Abstract
The nitrilase from Rhodococcus sp. CCZU10-1 catalyses the hydrolysis of dinitriles to acids without the formation of amides and cyanocarboxylic acids. It was induced by benzonitrile and its analogues (tetrachloroterephthalonitrile > ε-caprolactam > benzonitrile > phenylacetonitrile), and had activity towards aromatic nitriles (terephthalonitrile > tetrachloroterephthalonitrile > isophthalonitrile > tetrachloroisophthalonitrile > tetrafluoroterephthalonitrile > benzonitrile). After the optimization, the highest nitrilase induction [311 U/(g DCW)] was achieved with tetrachloroterephthalonitrile (1 mM) in the medium after 24 h at 30 °C after optimum enzyme activity was at pH 6.8 and at 30 °C. Efficient biocatalyst recycling was achieved by cell immobilization in calcium alginate, with a product-to-biocatalyst ratios of 776 g terephthalic acid/g DCW and 630 g isophthalic acid/g DCW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terephthalic acid and isophthalic acid are two key raw materials for the production of polyesters that are commercially required in large quantities for adhesives, beverage containers, fibers, films and paints. The most notable commercial processes to produce terephthalic acid and isophthalic acid involve the liquid-phase oxidation of p-xylene (Gabriele and Perathoner (2012) or m-xylene (Bramucci et al. 2001). Due to a high reaction temperature, solvent and product loss is common. Additionally, the corrosive nature of raw materials (e.g., bromides) at high temperatures requires the reaction be run in expensive titanium reactors. Although it is possible to oxidize xylenes by these processes, they are expensive and generate waste-streams containing environmental pollutants.
Biocatalytic synthesis of aromatic dicarboxylic acid is of current interest due to its mild reaction condition and substrate specificity. Bio-oxidation of methyl groups on aromatic rings, such as toluene and xylene, into aromatic dicarboxylic acids is well-known. For example, Comamonas testosterone could be used for biotransforming p-toluic acid into terephthalic acid (Bramucci et al. 2001; Wang et al. 2006). An isolate IR3 also could convert m-xylene into toluic and isophthalic acid (Bramucci et al. 2001). However, low concentrations of terephthalic acid and/or isophthalic acid were produced. Therefore, it is necessary to find an appropriate way to synthesize these aromatic dicarboxylic acids.
Nitrile-hydrolyzing biocatalysts are used for industrial production of some valuable acids (Bayer et al. 2011; He et al. 2012). There is a considerable industrial interest in bio-hydrolysis of nitrile owing to the desirability of conducting such conversion under mild conditions: (He et al. 2012). Microbial biotransformation of nitrile compounds proceeds by two distinct routes: the one-step reaction catalyzed by nitrilase (EC 3.5.5.1) transforms nitrile directly into corresponding acid; or the two-step reaction catalyzed by nitrile hydratase (EC 4.2.1.84; NHase) forms the corresponding amide, which is further hydrolyzed to the organic acid with the release of ammonia by an amidase (EC 3.5.1.4). Many dinitriles can also be hydrolyzed by nitrilase or nitrile hydratase/amidase. The nitrilase from Rhodococcus rhodochrous J1 converted isophthalonitrile and terephthalonitrile into 3- and 4-cyanobenzoic acids (Kobayashi et al. 1988). Rhodococcus rhodochrous NCIB 11216 selectively converted fumaronitrile into 3-cyano-acrylic acid (Bengis-Garber and Gutman 1989). Acidovorax facilis 72 W hydrolyzed 2-methylglutaronitrile into 4-cyanopentanoic acid and 2-methylglutaric acid (Gavagan et al. 1999). Nit1 nitrilase metabolized 2-methylglutaronitrile to the corresponding ω-cyanocarboxylic acid (Bayer et al. 2011). In these cases, cyanocarboxylic acids were produced from dinitrile. However, there is little information about biosynthesizing aromatic dicarboxylic acids effectively from the corresponding dinitriles by nitrilase without the formation of cyanocarboxylic acid. Fortunately, Rhodococcus sp. CCZU10-1 was isolated from soil by our group (He et al. 2012) with high nitrilase activity. Using it as a catalyst, terephthalic acid and isophthalic acid were synthesized from the hydrolysis of corresponding ditriles, and no cyanocarboxylic acids were detected. To the best of our knowledge, there is first report on the synthesis of terephthalic acid and isophthalic acid from the corresponding ditriles by nitrilase.
