Abstract
In this study, two different concentrations of fluorescent dye Rhodamine 6G (R6G) with two different strategies were used in a double layer flat panel photobioreactor (PBR) for investigation of its effect on growth parameters of microalgae Chlorella sp. Results showed that in the first strategy, when the light passed through the dye before reaching the broth, biomass productivity rate (P) and maximum specific growth rate (µmax) relative to control case, were increased up to 60 and 23% respectively. Increasing in these parameters were more, for the low dye concentration. Also, in the second strategy, when the light passed through the microalgae before reaching the dye solution, P and µmax for lower concentration increased about 9 and 15%, respectively. But using high dye concentration growth of the algae was decreased. Furthermore, using R6G caused increase in the lipid and chlorophyll content of the microalgae Chlorella sp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are potential sources of high-value chemical substances such as β-carotene, astaxanthin, and phycobilin pigments and have been considered as attractive sources, for production of biofuels and chemicals, due to the high efficiency of photosynthesis (PE). Photosynthesis is an important and vital phenomenon for the life cycle of plants and photosynthetic microorganisms. These creatures receive the potential energy of the sun and consume carbon dioxide as their energy sources and produce substances like carbohydrates. The produced carbohydrate is known as a factor in the life and growth of these microorganisms (Borowitzka 2013; de Mooij et al. 2016; Gharabaghi et al. 2015; Liu et al. 2016; Matthijs et al. 1996).
Increasing the productivity of microalgae biomass is necessary in order to achieve cost-effective production. Many efforts have been made in this regard, and extensive research has been carried out, including the design and construction of photobioreactors (PBRS) with optimal microalgae cultivation conditions (Ooms et al. 2016), as well as the use of spectrum converters, in the manufacture of PBRs (Delavari Amrei et al. 2014a, b ; Delavari Amrei and Ranjbar 2018; Delavari Amrei et al. 2015; Delavari Amrei et al. 2014a, b; Mohsenpour and Willoughby 2016; Wondraczek et al. 2013).
The growth of phototrophic microorganisms is highly dependent on various light parameters such as the intensity and wavelength of the light and the dark cycle of light (Khoobkar et al. 2019; Seo et al. 2014). Sunlight has a wide range of wavelengths that most of these wavelengths are unusable for microalgae, and so these wavelengths are wasted. The wavelengths of blue light (400–500 nm) and the wavelengths of red light (600–700 nm) are generally used by microalgae (Matthijs et al. 1996; Seo et al. 2014).
Spectral conversion of light causes more range of solar radiation used for photosynthesis. Different organic and inorganic colors are used to convert the unusable light spectra, into the light with a higher potential for photosynthesis.
Jang et al. (2018) cultivated Chlorella vulgaris and performed two different spectral correction strategies, where in the first strategy, the synthesized colors were in a separate layer and the light reached the culture medium containing microalgae after passing through this layer. In the second strategy, the synthesized colors were introduced into the microalgae culture chamber. The colors were dissolved in the two solvents of water and ethanol. In the first strategy, the lipid and biomass production were increased 13 and 31.65%, respectively, and in the second strategy, the lipid efficiency increased 17.5%, and the productivity of the produced biomass were increased 37.66% as compared to the control sample. In another study by Shin et al. (2018), the effect of using a light filter, based on the addition of different colors and their use as a layer of change in the spectrum were investigated. Biomass productivity and fatty acid methyl ester of Nannochloropsis gaditana increased by 44.6 and 22.6%, respectively, compared to the control sample.
