Abstract
Rhodamine-6-G (R6G) was used to investigate the effect of internal/external light conversion on Dunaliella salina in a 1000 mL double-layer flat plate photobioreactor (DLF-PBR) and a 900 mL cylindrical photobioreactor (C-PBR). External spectrum conversion was examined in the DLF-PBR where the outer layer was filled with four different dye solutions (water/ethanol as solvents, dye at 25/50 ppm) to select the appropriate dye level and solvent type. The highest biomass (2.1 g L-1) and productivity (116 mg L-1day-1) were obtained at low dye level in ethanol, while the highest productivities of storage materials (lipid:7.5, starch:18.73 mg L-1day-1) belonged to high level of dye in water. To benefit from conventional bioreactors with lower cost, internal conversion was carried out in C-PBR by addition of dye into medium aiming to compare growth and product formation with/without spectrum conversion. The highest level of biomass (3.49 g L-1) was achieved by light conversion in C-PBR. Additionally, the effect of adaption of cells to dye was investigated in the C-PBR. A long lag-phase was observed in the case of non-adapted cells while this considerably reduced in the case of adapted cells. Moreover, a two-stage cultivation was studied in the C-PBR in which mixed red-blue LEDs exposure allowing enhanced growth without dye was used in the 1st-stage and this was followed by addition of R6G and shifting to fluorescent exposure in the 2nd-stage, which resulted in 146 and 293% higher productivities of lipid and starch, respectively, compared to control. The highest cell contents of lipid and starch (9.91, 22.15% g g-1DCW) also corresponded to this strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Having versatile potentials, microalgae have found many applications all over the world. Microalgal cultivation yields cells rich in specific substances such as proteins, carbohydrates, lipids, pigments, and other useful organic materials. Among the various microalgae species, Dunaliella salina has been noted for its high ability to produce valuable metabolites such as polar lipids, carbohydrates, carotenoids, glycerol, and proteins (Liao et al. 2017; Monte et al. 2020). In the past few decades, production of biomass and the various products of microalgae have been the subject of many studies (Spolaore et al. 2006; Mata et al. 2010; Hamed and safety 2016). The effect of light as a crucial factor for growth of phototrophic microalgae and its attributes such as source type, intensity and dark-light cycle (Bezerra et al. 2011; Seo et al. 2015) as well as the quality of light having a key role in the efficiency of photosynthesis, have been widely examined (Katsuda et al. 2004; You and Barnett 2004; Pattanaik et al. 2018; Han et al. 2019).
Microalgal cells efficiently absorb light within the photosynthetically active range (PAR, 400-700 nm) due to chlorophylls a and b (abs. 600-700 nm, orange-red), and carotenoids (abs. 400-500 nm, blue) as key photosynthetic pigments, while green-yellow light (500-600 nm) is mostly reflected (Seo et al. 2015; Jang et al. 2018) .Artificial light has therefore been used in many studies to provide the proper wavelengths in accord with the cell pigments (Vadiveloo et al. 2017; McGee et al. 2020; Tayebati et al. 2021).
Effect of light wavelength (color) on growth of microalgae has been examined in many studies where red LEDs were found supportive of cell growth (Baba et al. 2012; Kim et al. 2014; Li et al. 2020). Even though blue light was not beneficial to cell growth in most cases, it has been shown to boost formation of particular products, i.e., carotenoids and storage materials (Mohebi Najafabadi and Naeimpoor 2023). Since red and blue wavelengths have a significant effect on the growth and production of microalgae products, respectively, the effect of the combination of red and blue LEDs has been investigated widely (Kim et al. 2013; Schulze et al. 2016; Ra et al. 2018). According to the purpose of microalgae cultivation, LEDs have been used in single-stage (Das et al. 2011) or two-stage (Xi et al. 2016) cultivations. Changing the exposure conditions by the onset of 2nd stage in two-stage strategy has been reported to act as a stress to cells which can direct cell metabolism towards formation of protective metabolites (Jung et al. 2019; Nagappan et al. 2019) or accumulation of storage materials. Substantial increases in formation of intracellular products (starch and lipid) and pigments have been reported in a two-stage study with the mixed red-blue LEDs exposure in the 1st stage for high biomass production followed by monochromatic blue exposure in the 2nd stage (Mohebi Najafabadi and Naeimpoor 2023).
Despite the advantages of artificial light in cultivation of microalgae, electricity consumption makes it costly and uneconomical, especially at industrial scale. Although the emergence of LEDs has made artificial illumination more affordable, microalgae cultivation may still be expensive especially for production of low-value biochemical such as biofuels (Nwoba et al. 2019). Sunlight, known as the cheapest sustainable light source, emits a wide range of wavelengths of which only specific wavelengths can be absorbed by algae. The unused wavelengths of sunlight lowering the photosynthesis efficiency could be shifted to photosynthetic wavelengths by using certain spectral conversion materials (Burak et al. 2019). This means that wavelengths with little or no photosynthetic functionality can be converted into effective wavelengths. A wide range of converters, including organic and inorganic dyes, have shown promising properties for wavelength conversion (Wondraczek et al. 2013; Seo et al. 2015). Light-converting dyes can increase the amount of PAR energy by modifying the spectral characteristics of light source (Jang et al. 2018). The process starts with the organic dye absorbing incident radiation followed by excitation of the luminescent species allowing a higher energy state. Excited dye molecules emit radiation until returning to their ground state, the decay of excited dye molecules. This leads to the emission of light through fluorescence, which is specific to the wavelength of the dye (Ramanna et al. 2018).
The absorption and emission spectra of light-converting dyes depend on the dye level and the type of used solvent. Typically, the wavelength of the peak of emission spectrum is 30-50 nm longer than that of the absorption (Prokop et al. 1984). To select the dye of the wavelength modifier, features such as the absorption of unused light wavelengths, overlap of the emitted wavelength with cell absorption wavelength, quantum efficiency of the emitted wavelengths, cost of production and decomposition of dye over time should be considered (Burak et al. 2019). Among the various examined dyes, some rhodamine dyes have shown high quantum efficiency. Rhodamine 6G (R6G) among rhodamines, has been reported to be the most stable against light and also the most suitable for enhancement of cell growth (Prokop et al. 1984). In algal cultivation, application of dyes with tunable frequency and different absorption and emission spectra is recommended to prevent re-absorption of light (Ramanna et al. 2018).
