Abstract
The focus of this study is the enhancement of microalgae growth rate using spectral conversion of green light. For this purpose, three reactors were considered and fluorescent pigment Rhodamine 6G was dissolved in a thermoplastic acrylic resin, the mixture was then applied on the front side of the first reactor, and on a mirror located at the rear side of the second one. Comparing their maximum specific growth rate (μ max) of Chlorella sp. to that in the third (uncoated) reactor, the former resulted in an increase up to 15% while the latter in decrease to at least 30%. Also, the rear side coated reactor showed up to 50% increase in biomass productivity rate (P) in early 4 days of experiment. However, this value decreased over time and the uncoated reactor in 12 days exhibited higher biomass productivity rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in large-scale cultivation of photosynthetic algae and bacteria has been increased due to their potential usefulness in the production of high-value products including carotenoids, long-chain polyunsaturated fatty acids, phycobilins, and biodiesel (Borowitzka 2013; Chisti 2013). Photosynthesis in photoautotrophic and mixotrophic microalgae can be driven by sunlight or artificial light. The properties of the light source, such as spectral power distribution and intensity, are critical for the growth of microalgae (Schulze et al. 2014; Singh and Singh 2015).
The energy of sunlight can be utilized as a source for carbon dioxide reduction by photosynthetic microorganisms. Although the spectrum of the sun at sea level includes wavelengths from 295 nm upwards, only the band within the range of 400–700 (nm) represents photosynthetically active radiation (PAR) (Kohen et al. 1995). Furthermore, although cyanobacteria (blue-green algae), rhodophytes (red algae), glaucocystophytes, and cryptophytes have phycobiliproteins which harvest green and yellow light, the absorption spectrum for chlorophylls absorbs better at the edge of 400–700 nm (violet, blue, orange, and red light) and not as well as that in the middle (green and yellow light) (Subramaniam et al. 1999; Ritchie 2010; Wondraczek et al. 2015). Therefore, spectral conversion of green light leads to a higher number of photons being in orange or red spectrum, which would increase the biomass productivity of microalgae.
Spectral conversion is one of the third-generation concepts suggested to overcome the classical efficiency limit of silicon solar cells (Strümpel et al. 2007; Klampaftis et al. 2009). Furthermore, some studies have examined the enhancement of microalgae and cyanobacteria growth rate using spectral converters (Mohsenpour and Willoughby 2013, 2016; Wondraczek et al. 2013; Delavari Amrei et al. 2014a, 2014b, 2015; Seo et al. 2014; Sforza et al. 2015;). In these studies, front side converters were used to enhance the biomass production; however, in one of them (Wondraczek et al. 2013), a back side converter was used. In this study, the rear side of the reactor, filled with Haematococcus pluvialis, was coated with a layer of red emitting phosphor Sr0.4Ca0.59 Eu0.01S as a spectral converter. A mirror was also placed on the rear side and the reactor was, then, put under solar radiation. Green light in the solar spectrum passing through the broth was converted to red and the mirror reflected the red light back into the broth. Therefore, an increase in cell generation around 36% was observed compared with the flat plate reactor without the spectral converter.
In the present study, the usage of Rhodamine 6G (R6G), as a fluorescent coating to enhance the growth rate of microalgae Chlorella sp., in a flat panel photobioreactor, and under metal halide lamp radiation was investigated. R6G is commercially produced, absorbs green light, and emits longer wavelengths. Two strategies were applied for this purpose. In the first one, the front side of reactor was coated and in the second one, coating was applied on the rear side mirror of the reactor.
Materials and methods
The green alga Chlorella sp. (PTCC 6010, Persian Type Culture Collection, Tehran, Iran) was used to test the efficiency of spectral conversion. This microorganism is provided by the Iranian Research Organization for Science and Technology and pre-cultivated in Rudic medium (pH ~ 8) (Rudic and Dudnicenco, 2000).
Light source
A metal halide lamp (MH-DE RX7S Daylight, Alite, Germany) was used as a full-spectrum light source. This lamp shows very sharp peaks in the visible region. The color rendering index (CRI) for this lamp is between 80 and 90%.
Coating
Solution of the organic dye R6G in a thermoplastic acrylic resin \( \left(\frac{0.1g-\mathrm{dye}}{30g-\mathrm{re}\sin}\right) \) was applied on the sheets and mirror through air spraying technique at 3.5 bar using an air spray gun (Iwata W101, Japan). The coatings were then dried after a short flash-off time at 60 °C for 5 min.