Some researchers investigated the growth parameters of microalgae under modified light spectra by coating on a wall of PBRs. Wondraczek et al. (2013) used the spectral converter Sr0.4Ca0.59Eu0.01s in a plane plate PBR filled with Haematococcus pluvialis microalgae to modify the spectrum. The converter was coated on a mirror and the mirror placed behind the reactor. Increasing cell production in this PBR was found to be 36% higher than flat-plate PBR without spectral converter. Delavari Amrei et al. (2014b) investigated the spectral shifting of UV–A to the photosynthetic active region (PAR) using polycarbonate and polymethyl methacrylate (PMMA) sheets coated with Uvitex OB (optical brightener) as a spectral converter for increasing the growth rate of microalgae Chlorella sp. The researchers reported that, using the Uvitex OB increased biomass productivity rate up to 74% compared to control sample. Also, removal of UV–A rays, led to an increase in chlorophyll a cell content. A previous study by these researchers showed that, the use of Uvitex OB fluorescence (to convert UV–A light to blue light) on the wall of a culture flask, increased the biomass productivity rate of Synechococcus sp. up to 38% (Delavari Amrei et al. 2014a). Also, Delavari Amrei and Ranjbar (2018) reported that the fluorescent color of Rhodamine 6G as a fluorescent coating, increased the biomass productivity rate of the microalgae Chlorella sp. up to 50%.
Given that the use of aqueous solution form of dyes have a better quantum yield (Zou et al. 2008), thus in this study, aqueous solution of commercial fluorescent dye Rhodamine 6G (R6G) with a high photostability was used, as spectral converter in a double-layer flat panel PBR for conversion of green light to longer wavelengths. The pigment solution was used at two different concentrations and with two different strategies. In the first strategy, light passes through R6G solution chamber then hit and after encountering microalgae cell culture and a mirror embedded behind the reactor, returns to the medium. In the second strategy, light passes through the microalgae, to the dye-containing compartment and then reflects on the mirror after encountering the mirror behind the reactor. Also, in this work chlorophyll and lipid content of different samples were measured.
Materials and methods
Microalgae and culture medium
The microorganism Chlorella sp. (PTCC 6010, Persian type culture collection) was used as a probe for assessment of wavelength shifting. This microalgae was provided by Iranian Research Organization for Science and Technology (IROST). The microorganism was pre-cultivated in Rudic’s culture medium (Delavari Amrei and Ranjbar 2018). For this purpose, 1800 mL of inorganic medium was inoculated with 2 mL of strain solution. The medium contained (in mg L−1) 300 NaNO3, 20 KH2PO4, 80 K2HPO4, 20 NaCl, 47 CaCl2, 10 MgSO4 ·7H2O, 0.1 ZnSO4·7H2O, 1.5 MnSO4·H2O, 0.08 CuSO4·5H2O, 0.3 H3BO3, 0.3 (NH4)·6Mo7O24·4H2O, 17 FeCl3·6H2O, 0.2 Co(NO3)2·H2O, and 7.5 EDTA. The Erlenmeyers were sealed and the mixture was aerated by a circulation pump. All chemicals used in this research were purchased from Merck Co. (Germany) with purity between 98 and 99.5% and the culture medium was autoclaved to be sterilized.
Fluorescent dye
Rhodamine 6G (R6G, 99% Sigma-Aldrich) used in this study as spectral converter, is a fluorescent dye with high photostability (Brackmann 1986; Prokop et al. 1984). For this purpose, two different concentration of 0.05 and 0.025 gr L−1 of the dye was prepared and poured in a layer of the reactor.
Experimental set-up
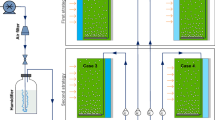
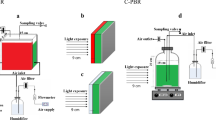
In this study, a double-layer flat panel PBR was made. The thin layer (thin chamber, width 1 cm) was filled with the dye solution and in other one (large chamber, width 4 cm) microalgae was cultivated. Also, a mirror was placed in the rear side of this PBR. Additionally, a PBR with the same design was setup as a control case filled up with distilled water in thin layer as dye solution. The light photon intensity of a white LEDs (NAMA LIGHT, Iran) at surface of each reactors were 490 μmol photons m−2 s−1. Figure 1 shows different cases of this experiment; case 1: high concentration dye in the first layer and broth in the second layer, case 2: low concentration dye in the first layer and broth in second layer, case 3: broth in the first layer, high concentration dye in the second layer, case 4: broth in the first layer, low concentration dye in the second layer.