The effect of spectrum converters, as coating on the wall of photobioreactors (Amrei et al. 2015; Seo et al. 2015; Ramanna et al. 2018; Burak et al. 2019) or as dye solution (Seo et al. 2015; Jang et al. 2018; Ramanna et al. 2018) on microalgae cultivation has been investigated by several researchers. These approaches allow exploitation of most of the sunlight wavelengths and reduction in energy consumption, which in turn increases the production efficiency of intercellular bioproducts (Ramanna et al. 2018). Application of dye solutions has been reported to have several selective benefits over solid-type converters, including higher quantum yield, lower cost, lesser toxicity, and easier control (Zou et al. 2008). Using dye solution as compared to solid-type converters is more cost-effective due to the higher quantum efficiency, less photodegradability, and dye recovery (Seo et al. 2015). Furthermore, using dye solution significantly reduces the cost due to not requiring special materials to cover the surface (Burak et al. 2019) with dye and solubility of dye in simple solvents such as water and ethanol.
Dye solution can be exploited in two forms of external and internal light converters where in the former dye solution is placed in the outer layer of a double-layer PBR while in the later dye is dissolved in the culture medium and hence conventional PBRs could be used. The effect of external light conversion on growth of Chlorella vulgaris has been studied using Rhodamine 101 dye solution in ethanol (Seo et al. 2015) where lower light intensity (100 W m-2) compared to that of control (150 W m-2 without dye) was required to obtain the highest amount of biomass (1.7 g L-1). Growth of microalgae has also been examined using internal conversion. In order to investigate the effect of internal light conversion, coumarin dye was dissolved in culture medium at 400 mg L-1, which increased the growth of microalgae by 100%, while applying R6G dye in the medium decreased cell level by about 40-64% (Prokop et al. 1984). Using dye solution (external/internal) therefore could reduce the overall production cost despite using dye compared to conventional cultivation method (no dye), since dye is effective at low levels. Moreover, the likely separation and reuse of the dye further reduces the overall cost.
Given the advantages of using dye solutions, we aimed to investigate the effect of dye level and solvent type as well as internal and external spectral light conversions on growth and products formation of D. salina. A double-layer flat panel photobioreactor (DLF-PBR) and a cylindrical PBR (C-PBR) were used to perform external and internal light conversions, respectively. R6G was used as spectrum converting dye allowing the conversion of green light to a longer wavelength. The effect of spectrum converting layer at low and high levels of dye was firstly investigated in the DLF-PBR, then considering the superiority of the low dye level, dye (low level) was added to culture medium to investigate internal conversion. As this cultivation method resulted in a long lag phase, the effect of using adapted cells to dye as inoculum on growth was also investigated. Additionally, to further enhance growth and product formation, a two-stage cultivation was designed. In the 1st stage, mixed red-blue LEDs exposure allowing enhanced growth was exploited and this was followed by addition of dye to the culture medium for light conversion alongside using white light in the 2nd stage. Culture was analyzed for chlorophylls, lipid, and starch contents as well as productivities under extra light at orange-red wavelength range.
Material and methods
Microalgae and culture medium
Dunaliella salina (CCAP 19/18) from the Culture Collection of Algae and Protozoa (CCAP) in Scotland was used. Modified Johnson (MJ) culture medium (Morowvat and Ghasemi 2016; Mohebi Najafabadi and Naeimpoor 2023) containing (in g L-1): MgCl2.6H2O: 1.5, MgSO4.7H2O: 0.5, KCl: 0.2, CaCl2: 0.2, KNO3: 1, NaHCO3: 0.035, KH2PO4: 0.035, NaCl: 12 was used for cell cultivation. To this medium were added 10 mL of Fe-solution (Na2 EDTA: 1.89, FeCl3.6H2O: 2.44 in g L-1) and 10 mL of trace elements solution (ZnCl2: 4.1, H3BO3: 61, (NH4)6Mo7O24.4H2O: 38, COCl2.6H2O: 5.1, CuCl2.2H2O: 4.1, MnCl2.4H2O: 4.1 in mg L-1) and culture pH was finally adjusted to 7.5 using hydrochloric acid. All solutions were separately autoclaved at 121°C for 20 min before use.
Fluorescent dye
Rhodamine 6G (R6G, 95% Sigma-Aldrich) was used as a spectrum converter due to its high quantum efficiency (Prokop et al. 1984). As a spectrum conversion layer, 25 and 50 ppm (Khoobkar and Amrei 2020) solutions of R6G in water and ethanol (Jang et al. 2018) as two different solvents were investigated. Additionally, 25 ppm of R6G in culture medium was used as a spectrum conversion medium in internal light conversion experiments.
Experimental set-up and experiments
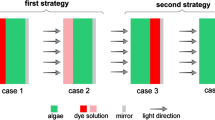
To investigate the effect of external and internal spectrum conversion on cell growth and product formation, two bioreactors (DLF-PBR and C-PBR) were designed as shown in Fig. 1. The DLF-PBR (Fig. 1-a, b, c) was designed to contain dye solution acting as spectrum converter in a front layer and cultivation medium in a rear layer with 1000 mL working volume. The front layer was filled with the dye solution prepared by dissolving R6G separately in ethanol and water (Fig. 1-a, b). Illumination was carried out by a fluorescent lamp placed at 9 cm away from the center of the front panel (Fig. 1-b). A mirror was placed in the back of PBR as shown in Fig. 1-c. The transmitted light from the source passed firstly through the R6G solution for spectrum conversion and then through cultivation chamber before hitting the mirror where light was redirected back into medium. This bioreactor was equipped with two air inlets and an outlet as well as a sampling port. After passing through a flowmeter and a filter for sterilization, air was humidified and introduced into bioreactor. C-PBR was a cylindrical Pyrex glass vessel with 900 mL working volume, equipped with three ports for sampling, aeration, and air outlet, which was placed on a magnetic stirrer as shown in Fig. 1-d. Air introduction into the C-PBR was as described for the DLF-PBR. Dye was dissolved in the culture medium to provide an internal light spectrum converter in the C-PBR.