Experimental setup
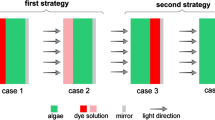
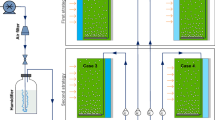
After pre-cultivation in 250-mL flasks (100 mL working volume) under cool white fluorescent light with 25 μmol photons m−2 s−1, Chlorella sp. was inoculated into a 2000-mL flask (1000-mL working volume) containing 900 mL Rudic medium. Then, the broth was distributed equally in three polycarbonate-based (PC) flat panel reactors with a capacity of 600 mL each (working volume of 400 mL or 4 × 10 × 10 cm3). For each reactor, a mirror was placed, as light reflector, at rear side (Fig. 1). In one of these reactors, the mirror was coated with the fluorescent dye R6G (Fig. 1, case Ι). Also, broth was grown at 27 ± 1 °C and irradiances of 25 and 150 μmol photons m−2 s−1.
In Fig. 1, three modes of cultivation are presented. For cases (Ι) and (ΙΙ), rear and front side of reactors were coated, respectively, while there was no coating for case (ΙΙΙ) (control sample).
Measurement of the growth
The optical density (OD) of the broth was determined by measuring the absorbance at 560 nm in a double beam UV/Vis spectrophotometer (V-550, JASCO, USA) with cell path length of 1 cm. The relationship between the biomass concentration (X, g L−1) or dry weight and OD560 follows the equation X = 0.49 × OD 560 (Delavari Amrei et al. 2014a)
The specific growth rate (day−1) of the culture was calculated using Eq. (1):
where μ is the specific growth rate (day−1), X t and X 0 are the biomass concentration at time t and at the beginning, respectively.
The biomass productivity in n days of experiment (P n , g L−1 day−1) was estimated by Eq. (2):
where X n is the biomass concentration at day n or t n .
To determine absorption spectra of extracted pigments, cells collected in 2-mL centrifuge tubes were disrupted in an ultrasonic bath (LBS1, ALC; Italy) for 45 min. The pigments were extracted with 2 mL of 90% acetone overnight in an ice bath, and then centrifuged at 2500×g for 10 min to sediment the cell debris. The supernatant was used to determine the absorption curve of the pigments using a UV/Vis spectrophotometer (Li et al. 2008).
Absorption, emission, and transmittance spectra
The absorption spectrum of the dye was determined with air as reference by the spectrophotometer. Using a high-resolution spectrometer (HR4000 Ocean Optics, USA), the emission and transmittance spectra of the dye solution, coated panel, and output spectrum of the light source were obtained. The intensity of visible light was measured with a light meter (TES-1330A, Taiwan).
Results
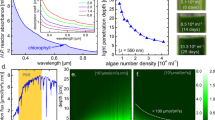
The emission spectrum of metal halide lamp—as a light source—showed sharp peaks in the blue, green, yellow, and orange regions (Fig. 2(A)) and a weak and very sharp peak exists in the red region (about 670 nm). The peaks at the green region of spectrum (495–570) are higher than others. This spectrum is different from that of the continuous one from the sun (Fig. 2(S)). Although the emission spectrum of the light source is not continuous, it showed some peaks in the photosyntethically active region (PAR). The absorption spectrum of the extracted pigment shows that absorption in the green region is very low. This region almost coincided with absorption band of the dye (gray area in Fig. 2). For wavelengths > 470 nm (yellow and orange) absorption of the light is higher than that of the green region and the emission band of the dye is within this region (dark gray are in Fig. 2). Furthermore, the absorption and emission bands of the dye overlapped in a short wavelength range (~ 535–550 nm).
The spectral power distributions when the light passes uncoated and coated PC surfaces and before reaches the broth are presented in Fig. 3(C and D). As can be seen, most of the green light was absorbed by coating. Considering spectra C and D, most of the green light reaches the broth in the uncoated reactor, whereas for the front side coated reactor, most of the green light was absorbed by the coated wall. The total transmittances of uncoated and coated reactors are presented in Fig. 3(E and F).