Measurement of growth parameters
3 mL of the microalgae was analyzed at each time to determine the optical density (OD) as a parameter of the growth rate of the microalgae. Absorbance measurement of the broth was at 560 nm in a double beam UV–Vis spectrophotometer (Philler Scientific, SU6100). Biomass dry weight was measured according to previous study of Delavari Amrei et al. (2014a, b). The relationship between the biomass concentration (X, mg L−1) or dry weight and OD at 560 nm is as follows (Delavari Amrei et al. 2014a, b):
The specific growth rate of the broth was calculated using Eq. (2):
where μ is the specific growth rate (day−1) and Xt and X0 are the biomass concentration at time t and at the beginning, respectively. Also, the biomass productivity rate (P, mg L−1 day−1) was also estimated by Eq. (3):
where Xf is biomass concentration at the end of experiment (tf).
In order to obtain the chlorophyll pigment concentration, the cells were collected in 2 mL centrifuge tubes. The pigments were extracted with 2 mL ethanol (98%) overnight in an ice bath. Then the mixture was centrifuged at 2500×g for 10 min to sediment the cell debris. The Supernatant liquid was used for the determination of the absorption curve of the pigments using the UV–Vis spectrophotometer (Li et al. 2008). Finally, the amount of chlorophyll a and b content (mg mL−1) was calculated according to following equations (Wellburn 1994):
Furthermore, total lipid content was measured and calculated using modified Bligh and Dyer (1959) by using chloroform–methanol as solvent.
Absorption and emission spectra
Absorption spectra of the dye solution was determined using the double beam UV–Vis spectrophotometer (Philler Scientific, SU6100) with air as reference. Emission spectra of the light source and dye solution were determined by a spectrometer (Lasertech, England). The light intensity was set at 490 µmol photon m−2 s−1 and was measured by a quantameter (QUA SKP215 SKYE Instruments Ltd, UK).
Statistical analyses
All experiments in this study were carried out in triplicate, in two different runs. One-way analysis of variance (ANOVA) and Bonferroni analysis were performed using (SPSS, V25) package for social Science software. P values < 0.05 were considered as significant difference.
Results
Absorption and emission spectra
Absorption and emission spectra of the R6G solution (B and C) and emission spectrum of LED lamp (A) are presented in Fig. 2. As can be seen in this figure, emission spectrum of R6G solution (C) has a peak at 615 nm in the region of orange light spectrum. The R6G have shown two absorption peaks at 497 and 534 nm in green light spectrum. Also, light source showed a sharp peak at about 440 nm in blue light region and a broad peak at about 568 nm in green light.
Algal growth parameters and lipid content
Time course of cell density is shown in Fig. 3 and the growth parameters of this microalgae is presented in Table 1. Also, the statistical analysis of biomass concentration is presented in Table 2. The biomass production rate in the case 2 was the highest one. In fact, using R6G solution with lower concentration in the first layer (front side) of the reactor after 14 days of the cultivation increased P about 60% compared to control sample. The biomass production profiles of all the cases were almost similar to each other in the first 4 days of the cultivation, but after that, the biomass production occurred for case 2 and case 1 showed a meaningful increase in comparison with the other cases. The highest and lowest value of μmax and P was occurred for case 2 and case 3, respectively. In fact, the algal growth in case 3 was even lower than control one.
Total chlorophyll content \(C = C_{a} + C_{b}\) of different cases are shown in Fig. 4. Also, the statistical analysis of C is presented in Table 3. As shown in Fig. 4, total chlorophyll content for control case was the lowest one. In fact, spectral modification using R6G has increased C content of Chlorella sp. Also, the highest value of C was occurred for case 3. A closer look at data shows that using R6G solution in second layer of reactor (second strategy) causes more increase in C. Furthermore, a higher concentration of the solution has more effect. A higher dye concentration has more effect on C production.