Cells were pre-cultured in MJ medium to prepare the cell suspension for bioreactor inoculation. Exponentially growing pre-culture at OD680 = 1 was used for 10% v/v inoculation of all bioreactor experiments. The only exception was using an adapted inoculum prepared by pre-culturing cells in MJ medium supplemented with 25 ppm R6G to shorten the lag phase in one of C-PBR experiments. A white fluorescent lamp (intensity: logarithmic average of 350 µmol photons m-2 s-1) was used for illumination in all experiments, apart from the two-stage C-PBR experiment with red-blue LEDs (logarithmic average of 140 µmol photons m-2 s-1, 60% red + 40% blue) illumination in the 1st and fluorescent (same intensity) illumination in the 2nd stage. All cultures were aerated at 500 mL min-1 and temperature was 25 \(\pm\) 2 ℃, room temperature. Photobioreactors and all their connections were sterilized at 121 °C for 20 min before use.
Four individual experiments were carried out in the DLF-PBR using four different dye solutions in water and ethanol as solvents as given in Table 1. The C-PBR was used to perform two experiments (see Table 1) to investigate the effect of spectrum-converting medium containing 25 ppm R6G using non-adapted and adapted inoculum. A two-stage C-PBR experiment was also designed to allow cell growth in the absence of R6G under mixed red-blue LED illumination (1st stage) followed by addition of R6G (25 mg L-1) into culture medium under fluorescence illumination (2nd stage). Additionally, control experiments were separately carried out in the DFL-PBR and C-PBR in the absence of dye for comparisons.
Analytical methods
Measurement of growth parameters
The optical density (OD) of the culture sample was determined at 680 nm. Culture samples (10 mL) were centrifuged at 4,000 rpm for 10 min followed by washing cells with distilled water and drying cells at 70 ℃ for 24 h to obtain a correlation between culture cell dry weight and OD680 as given in Eq. 1 (Mohebi Najafabadi and Naeimpoor 2023).
This correlation was used to report culture biomass level (g L-1) based on the measured OD680.
Pigments analyses
Using an ethanol solvent extraction method, concentrations of chlorophyll (-a, -b, -total) and carotenoids in cells were determined (Khoobkar and Amrei 2020). After centrifugation of 2 mL of sample at 4000 rpm for 10 min the precipitated cells were stored in a Bain-Marie for 2 min to rupture the cell wall. To extract the pigments, 2 mL of ethanol (96% v/v) was added to the harvested algal biomass. After mixing and centrifugation at 4000 rpm for 10 min the absorbance of the supernatant was measured at 470, 669 and 645 nm and Eqs. 2-5 were t used to calculate pigment levels:
where v1 and v2 represent the sample and supernatant volume, and Ca and Cb refer to the amount of Chla and Chlb, respectively.
Starch Measurement
To measure the amount of starch in cells, the remaining cells from the pigment extraction received 0.25 mL of HClO4 (60%) and the suspension was agitated for 15 min followed by addition of 0.6 mL H2O and centrifugation at 4000 rpm for 10 min. To 0.4 mL of the supernatant was added 2 mL of anthrone reagent (0.2 g of anthrone in 0.1 L of 72% H2SO4). This mixture was well mixed and maintained at 100 °C for 8 min. After cooling to room temperature, the absorbance was measured at 620 nm. Standard solutions of glucose (0-5 mg L-1) were used to develop the calibration curve (Hu and Sato 2017).
Lipid analyses
Lipid content was determined using the sulfo-phospho-vanillin (SPV) reagent according to Mishra et al. (2014). The cells were re-suspended in 100 mL water after being centrifuged at 4000 rpm for 5 min, and then 2 mL concentrated sulfuric acid was added (98 %). This combination was held at 100°C for 10 min in a thermo-reactor before cooling in an ice bath for 5 min. The mixture was then incubated for 15 min at 37 °C and 200 rpm with 5 mL of freshly made phospho-vanillin reagent (0.6 g vanillin in 10 mL pure ethanol; 90 mL water; 400 mL concentrated phosphoric acid). The absorbance was measured at 530 nm. The calibration curve was made with commercial canola oil (0-2 mg L-1).
Absorption and emission spectra
The absorption and emission spectra of R6G solution were measured for two different solvents of water and ethanol. A UV/vis spectrophotometer (Cecil BioQuest CE 2501) was used to measure the pigment absorption spectrum and the pigment emission spectrum was measured by a fluorescence spectrometer (RF 6000, Shimadzu), with solvents (ethanol and water) being the reference points.
Statistical analysis
One-way ANOVA followed by Tukey’s test with the significant level (P < 0.05) was used to analyze the data when comparing the results obtained using different conditions. All statistical analyses were performed using IBM SPSS software (ver. 26).
All measurements in this study were repeated twice and the reported values are the average of the two samples alongside the relevant error bars.
Results and discussion
Absorption and emission spectra of dye solutions
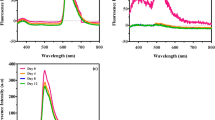
R6G is generally known for the absorption of green wavelength and emission in the orange-red light spectrum. To investigate the spectral conversion of R6G, absorption and emission spectra of the dye solutions were measured as shown in Fig. 2.
Figure 2 shows that the absorption peak (at 520 nm) of ethanol solution in the green light range was shifted to the longer wavelength of 590 nm, the lower limit of orange light range. This can support microalgae growth by shifting ineffectual green spectrum into useful orange-red. Wavelengths corresponding to peaks of absorption and emission (532 and 610 nm) for water were slightly higher compared to ethanol. This can be attributed to the difference in polarity of solvents, water being more polar than ethanol (Jang et al. 2018). Overall, our results confirmed wavelength conversion by R6G dye when dissolved in ethanol and water and hence these two solvents were used in further spectrum conversion experiments.