The spectra of transmitted light; C: spectrum after the light passes uncoated PC surface before falling onto the broth, D: spectrum after the light passes coated PC before falling onto the broth, E: total transmittance of the photobioreactor without coating, F: total transmittance of the photobioreactor with coating
Because of the different conditions for each sample, different productivities and maximum specific growth rates were observed. The time courses for cell growth of samples under 25 and 150 μmol photons m−2 s−1 at the surface of each reactor are illustrated in Figs. 4 and 5, respectively, and Tables 1 and 2 show the growth parameters of Chlorella sp.
Sample case (Ι) had the highest maximum specific growth rate (μ max) for both light intensities of 25 and 150 μmol photons m−2 s−1. However, for case (ΙΙ), this value was lower than that of the control (case ΙΙΙ). The biomass productivity (P n ) for case (I) was higher than the other samples initially. Considering Tables 1 and 2, biomass productivity for case (I) is about 50% more than that of the control during the first 4 days, while the control showed the highest P n over time and, during the 12 days of the experiment, this value for the control rose higher than case (I).
Considering spectra C and E (Fig. 3), the transmittance of green wavelengths through the reactor was higher than other wavelengths, and absorption spectrum of extracted pigments (Fig. 2(B)) confirms this. In case (I), when green light hits the coated surface, because of fluorescent property of R6G and mirror reflection, green light was converted to longer wavelengths (yellow and orange) and reflected back to the reactor (Fig. 6). Therefore, μ max in case (Ι) was more than that of control. But for case (ΙΙ), by considering spectrum D, coated layer on the front side of the reactor, not only absorbed green light, but also decreased the intensity of other usable wavelengths in the photosynthetically active region. Therefore, total light intensity hitting the broth, decreased and μ max in case (II) was the lowest. In fact, by comparing μ max in case (II) and control for both of light intensity, it is decreased to about 30%.
Growth of algae over time decreased transmittance of the green wavelengths through the reactor. This caused the intensity of green light that hit the fluorescent coating on the rear side of reactor to decrease and conversion of green light to longer wavelengths did not happen. Therefore, biomass concentration and P n for case (I) were higher than the control sample during the first 10 and 8 days under illumination of 25 and 150 μmol photons m−2 s−1, respectively. Furthermore, the greater intensity of the light that hit the surface of the system of case (I) resulted in a higher growth and turbidity of the system. This caused a considerable decrease in average light intensity in the system. Therefore, under 150 μmol photons m−2 s−1, the biomass concentration of the control sample reached that of case (I) sooner compared to that in under 25 μmol photons m−2 s−1.
Discussion
Wondraczek et al. (2013) used the phosphor Sr0.4Ca0.59Eu0.01S as a backside converter in a flat panel reactor growing Haematococcus pluvialis. They observed an increase about 36% in cell generation compared to the flat plate reactor without the backside converter under solar radiation. It is important to note that synthesis of the phosphor requires complex procedures while R6G is produced commercially and the results of the present study showed that using R6G as a backside converter increased productivity in early days of the culture by up to 50%.
Considering spectra E and F, total intensity of the light which hit the broth in case (II) was lower than the other two cases. In fact, although coating the front side of the reactor absorbs the green light and converts it to longer and more useful wavelengths, the coating filters some useful wavelengths in photosynthetically active region. Therefore, P n and μ max in this case were the lowest. Mohsenpour and Willoughby (2013) used a bubble column photobioreactor constructed from luminescent acrylic polymer for cultivation of Chlorella vulgaris. The photobiorector was lit by 150 W xenon lamps providing an intensity of 250 μmol photons m−2 s−1. They reported that for some luminescent dyes, biomass productivity increased. Seo et al. (2014) used organic dye solutions of Rhodamine101 (R101) and 9,10-diphenylanthracene (DPA) as front side wavelength converters. They used a reactor with two layers: one for algae cultivation and the other for the organic dye solution. C. vulgaris was cultivated in the reactor under a solar simulator radiation (UV+Visible) with different intensities. They showed that using R101 and DPA increased photosynthetic efficiency up to 40 and 20%, respectively. However, in our work, coating the front side of the reactor decreased the biomass productivity of Chlorella sp. under metal halide lamp radiation.