Total lipid content of different cases are presented in Fig. 5. The results showed that the highest lipid accumulation was in case 3, in the 1st week of cultivation. Anyway, in the 2nd week of cultivation, the lipid accumulation noticeably decreased for case 3. At the end of cultivation, the lipid accumulation in case 1, was the highest, relative to the other cases. Therefore, it seems that using R6G caused increase in lipid content of Chlorella sp.
Discussion
Microalgae aren’t capable of absorbing all of the light wavelengths. In fact, they are only accepting photosynthetically active radiation (PAR). The orange–red light region (600–700 nm), is the most effective light energies, for the growth of microalgae (Seo et al. 2014). Rhodamine 6G solution, as spectral converter, is capable to shift the light wavelengths from 400 to 500 nm to the most effective region for photosynthesis (600–700 nm) and make them as most useful energy for the growth of microalgae.
In fact, fluorescent dye R6G is capable of absorbing the green and emitting the red light wavelengths. The emitting peak of this dye solution, was in the red region (Fig. 2, spectrum C). The converted spectrum by R6G is more suitable for the microalgae growth. That is why, the three cases used in these study, caused the increase of biomass productivity and specific growth rate. In the second strategy light hits the pigment solution after passing through the broth therefore its intensity is reduced and, when hit the solution fewer fluorescent molecules are excited. In fact, this phenomenon causes decrease in algal growth for the second strategy.
Delavari Amrei and Ranjbar (2018) used R6G to coat rear and front side of a panel PBR. Coating front side of the reactor caused decrease in the growth of microalgae Chlorella sp. In fact, this coating showed that filtering of a suitable wavelengths for photosynthesis. Also, coating the rear side of the reactor had increased P up to 50%. But in the present study using R6G as a solution in front side (first layer) and rear side (second layer) has increased P in the reactor up to 60% and 9%, respectively. The fluorescent dyes used in the solution form relative to the other type like layer coating technique on the reactor surfaces has got higher quantum efficiency (Zou et al. 2008).
Seo et al. (2014) studied the performance of the fluorescent red, yellow, green and blue dyes solutions in a double layer PBR. The effect of the converted light by these dye solutions on C. vulgaris showed that, maximum biomass production for the red and blue light were 1.7 and 1.45 g/L, respectively and for both, the green and yellow dyes were 1 g/L. The peak of emission of the red dye was closer to R6G. In another work Jang et al. (2018) synthesized the fluorescent dyes and used that as light convertor solution. They reported that the growth rate of the C. vulgaris had improved. Although they synthesized a new dye but the dye is yet available commercially. Generally, in the two previous studies researchers did not use the dye solutions in the second layer or rear side of reactors.
Statistical analyses of the present study showed that, a significant difference in chlorophyll content in all cases. Results showed that the maximum chlorophyll content, in case 1 was the highest. It seems that, the use of concentrated dye in the 1st week, having low amount of biomass, has got positive effect, on cellular chlorophyll content, but gradually as the biomass increases, the light reaching the microalgae cells decreases. Thus the chlorophyll content, in the 2nd week decreased. In any way, the chlorophyll content of the control sample, was lowest relative to all other cases.
Cheirsilp and Torpee (2012) reported that, chlorophyll content in photoautotrophic condition, relative to heterotrophic and mixotrophic was increased. Also, they reported that, in heterotrophic condition, in which glucose was used as a carbon source needed in culture medium, the color of cells during the cultivation time changed, from green to yellow, which is the indication of decrease in chlorophyll content. Therefore, it can be concluded that chlorophyll content, is highly depended on light and the exact amount of light causes, increase in chlorophyll content. Microalgae are only absorbing PAR, which is due to the presence of inter cellular chlorophyll, like chlorophyll a and b, which absorbs light in the region of orange–red, but it is not able to absorb green light well, therefore, increase of inter cellular chlorophyll pigments are due to increase in red light.