External spectral light conversion in DLF-PBR
Microalgae are not capable of absorbing all wavelengths of light, in fact, they can only absorb light within the range of the active photosynthesis spectrum. Orange-red light range (600-700 nm) has been reported to be the most effective light for microalgal growth (Seo et al. 2014). R6G dye rendering the transmitted spectrum highly efficient for microalgae growth was used in this study as an external spectral converter in a DLF-PBR. This allowed the transfer of the green wavelength (500-550 nm) of a fluorescent lamp (a full-spectrum light source) towards longer wavelengths in the optimal photosynthesis range (600-700 nm). Growth and product formation by D. salina were examined using separate dye solutions at 25 and 50 ppm in water and ethanol in front layer of the DLF-PBR. Batch time courses of the five culture variables (cell, lipid, starch, chlorophyll and carotenoids concentrations) for the five experiments carried out in the DLF-PBR including the control case are shown in Fig. 3a-e.
Figure 3a illustrates the results of control mode of DLF-PBR containing water in the front layer. Almost sharp and balanced increases were observed in concentrations of biomass, pigments and storage materials till day 4 of cultivation. Thereafter, the rate of change of pigments slowed down, while the rate of change of storage materials and biomass remained almost constant.
Higher levels of variables, though at various extents, observed in all examined cases with external light conversion (Fig. 3b-e) compared to control case, confirmed the positive effect of light conversion with R6G dye. It should however be mentioned that the highest level of each culture variable occurred at dissimilar solvent types and dye levels.
At 25 ppm dye in ethanol (Fig. 3b), increases were observed in photosynthesis and cell division compared to control case due to the intensification of orange-red light available to the cell. The highest rate and level of biomass formation as well as the lowest concentration of chlorophylls among the examined cases corresponded to this case. Using 25 ppm R6G in water (Fig. 3c), ranked the second in terms of biomass production, led to the highest chlorophylls level due to the availability of more red light intensity, the suitable wavelength of absorption of chlorophylls. Additionally, lipid and starch production increased compared to 25ppm dye in ethanol. Carotenoid production showed sharp increases till day 6 and slowed down thereafter, with a fluctuating trend at 25 ppm R6G in water (Fig. 3c).
Similarly, at 50 ppm R6G all variables showed increases for both ethanol (Fig. 3d) and water (Fig. 3e), compared to the control mode. But due to the increased level of dye and hence reduced light passing through the culture, decreases were observed in cell growth compared to lower dye level (25 ppm) (Fig. 3b-c). Interestingly, the highest lipid and starch production was obtained at the high level of R6G (50 ppm in water). This confirms the decrease in cell growth due to light limitations resulting in cell metabolism directing towards storing intracellular products for self-preservation (Oldenhof et al. 2004). Chlorophyll production showed almost similar trends to growth, showing initial sharp rates followed by slower rates till the end. For both cases of 50 ppm R6G, carotenoid production along with cell growth experienced a steep increasing trend up to day 6 and thereafter fluctuations were observed.
In the following sections, cell growth as well as production of pigments (carotenoids and chlorophylls) and storage materials (starch and lipid) will be separately analyzed for the examined cases and the results will be compared with the control case.
Effect of external light spectrum conversion on cell growth
The type of solvent has been reported to affect the spectrum of the emitted light (Jang et al. 2018). Therefore, we used ethanol and water (a more polar solvent compared to ethanol) to investigate this effect. Ethanol compared to water showed a greater effect on growth at both levels of R6G. On the most fundamental level, it may be claimed that the solvent's polarity has a significant impact on the dye's ability to emit light. It has been previously reported that in a more polar solvent, the emitted color will be redder (Jang et al. 2018). In our case, the slightly higher polarity of water led to the redder emission, while emission of dye in ethanol was closer to orange. In an study on the growth of Chlorella vulgaris, where two synthetic dyes were used as light converting layer, higher biomass productivity was also obtained using the dye emitting in the range of orange spectrum compared to the dye emitting light in the red range (Jang et al. 2018). This effect was also observed in growth of C. vulgaris using the coated plates with orange dye (color emission in the range of 585-620 nm) among green, red, and purple dyes (Mohsenpour et al. 2012). Actually, absorption and conversion of green light is greater for dye in ethanol due to the dye absorption peak (520 nm) being closer to green and hence more availability of light in the range of orange-red supporting microalgal growth compared to dye in water case with absorption peak of 532 nm (Jang et al. 2018).
For both solvents, growth was adversely affected by the higher level of dye, implying that the dye acted as a filter attenuating other wavelengths required for growth. Similarly, higher biomass productivity was reported for Chlorella sp. at 25 ppm of R6G compared to 50 ppm in a double-layer PBR (Khoobkar and Amrei 2020). Lowered growth of Chlorella sp. was also reported for R6G coated plate, placed between the light source and the PBR as a light converter, due to filtering the useful wavelengths for photosynthesis (Delavari Amrei and Ranjbar 2018).
The highest (2.1 g L-1) and lowest (1.4 g L-1) levels of biomass in this study corresponded to the case of 25 ppm R6G in ethanol and control case, respectively, showing significant difference on the last day (18th) (p-value < 0.05, ANOVA followed by Tukey’s test). Fig. 3a (control) and Fig. 3b (25 ppm ethanol) show no significant difference in biomass at the beginning, while a significant difference can be observed from day 12 (p-value < 0.05, ANOVA followed by Tukey’s test). In fact, useless short wavelengths of light such as green were absorbed by the fluorescent dye and were converted into longer wavelengths which could be absorbed by cells leading to the optimal use of energy. Rhodamine 101 dye in ethanol in a double-layer PBR containing C. vulgaris culture also resulted in the highest biomass level of 1.7 g L-1, while achieving this value with no spectrum conversion required 1.5 fold higher light intensity (Seo et al. 2015).
Overall, our results indicated that R6G functions as a spectral converter by transferring the wavelength range between 500-550 nm to the optimal photosynthesis range of 600-700 nm, thereby enhancing the utilization of light energy for microalgae growth. Essentially, R6G, as a fluorescent dye, has the ability to absorb green wavelengths and release red wavelengths, rendering the transmitted spectrum highly efficient for microalgae growth. Although color alteration could be detected, the molecular processes causing color shifting due to the multitude of intra- and inter-molecular interactions are quite complex (Jang et al. 2018).
