Mutual predation between and cannibalism within several freshwater gammarids: Dikerogammarus villosus versus one native and three invasives. Aquat Ecol 43:457–464. doi:10.1007/s10452-008-9206-7
Kley A, Kinzler W, Schank Y, Mayer G, Waloszek D, Maier G (2009) Influence of substrate preference and complexity on co-existence of two non-native gammarideans (Crustacea: Amphipoda). Aquat Ecol 43:1047–1059. doi:10.1007/s10452-009-9242-y
Klüttgen B, Dülmer U, Engels M, Ratte H (1994) ADaM, an artificial freshwater for the culture of zooplankton. Water Res 28:743–746
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. doi:10.1016/S0169-5347(01)02101-2
Leuven REW, van der Velde G, Baijens I, Snijders J, van der Zwart C, Lenders HJR, Bij de Vaate A (2009) The river Rhine: a global highway for dispersal of aquatic invasive species. Biol Invasion 11:1989–2008. doi:10.1007/s10530-009-9491-7
Lockwood JL, Hoopes MF, Marchetti MP (2013) Invasion ecology. Wiley, New York
Maazouzi C, Piscart C, Legier F, Hervant F (2011) Ecophysiological responses to temperature of the “killer shrimp” Dikerogammarus villosus: is the invader really stronger than the native Gammarus pulex? Comp Biochem Phys A 159:268–274. doi:10.1016/j.cbpa.2011.03.019
MacNeil C, Boets P, Platvoet D (2012) ‘Killer Shrimps’, dangerous experiments and misguided introductions: how freshwater shrimp (Crustacea: Amphipoda) invasions threaten biological water quality monitoring in the British Isles. Freshw Rev 5:21–35. doi:10.1608/frj-5.1.457
Magurran AE (1986) Individual differences in fish behaviour. In: Pitcher TJ (ed) The behaviour of teleost fishes. Croom Helm, London, pp 338–365
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. doi:10.1111/j.1469-185X.2010.00141.x
Perrot-Minnot M-J, Maddaleno M, Balourdet A, Cézilly F (2012) Host manipulation revisited: no evidence for a causal link between altered photophobia and increased trophic transmission of amphipods infected with acanthocephalans. Funct Ecol 26:1007–1014. doi:10.1111/j.1365-2435.2012.02027.x
Phillips BL, Suarez A (2012) The role of behavioural variation in the invasion of new areas. In: Candolin U, Wong BBM (eds) Behavioural responses to a changing world: mechanisms and consequences. Oxford University Press, Oxford
Platvoet D, Dick JTA, MacNeil C, van Reil M, van der Velde G (2009) Invader-invader interactions in relation to environmental heterogeneity leads to zonation of two invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus Sexton: amphipod pilot species project (AMPIS) report 6. Biol Invas 11:2085
R_Core_Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. doi:10.1111/j.1469-185X.2007.00010.x
Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS (2010) Population diversity and the portfolio effect in an exploited species. Nature 465:609–612
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. doi:10.1016/j.tree.2004.04.009
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289. doi:10.1111/j.1461-0248.2011.01731.x
Truhlar A, Aldridge D (2015) Differences in behavioural traits between two potentially invasive amphipods, Dikerogammarus villosus and Gammarus pulex. Biol Invasion 17:1569–1579. doi:10.1007/s10530-014-0816-9
Tsutsui ND, Suarez AV, Holway DA, Case TJ (2000) Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci USA 97:5948–5953. doi:10.1073/pnas.100110397
van Riel MC, van der Velde G, Rajagopal S, Marguillier S, Dehairs F, Bij de Vaate A (2006) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. In: Leuven RSEW, Ragas AMJ, Smits AJM, van der Velde G (eds) Living rivers: trends and challenges in science and management. Springer, Netherlands, pp 39–58. doi:10.1007/1-4020-5367-3_3
van Riel MC, van der Velde G, Bij de Vaate A (2011) Dispersal of invasive species by drifting. Curr Zool 57:818–827
Wallace JB, Eggert SL, Meyer JL, Webster JR (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104. doi:10.1126/science.277.5322.102
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–461
Wolf M, van Doorn GS, Weissing FJ (2008) Evolutionary emergence of responsive and unresponsive personalities. Proc Natl Acad Sci USA 105:15825–15830. doi:10.1073/pnas.0805473105
Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA (2010) Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 22:393–404. doi:10.1080/03949370.2010.505580
Acknowledgments
We like to thank H. Geupel and E.-M. Wörner for help with animal care as well as all participants of the 2013 student class “Experimental Ecology” for their help in the field and with data collection. We received financial support by the Biodiversity and Climate Research Centre (BiK-F), Frankfurt a.M., the research funding program “LOEWE – Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of the Hessian Ministry of Higher Education, Research, and the Arts as well as by the Leibniz Competition (SAW-2013-IGB-2) and by the DFG (LA 3778/1-1). The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Rights and permissions
About this article
Cite this article
Bierbach, D., Laskowski, K.L., Brandt, AL. et al. Highly variable, unpredictable activity patterns in invasive, but not native amphipod species. Aquat Ecol 50, 261–271 (2016). https://doi.org/10.1007/s10452-016-9573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-016-9573-4