In case of lipid content recent studies showed that, the blue light has got more ability, for the lipid accumulation, than the red light. Among this studies, it can be referred to Mohsenpour and Willoughby (2016) studies. They reported that, in blue converter dye with 15% CO2 aeration, the amount of lipid was 36%, and the lowest lipid amount was, in red and orange converter dyes. Shu et al. (2012) reported the effect of light quantity, on the lipid accumulation in Chlorella sp. that, the blue light had got better effects relative to the red light, on lipid accumulation. Teo et al. (2014) reported that, microalgae cultivation under the blue light irradiation, had highest lipid content. Also, Yoshioka et al. (2012) found that, Isochrysis galbana under the blue LED light, produced highest lipid content and in other research done by Cheirsilp and Torpee (2012) under the mixotrophic culture with glucose as a carbon source, resulted in higher lipid content, relative to photoautotrophic and heterotrophic conditions. Seo et al. (2014) reported, the maximum lipid content was 30% of the biomass in blue light PBR.
In conclusion, using the fluorescent dye R6G as a spectral converter caused an increase in the growth rate and lipid content of microalgae Chlorella sp. The results have been shown clearly that the decrease in dye concentration caused a pronounced increase in the biomass production of Chlorella sp. Also, the first strategy, in which the light passes through dye solution before irradiating the broth, has got a better effects in the growth and biomass production rather than in the second strategy. Furthermore, using R6G in both strategies increases lipid content of the microalgae.
References
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917. https://doi.org/10.1139/y59-099
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756. https://doi.org/10.1007/s10811-013-9983-9
Brackmann U (1986) Lambdachrome laser dyes. Lambda Physik, Goettingen
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516. https://doi.org/10.1016/j.biortech.2012.01.125
de Mooij T, de Vries G, Latsos C, Wijffels RH, Janssen M (2016) Impact of light color on photobioreactor productivity. Algal Res 15:32–42. https://doi.org/10.1016/j.algal.2016.01.015
Delavari Amrei H, Ranjbar R (2018) Influence of fluorescent coating at rear and front side of a flat panel photobioreactor on algal growth. J Appl Phycol 30:901–907. https://doi.org/10.1007/s10811-017-1303-3
Delavari Amrei H, Nasernejad B, Ranjbar R, Rastegar S (2014a) Spectral shifting of UV–A wavelengths to blue light for enhancing growth rate of cyanobacteria. J Appl Phycol 26:1493–1500. https://doi.org/10.1007/s10811-013-0187-0
Delavari Amrei H, Nasernejad B, Ranjbar R, Rastegar S (2014b) An integrated wavelength-shifting strategy for enhancement of microalgal growth rate in PMMA-and polycarbonate-based photobioreactors. Eur J Phycol 49:324–331. https://doi.org/10.1080/09670262.2014.919030
Delavari Amrei H, Ranjbar R, Rastegar S, Nasernejad B, Nejadebrahim A (2015) Using fluorescent material for enhancing microalgae growth rate in photobioreactors. J Appl Phycol 27:67–74. https://doi.org/10.1007/s10811-014-0305-7
Gharabaghi M, Delavari Amrei H, Zenooz AM, Guzullo JS, Ashtiani FZ (2015) Biofuels: bioethanol, biodiesel, biogas, biohydrogen from plants and microalgae. In: Lichtfouse E, Schwarzbauer J, Robert D (eds) CO2 sequestration biofuels and depollution. Springer, Cham, pp 233–274
Jang H, Namgoong J, Sung M-G, Chang Y, Kim J (2018) Synthesis and characterization of fluorescent dyes and their applications for the enhancement of growth rate of Chlorella vulgaris. Dyes Pigment 158:142–150. https://doi.org/10.1016/j.dyepig.2018.05.003