Effect of external spectrum conversion on pigments production
Pigments such as chlorophylls are used as a measure of microalgae growth due to their importance in absorbing energy for microalgae growth. Chlorophylls a and b are the most important chlorophylls promoting photosynthesis, with the best absorption range of 680-700 nm spectrum. Carotenoids as accessory pigments can help microalgae to absorb light at wavelength range of 400-500 nm (Koller et al. 2014; Maltsev et al. 2021). Certain wavelengths of light (especially blue light) increase photosynthetic electron transfer and oxidative damage, leading to the production of reactive oxygen species (ROS). Therefore, cells start to produce carotenoids to prevent photo-oxidative damage and physiological stresses (Emeish 2012; Havaux 2014; Xu and Harvey 2019). To investigate the effect of light spectral conversion on pigments production, cell pigments content as well as pigments productivity for the five examined cases at the end of cultivation (day 18) are compared in Fig. 4.
Effect of solvent and R6G dye level in external light conversion experiments on cell pigments content (Cont.) and productivities (Prod.). Et. and Wa. in horizontal axis refer to ethanol and water, respectively. Data shown are mean ± SD, n=2. Asterisk (*) over the content bars represent significant differences in chlorophylls content on day 18 (p-value <0.05, ANOVA followed by Tukey’s test)
The highest chlorophyll content (15.5 mg g-1) and productivity (1.51 mg L-1 day-1) were observed at 25 ppm dye in water, showing 30 and 68% increases compared to control, respectively. At 25 ppm dye, significant differences were observed on day 18 between chlorophylls content by using water as solvent as compared to ethanol as well as control (p-value < 0.05, ANOVA followed by Tukey’s test). Superiority of water as solvent can be attributed to the redder emission of dye dissolved in water compared to ethanol. Although the two solutions containing 50 ppm dye illustrated only slightly lower chlorophylls content compared to the maximum, productivities were nearly 17% lower for these cases, due to the lower biomass level. In contrast to the highest biomass at 25 ppm dye in ethanol solution, chlorophylls content was the lowest in this case. This means that the availability of specific wavelengths does not simultaneously increase biomass and pigment production. The effect of red dye on pigment content of cells has also been observed in cultivation of C. vulgaris where among the used coated plates with various dyes as light converters, the red coated plate has resulted in the increased pigments content of cell, especially for chlorophyll a and b by 1.29% and 0.38%, respectively (Mohsenpour et al. 2012).
As can be seen in Fig. 4, cell content of carotenoid in all examined cases was lower compared to chlorophylls, showing a ratio of carotenoids/chlorophylls of about 0.25-0.38 with the lowest and highest belonging to 25 ppm dye in water and control case, respectively. Despite variations observed in carotenoids content from 3.5-4.67 mg g-1 in this study, carotenoids productivity showed insignificant changes (0.34-0.39 mg L-1 day-1) with the counterbalance of biomass level. The use of R6G dye has no effect on carotenoid production due to the emission of more photons in the orange-red spectrum range, which was not in accordance with the absorption range of carotenoids.
Effect of external spectrum conversion on storage materials production
Lipids and starch are intracellular products produced as storage materials to maintain cells in the absence of light energy. Cell content of storage materials and their productivities are functions of culture conditions such as lack of nutrients (nitrogen and phosphor), salinity, and the length of light-dark period (Junying et al. 2013; Zhao and Su 2014; Hsieh-Lo et al. 2019). Additionally, the intensity and spectrum of light affect their production. Starch and lipid content as well as their productivities at the end of cultivation for the examined spectrum conversion cases and the control are illustrated and compared in Fig. 5, showing significant differences in storage materials content on day 18 (p-value < 0.05, ANOVA followed by Tukey’s test).
Effects of R6G dye on cell lipid and starch contents (Cont.) and productivities (Prod.). Et. and Wa. in horizontal axis refer to ethanol and water, respectively. Data shown are mean ± SD, n=2. Asterisk (*) over the bar represent significant differences in lipid and starch content on 18th day (p-value <0.05, ANOVA followed by Tukey’s test)
The spectrum-converting layer containing 50 ppm R6G in water led to the highest increases in cell contents of starch (55%) and lipid (53%) as well as productivities of lipid (66.4%) and starch (68.8%) as compared to control. At the low level of dye (25 ppm), water and ethanol as solvents resulted in 12 and 28% decreases in the cell content of total storage materials compared to control, while their productivities increased by 14 and 9%, respectively. At lower cell growth, the highest cell contents of lipid and starch were observed due to the lack of continuous cell division. Our results showed higher cell content of starch than that of lipid for all examined cases, a starch/lipid ratio in a range of 2.4-2.8. This difference has been attributed to the lower energy requirement for carbohydrate production, though lipid and starch productions follow similar processes (Subramanian et al. 2013; Li et al. 2015).
Although in recent studies the use of blue light stress has led to an increase in lipid and starch production (Seo et al. 2014; Teo et al. 2014; Mohebi Najafabadi and Naeimpoor 2023), in this study an increase in lipid and starch production was observed using the spectrum conversion method. Red Rhodamine101 organic dye used as spectrum-converting liquid layer in cultivation of C. vulgaris also resulted in a 23% increase in lipid production while the highest increase (30%) was obtained using the blue dye 9,10-diphenylanthracene (Seo et al. 2015). This was attributed to the higher energy level of blue photons, compared to other colors, providing the easy access to the activation energy.
Internal spectral light conversion in C-PBR
Construction and application of flat panel and especially DLF-PBRs for cultivation of microalgae and spectrum conversion is laborious and costly compared to the conventional cylindrical photobioreactors (C-PBR). Alternatively, microalgae cultivation can be carried out in C-PBRs with internal spectrum conversion by addition of the dye as converter material into the culture medium provided that it shows no adverse effect on cell viability. This method has advantages such as no need for additional space to make layers, cost-effectiveness and being environmentally friendly with easy separation of the dye by salt formation as well as reduction of light absorption or reflection by bioreactor walls and hence lower light loss (Jang et al. 2018). However, adding dye to the medium has disadvantages such as the need for dye separation from the medium, the possibility of dye penetration onto the cell, decomposition of cells, and long lag phase due to the increased time of cells adaption (Prokop et al. 1984).