In conclusion, converting the green light into longer wavelengths using rear side fluorescent coating increased maximum specific growth rate and biomass productivity in the early days of the culture. Coating the rear of the reactor, when the turbidity of the broth is low, increases algal growth. However, coating the front side of the reactor showed unfavorable results. In this case, the coating filtered some useful wavelengths in the photosynthetically active region resulting in a decrease in biomass productivity. However, using Rhodamine 6G as converter material on rear side of the flat panel reactor with a mirror increased algal growth.
References
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167:201–214
Delavari Amrei H, Nasernejad B, Ranjbar R, Rastegar S (2014a) An integrated wavelength-shifting strategy for enhancement of microalgal growth rate in PMMA- and polycarbonate-based photobioreactors. Eur J Phycol 49:324–331
Delavari Amrei H, Nasernejad B, Ranjbar R, Rastegar S (2014b) Spectral shifting of UV-A wavelengths to blue light for enhancing growth rate of cyanobacteria. J Appl Phycol 26:1493–1500
Delavari Amrei H, Ranjbar R, Rastegar S, Nasernejad B, Nejadebrahim A (2015) Using fluorescent material for enhancing microalgae growth rate in photobioreactors. J Appl Phycol 27:67–74
Klampaftis E, Ross D, McIntosh KR, Richards BS (2009) Enhancing the performance of solar cells via luminescent down-shifting of the incident spectrum: a review. Solar Energy Mat Solar Cell 93:1182–1194
Kohen E, Santus R, Hirschberg JG (1995) Photobiology. Academic Press, New York
Mohsenpour SF, Willoughby N (2013) Luminescent photobioreactor design for improved algal growth and photosynthetic pigment production through spectral conversion of light. Bioresour Technol 142:147–153
Mohsenpour SF, Willoughby N (2016) Effect of CO2 aeration on cultivation of microalgae in luminescent photobioreactors. Biomass Bioenergy 85:168–177
Ritchie RJ (2010) Modelling photosynthetic photon flux density and maximum potential gross photosynthesis. Photosynthetica 48:596–609
Rudic V, Dudnicenco T (2000) Process for cultivation of green alga Haematococcus pluvialis (Flotow). Moldova Patent 2000 0154
Schulze PSC, Barreira LA, Pereira HGC, Perales JA, Varela JCS (2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotech 32:422–430
Seo YH, Cho C, Lee J-Y, Han J-I (2014) Enhancement of growth and lipid production from microalgae using fluorescent paint under the solar radiation. Bioresour Technol 173:193–197
Sforza E, Barbera E, Bertucco A (2015) Improving the photoconversion efficiency: an integrated photovoltaic-photobioreactor system for microalgal cultivation. Algal Res 10:202–209
Singh SP, Singh P (2015) Effect of temperature and light on the growth of algae species: a review. Renew Sust Energy Rev 50:431–444
Strümpel C, McCann M, Beaucarne G, Arkhipov V, Slaoui A, Švrček V, del Cañizo C, Tobias I (2007) Modifying the solar spectrum to enhance silicon solar cell efficiency—an overview of available materials. Solar Energy Mat Solar Cell 91:238–249
Subramaniam A, Carpenter EJ, Karentz D, Falkowski PG (1999) Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnol Oceanogry 44:608–617
Wondraczek L, Batentschuk M, Schmidt MA, Borchardt R, Scheiner S, Seemann B, Schweizer P, Brabec CJ (2013) Solar spectral conversion for improving the photosynthetic activity in algae reactors. Nat Commun 4:2047. https://doi.org/10.1038/ncomms3047
Wondraczek L, Tyystjärvi E, Méndez-Ramos J, Müller FA, Zhang Q (2015) Shifting the sun: solar spectral conversion and extrinsic sensitization in natural and artificial photosynthesis. Adv Sci 2(12). https://doi.org/10.1002/advs.201500218
Acknowledgements
We would like to thank Dr. Madanipour and Mr. Hosseini from the Optical Measurement Central Laboratory of the Optics, Laser and Photonics Institute of Amirkabir University of Technology for assisting us with light-measuring devices and methods. Also, the effort of engineers Atefeh Nejadebrahim and Oberon Bolouri in making coated sheet and cooperation in conducting experiment is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delavari Amrei, H., Ranjbar, R. Influence of fluorescent coating at rear and front side of a flat panel photobioreactor on algal growth. J Appl Phycol 30, 901–907 (2018). https://doi.org/10.1007/s10811-017-1303-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1303-3