Khoobkar Z, Shariati FP, Safekordi AA, Delavari Amrei H (2019) Performance assessment of a novel pyramid photobioreactor for cultivation of microalgae using external and internal light sources. Food Technol Biotechnol 57(1):68–76. https://doi.org/10.17113/ftb.57.01.19.5702
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636. https://doi.org/10.1007/s00253-008-1681-1
Liu W, Pearce CM, Mckinley RS, Forster IP (2016) Nutritional value of selected species of microalgae for larvae and early post-set juveniles of the Pacific geoduck clam, Panopea generosa. Aquaculture 452:326–341. https://doi.org/10.1016/j.aquaculture.2015.10.019
Matthijs HC, Balke H, Van Hes UM, Kroon BM, Mur LR, Binot RA (1996) Application of light-emitting diodes in bioreactors: Flashing light effects and energy economy in algal culture (Chlorella pyrenoidosa). Biotechnol Bioeng 50:98–107. https://doi.org/10.1002/(SICI)1097-0290(19960405)50:1%3c98:AID-BIT11%3e3.0.CO;2-3
Mohsenpour SF, Willoughby N (2016) Effect of CO2 aeration on cultivation of microalgae in luminescent photobioreactors. Biomass Bioenergy 85:168–177. https://doi.org/10.1016/j.biombioe.2015.12.002
Ooms MD, Dinh CT, Sargent EH, Sinton D (2016) Photon management for augmented photosynthesis. Nat Commun 7:12699. https://doi.org/10.1038/ncomms12699
Prokop A, Quinn M, Fekri M, Murad M, Ahmed S (1984) Spectral shifting by dyes to enhance algae growth. Biotechnol Bioeng 26:1313–1322. https://doi.org/10.1002/bit.260261108
Seo YH, Cho C, Lee J-Y, Han J-I (2014) Enhancement of growth and lipid production from microalgae using fluorescent paint under the solar radiation. Bioresour Technol 173:193–197. https://doi.org/10.1016/j.biortech.2014.09.012
Shin W-S, Jung SM, Cho C-H, Woo D-W, Kim W, Kwon J-H (2018) Use of tar color additives as a light filter to enhance growth and lipid production by the microalga Nannochloropsis gaditana. Environ Eng Res 23:205–209. https://doi.org/10.4491/eer.2017.162
Shu CH, Tsai CC, Liao WH, Chen KY, Huang HC (2012) Effects of light quality on the accumulation of oil in a mixed culture of Chlorella sp. and Saccharomyces cerevisiae. J Chem Technol Biotechnol 87:601–607. https://doi.org/10.1002/jctb.2750
Teo CL, Atta M, Bukhari A, Taisir M, Yusuf AM, Idris A (2014) Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol 162:38–44. https://doi.org/10.1016/j.biortech.2014.03.113
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wondraczek L et al (2013) Solar spectral conversion for improving the photosynthetic activity in algae reactors. Nat Commun 4:2047. https://doi.org/10.1038/ncomms3047
Yoshioka M, Yago T, Yoshie-Stark Y, Arakawa H, Morinaga T (2012) Effect of high frequency of intermittent light on the growth and fatty acid profile of Isochrysis galbana. Aquaculture 338:111–117. https://doi.org/10.1016/j.aquaculture.2012.01.005
Zou L, Gu Z, Zhang N, Zhang Y, Fang Z, Zhu W, Zhong X (2008) Ultrafast synthesis of highly luminescent green-to near infrared-emitting CdTe nanocrystals in aqueous phase. J Mater Chem 18:2807–2815. https://doi.org/10.1039/B801418C
Acknowledgements
We are grateful to Dr. J. Hashemi for his assistance in the Statistical Analysis and also we would wish to thank Dr. K. Sobhani director of Hourateb Co. for his assistance and valuable work with light-measuring devices and methods. Also, the effort of experts at the “Environmental and Energy Lab” in the Central Laboratory of the University of Bojnord is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest regarding the publication of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khoobkar, Z., Delavari Amrei, H. Effect of fluorescent dye positioning and concentration on the growth parameters and lipid content of Chlorella sp. in a flat panel photobioreactor. Biotechnol Lett 42, 1397–1405 (2020). https://doi.org/10.1007/s10529-020-02862-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02862-9