To investigate the effect of internal spectrum conversion on culture variables, cultivations were carried out in a 1 L cylindrical photo-bioreactor with and without (control) addition of R6G dye into the culture medium. The time courses of biomass and intracellular products for C-PBRs with no dye (control), 25 ppm of R6G dye in non-adapted MJ, two-stage cultivation and 25 ppm of R6G dye in non-adapted MJ are illustrated in Fig. 6a, d.
Time courses of culture variables in C-PBR. Control mode (no R6G) (a), 25 ppm R6G dissolved in MJ medium (b), two-stage cultivation: 1st stage illuminated with mixed RB LEDs and 2nd stage: addition of 25 ppm R6G into culture medium and illumination with fluorescent lamp and media inoculated with adapted cells to 25 ppm R6G (c). Error bars denote standard error for n=2
Time courses of culture variables in the control mode of C-PBR depicted in Fig. 6a illustrate increasing trends in concentration of biomass, storage materials (starch and lipid) and pigments (carotenoid and chlorophylls) till day 6 as a result of balanced growth. Thereafter, the level of storage materials continuously increased till day 18, while carotenoid level remained almost constant and chlorophylls level displayed some fluctuations. The highest biomass (1.62 g L-1) was achieved on the last day of cultivation.
Results for the case with spectral conversion in presence of R6G given in Fig. 6b demonstrate a lag/very slow growth phase lasting till day 6 due to the cells detecting the dye as a disturbing factor in the culture medium. In fact, acclimated their metabolism to the new medium containing R6G and hence only minor changes were observed in culture variables during this long lag phase. After acclimation, cells started to grow with mild increases in biomass and storage materials as well as sharp increases in pigments levels. These increasing trends continued till the end of cultivation, though at various extents. Decreased formation rates of pigments were observed after day 12, while almost linear increases were observed in the levels of biomass and storage materials till day 16 followed by sharp increases in biomass and lipid levels. The highest concentration of biomass (3.49 g L-1) obtained on day 18 was 215% higher than that in control.
Using internal conversion resulted in a high final biomass level however a long-lag phase was observed in cell growth due to cells pre-cultured in normal MJ medium used to inoculate MJ medium supplemented with R6G. Therefore, a two-stage cultivation method was used to allow favorable cell growth using MJ medium (no dye) and mixed red-blue illumination (1st stage) followed by the fluorescent illumination after addition of dye on day 9 (2nd stage) to exploit spectrum light conversion supporting storage material production. The strategy used in the 1st stage was according to on our previous findings on the efficiency of the mixed R-B LEDs illumination in biomass formation as compared to fluorescent, showing almost two fold increase in final biomass level (Mohebi Najafabadi and Naeimpoor 2023). Fig. 6c shows higher level of variables on day 8 as compared to control, especially for starch which was almost doubled (Fig. 6a). Biomass concentration on day 8 (1.3 g L-1) of this two-stage experiment was 152% higher than that in control. Addition of dye into culture medium in the 2nd stage (day 9) led to a dramatic decrease in biomass concentration due to the dye functioning as a disturbing factor and hence growth was ceased. However, net growth recommenced after 5 days of cell adaptation to new medium component. During the 2nd stage, lipid and starch levels showed increasing trends while pigments level remained almost constant during the lag phase followed by slight increases by the onset of growth after the lag phase.
To examine the effect of cell adaption to R6G on culture performance, an internal light conversion experiment was also performed in the C-PBR using adapted cells as inoculum. As expected, in the results (Fig. 6d) show elimination of lag phase due to cell adaption as well as monotonic increases in biomass and products levels from beginning till the end. There were 31 and 151% increases in biomass and starch levels compared to control, respectively. Despite the lower biomass level (30%) compared to non-adapted cultivation, starch level interestingly showed 42% enhancement by this method. Similarly, 47% higher lipid level was recorded compared to control, while 16% decrease was seen compared to non-adapted case.
Effect of internal spectral conversion on product formation
To compare the final results (day 18) of internal light conversion experiments, cell contents and productivities of storage materials, chlorophylls and carotenoid are presented in Figs. 7 and 8. A significant difference (p-value < 0.05, ANOVA followed by Tukey’s test) on day 18 was observed for the content of pigments, lipids and starch in the two-stage mode compared to other modes.
Effects of R6G dye on cell pigments contents (Cont.) and productivities (Prod.) on day 18. Data shown are mean ± SD, n=2. The experimental conditions are according to Table 1. Asterisk (*) over the content bars represent significant differences in chlorophylls content on day 18 (p-value <0.05, ANOVA followed by Tukey’s test)
Effects of R6G dye on cell lipid and starch contents (Cont.) and productivities (Prod.) on 18th day. Data shown are mean ± SD, n=2. The experimental conditions are according to Table 1. Asterisk (*) over the content bars represent significant differences in lipid and starch content on day 18 (p-value <0.05, ANOVA followed by Tukey’s test)
Figure 7 designates two-stage cultivation as the most appropriate case for high chlorophylls (14.46 mg g-1 DCW) and carotenoid (5.07 mg g-1 DCW) contents of cell. This could be the reason for the superiority of blue and red light in the 1st stage of cultivation in the production of pigments (Zhao et al. 2013). Although in non-adapted cells mode containing 25 ppm R6G the red light available to the cell increases, but due to the long-lag phase and subsequent cell division, the pigments content remains at the lowest level. Use of adapted cells enhanced the content of chlorophylls (10.5 mg g-1 DCW) and carotenoids (3.12 mg g-1 DCW) compared to non-adapted cells. The highest chlorophyll productivity (1.24 mg L-1 day-1) also corresponded to this strategy. Despite the changes observed in cell content of carotenoids in internal conversion experiments, almost constant carotenoids productivities in a range of 0.35-0.38 mg L-1 day-1 were obtained.
Figure 8 compares the cell content of storage materials as well as their productivities in internal conversion experiments. As compared to control, cell contents of lipid and starch with two-stage cultivation were 176 and 354% higher, while non-adapted cells led to 67 and 81% decreases in lipid and starch, respectively. Taking into account the higher biomass level in the non-adapted case (Fig. 6b), one can conclude that the nutrients were directed towards cell replication rather than storage of higher amounts of lipid and starch within the existing cells. This can be justified by the environmental conditions being favorable for cell growth and replication which counterbalanced the lower cell contents of storage materials. Actually, R6G in the culture medium acted as an internal lighting method allowing the emitted light reaching the interior of PBR boosting cell growth. Additionally, shading as a result of increased cell concentration was alleviated. Comparing results of adapted and non-adapted cells show two-fold higher starch and lipid contents of cell for adapted cells, showing balanced growth and product formation with no long lag phase. Overall productivities of storage materials for all internal light conversion cases were higher than that of control case in C-PBR. In particular, starch productivity was most affected by the two-stage strategy, showing the highest increase of 293% compared to control.
Internal spectrum conversion has been previously examined for some microalgae, the results being dependent on the type of dye. Despite expecting improvements in exposure conditions, rhodamine dyes resulted in 40-64% reduction in biomass level due to cell staining (solubility of rhodamines dye in lipid) while growth enhancement by 100% was observed using coumarin dyes (Prokop et al. 1984). In another study where two synthetic dyes of 1′ (λemission=599 nm) and 2′ (λemission=619 nm) were used as internal converter in culture medium, light conversion enhanced biomass productivity by more than 30% compared to control (Jang et al. 2018). Therefore, effectiveness of internal conversion in cell cultivation and product formation relies not only on light conversion but also on toxicity of dye for the species.
Conclusion
The effect of conversion of green spectrum to the orange-red range using R6G dye on growth and product formation by D. salina was separately examined in double-layer flat panel (as external converter in outer layer) and cylindrical (as internal converter in culture medium) photo-bioreactors. Overall, light conversion can be concluded to have significant effect on cell growth and/or production of intracellular materials. The level of dye and the type of solvent were found effective in the performance of the DLF-PBR. Lower level of dye in ethanol enhanced biomass formation due to the greater conversion of the green spectrum into the orange range, while higher dye level in water increased the content of intracellular products due to the increased emission in the red range. Compared to control, internal spectral light conversion at lower level of dye carried out in a conventional C-PBR expressively enhanced biomass level and productivities of intracellular products due to the internal emission and hence lower loss of light through the walls. Two-stage cultivation in the C-PBR resulted in the highest productivity and cell content of intracellular products. Considering the advantages of the C-PBR over the DLF-PBR, including construction cost reduction and the ease of design and application as well as the significant impact of internal light conversion on productivities of both biomass and products, internal conversion in the C-PBR proves itself as an effective choice in microalgal growth and product formation. This can have generic application in all phototrophic microorganisms provided that appropriate dye type and level are exploited.
Data availability
The datasets generated and analyzed in the current study may be made available on demand and reasonable request.
Reference
Amrei HD, Ranjbar R, Rastegar S, Nasernejad B, Nejadebrahim A (2015) Using fluorescent material for enhancing microalgae growth rate in photobioreactors. J Appl Phycol 27:67–74
Baba M, Kikuta F, Suzuki I, Watanabe MM, Shiraiwa Y (2012) Wavelength specificity of growth, photosynthesis, and hydrocarbon production in the oil-producing green alga Botryococcus braunii. Bioresour Technol 109:266–270
Bezerra RP, Montoya EYO, Sato S, Perego P, de Carvalho JCM, Converti A (2011) Effects of light intensity and dilution rate on the semicontinuous cultivation of Arthrospira (Spirulina) platensis. A kinetic Monod-type approach. Bioresour Technol 102:3215–3219
Burak H, Dunbar A, Gilmour DJ (2019) Enhancement of Dunaliella salina growth by using wavelength shifting dyes. J Appl Phycol 31:2791–2796
Das P, Lei W, Aziz SS, Obbard J (2011) Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour Technol 102:3883–3887
Delavari Amrei H, Ranjbar R (2018) Influence of fluorescent coating at rear and front side of a flat panel photobioreactor on algal growth. J Appl Phycol 30:901–907
Emeish S (2012) Production of natural β-carotene from Dunaliella living in the Dead Sea. Jordan J Earth Environ Sci 4:23–27
Hamed (2016) The evolution and versatility of microalgal biotechnology: a review. Compr Rev Food Sci Food Saf 15:1104–1123
Han S-I, Kim S, Lee C, Choi Y (2019) Blue-red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J Microbiol 57:101–106
Havaux M (2014) Carotenoid oxidation products as stress signals in plants. Plant J 79:597–606
Hsieh-Lo M, Castillo G, Ochoa-Becerra MA, Mojica L (2019) Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res 42:101600
Hu J-Y, Sato T (2017) A photobioreactor for microalgae cultivation with internal illumination considering flashing light effect and optimized light-source arrangement. Energy Convers Manage 133:558–565
Jang H, Namgoong JW, Sung M-G, Chang Y, Kim J (2018) Synthesis and characterization of fluorescent dyes and their applications for the enhancement of growth rate of Chlorella vulgaris. Dyes Pigm 158:142–150
Jung J-H, Sirisuk P, Ra CH, Kim J-M, Jeong G-T, Kim S-K (2019) Effects of green LED light and three stresses on biomass and lipid accumulation with two-phase culture of microalgae. Process Biochem 77:93–99
Junying Z, Junfeng R, Baoning Z (2013) Factors in mass cultivation of microalgae for biodiesel. Chin J Catal 34:80–100
Katsuda T, Lababpour A, Shimahara K, Katoh S (2004) Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enz Microb technol 35:81–86
Khoobkar Z, Amrei HD (2020) Effect of fluorescent dye positioning and concentration on the growth parameters and lipid content of Chlorella sp. in a flat panel photobioreactor. Biotechnol Lett 42:1397–1405
Kim T-H, Lee Y, Han S-H, Hwang S-J (2013) The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour Technol 130:75–80
Kim DG, Lee C, Park S-M, Choi Y (2014) Manipulation of light wavelength at appropriate growth stage to enhance biomass productivity and fatty acid methyl ester yield using Chlorella vulgaris. Bioresour Technol 159:240–248
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6:52–63
Li T, Gargouri M, Feng J, Park J-J, Gao D, Miao C, Dong T, Gang DR, Chen S (2015) Regulation of starch and lipid accumulation in a microalga Chlorella sorokiniana. Bioresour Technol 180:250–257
Li Y, Cai X, Gu W, Wang G (2020) Transcriptome analysis of carotenoid biosynthesis in Dunaliella salina under red and blue light. J Oceanol Limnol 38:177–185
Liao Q, Sun Y, Huang Y, Xia A, Fu Q, Zhu X (2017) Simultaneous enhancement of Chlorella vulgaris growth and lipid accumulation through the synergy effect between light and nitrate in a planar waveguide flat-plate photobioreactor. Bioresour Technol 243:528–538
Maltsev Y, Maltseva K, Kulikovskiy M, Maltseva SJB (2021) Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 10:1060
Mata TM, Martins AA, Caetano N (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
McGee D, Archer L, Fleming GT, Gillespie E, Touzet N (2020) Influence of spectral intensity and quality of LED lighting on photoacclimation, carbon allocation and high-value pigments in microalgae. Photosynth Res 143:67–80
Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang J-W (2014) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155:330–333
MohebiNajafabadi M, Naeimpoor F, Naeimpoor (2023) Boosting β-carotene and storage materials productivities by two-stage mixed and monochromatic exposure stresses on Dunaliella salina. Int J Phytoremed 25:609–620
Mohsenpour SF, Richards B, Willoughby N (2012) Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour Technol 125:75–81
Monte J, Ribeiro C, Parreira C, Costa L, Brive L, Casal S, Brazinha C, Crespo J (2020) Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour Technol 297:122509
Morowvat MH, Ghasemi Y (2016) Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal Agric Biotech 7:217–223
Nagappan S, Devendran S, Tsai P-C, Dahms H-U, Ponnusamy VK (2019) Potential of two-stage cultivation in microalgae biofuel production. Fuel 252:339–349
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2019) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:101433
Oldenhof H, Zachleder V, Van Den Ende H (2004) Blue light delays commitment to cell division in Chlamydomonas reinhardtii. Plant Biol 6:689–695
Pattanaik A, Sukla LB, Pradhan D (2018) Effect of LED Lights on the growth of microalgae. Inglomayor 14:17–24
Prokop A, Quinn M, Fekri M, Murad M, Ahmed S (1984) Spectral shifting by dyes to enhance algae growth. Biotechnol Bioeng 26:1313–1322
Ra CH, Sirisuk P, Jung J-H, Jeong G-T, Kim S-K (2018) Effects of light-emitting diode (LED) with a mixture of wavelengths on the growth and lipid content of microalgae. Bioproc Biosyst Eng 41:457–465
Ramanna L, Rawat I, Zerrouki D, Bux F (2018) A novel organic dye-based approach to increase photon flux density for enhanced microalgal pigment production. J Cleaner Product 198:187–194
Schulze PS, Pereira HG, Santos TF, Schueler L, Guerra R, Barreira LA, Perales JA, Varela JC (2016) Effect of light quality supplied by light emitting diodes (LEDs) on growth and biochemical profiles of Nannochloropsis oculata and Tetraselmis chuii. Algal Res 16:387–398
Seo YH, Cho C, Lee J-Y, Han J-I (2014) Enhancement of growth and lipid production from microalgae using fluorescent paint under the solar radiation. Bioresour Technol 173:193–197
Seo YH, Lee Y, Jeon DY, Han J-I (2015) Enhancing the light utilization efficiency of microalgae using organic dyes. Bioresour Technol 181:355–359
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Subramanian S, Barry AN, Pieris S, Sayre RT (2013) Comparative energetics and kinetics of autotrophic lipid and starch metabolism in chlorophytic microalgae: implications for biomass and biofuel production. Biotech Biofuels Bioprod 6:150
Tayebati H, Pajoum Shariati F, Soltani N, Sepasi Tehrani H (2021) Effect of various light spectra on amino acids and pigment production of Arthrospira platensis using flat-plate photobioreactor. Prep Biochem Biotech. https://doi.org/10.1080/10826068.2021.1941102
Teo CL, Atta M, Bukhari A, Taisir M, Yusuf AM, Idris A (2014) Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol 162:38–44
Vadiveloo A, Moheimani NR, Cosgrove JJ, Parlevliet D, Bahri P (2017) Effects of different light spectra on the growth, productivity and photosynthesis of two acclimated strains of Nannochloropsis sp. J Appl Phycol 29:1765–1774
Wondraczek L, Batentschuk M, Schmidt MA, Borchardt R, Scheiner S, Seemann B, Schweizer P, Brabec CJ (2013) Solar spectral conversion for improving the photosynthetic activity in algae reactors. Nature Commun 4:2047
Xi T, Kim DG, Roh SW, Choi J-S, Choi Y-E (2016) Enhancement of astaxanthin production using Haematococcus pluvialis with novel LED wavelength shift strategy. Appl Microbiol Biotechnol 100:6231–6238
Xu Y, Harvey P (2019) Carotenoid production by Dunaliella salina under red light. Antioxidants 8:123
You T, Barnett S (2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J19:251–258
Zhao B, Su Y (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sustain Energy Rev 31:121–132
Zhao Y, Wang J, Zhang H, Yan C, Zhang Y (2013) Effects of various LED light wavelengths and intensities on microalgae-based simultaneous biogas upgrading and digestate nutrient reduction process. Bioresour Technol 136:461–468
Zou L, Gu Z, Zhang N, Zhang Y, Fang Z, Zhu W, Zhong X (2008) Ultrafast synthesis of highly luminescent green-to near infrared-emitting CdTe nanocrystals in aqueous phase. J Mater Chem 18:2807–2815
Funding
The authors declare no funding was received.
Author information
Authors and Affiliations
Contributions
Both authors contributed to research ideas and method, as well as laboratory work and funding acquisition, wrote the main manuscript text and performed statistical analysis. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interest. This project was supported by the Iran University of Science and Technology (IUST).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Najafabadi, M.M., Naeimpoor, F. Internal and external spectral light conversion amplifying growth/bio-products formation of Dunaliella salina. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03304-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03304-z