In this study, we have used Rhodococcus sp. CCZU10-1 for the effective bioproduction of terephthalic acid, isophthalic acid and their derivatives from the corresponding ditriles. With the optimization of induction and reaction conditions, a more simplified, practical and highly productive process for the manufacture of terephthalic acid and isophthalic acid was attempted to develop.
Materials and methods
Materials
Isophthalonitrile, tetrachloroterephthalonitrile, tetrachloroisophthalonitrile, tetrafluoroterephthalonitrile and terephthalonitrile were obtained from Aladdin Chemical Reagent Co. Ltd. (Shanghai, China). Other chemicals were obtained from commercial sources and were of analytical grade.
Microorganism
Rhodococcus sp. CCZU10-1 with the accession number of JF272470 was used.
Effect of inducers on nitrilase production by Rhodococcus sp.
To choose an appropriate inducer, various compounds were investigated (Fig. 1). Rhodococcus sp. was cultured in 500 ml Erlenmeyer flasks containing 100 ml growth medium (glucose 1 g, peptone 1 g, yeast extract 0.5 g, KH2PO4 0.2 g, NaCl 0.1 g, MgSO4 0.02 g plus inducer at 0.5 mmol, pH 7.0) at 30 °C with shaking (180 rpm). After 48 h, cells were harvested for assaying nitrilase activity. To enhance the nitrilase activity, different addition time of inducer and inducer concentration were also investigated, respectively.
Different compounds were chosen as potential inducers in this study. Compounds: acetonitrile (a), acrylonitrile (b), butylonitrile (c), benzonitrile (d), phenylacetonitrile (e), 4-chlorophenylacetonitrile (f), 3-chlorophenylacetonitrile (g), benzyol cyanide (h), terephthalonitrile (i), isophthalonitrile (j), tetrafluoroterephthalonitrile (k), tetrachloroterephthalonitrile (l), tetrachloroisophthalonitrile (m), ε-caprolactam (n)
Optimization of reaction conditions
The effects of reaction pH (6–8), reaction temperature (20–40 °C), terephthalonitrile concentration (100–600 mM) and metal ions (Ca2+, Co2+, Cu2+, Fe2+, Mg2+, Mn2+ and Zn2+) on the nitrilase activity were investigated.
Immobilization of Rhodococcus sp.
Immobilized cells and biocatalyst recycling were performed as given by He et al. (2012).
Analytical methods
Hydrolysis reactions of nitrile were quenched by adding an equal volume of 2 M NaOH. The mixture was passed through a 0.22 μm filter prior to analysis. Terephthalic acid and isophthalic acid were assayed by HPLC equipped with a C18 reversed-phase column with NH4H2PO4 (0.25 M)/acetonitrile (10 %, v/v) as mobile phase at 0.8 ml/min. (Wang et al. 2006). Absorption at 214 nm was monitored (Bramucci et al. 2001).
Nitrilase assays
Nitrilase activity in resting cells was measured by using the ammonia release assay (He et al. 2010). One unit of activity is defined as the amount of enzyme required to catalyze the formation of 1 μmol ammonia per min at 30 °C under standard assay conditions. All experiments were performed in triplicate.
Results and discussion
Effects of inducers
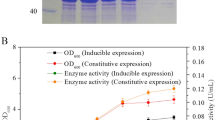
Nitrilase is an inducible enzyme during microbial cultivation (Banerjee et al. 2006; He et al. 2012). Various compounds (Fig. 1a–n) were used to examine the induction efficiency for Rhodococcus sp. with no inducer as the negative control. Without inducer, the biomass and nitrilase activity could reach 6.5 g DCW/L and 20.9 U/(g DCW), respectively. As shown in Fig. 2, benzonitrile and phenylacetonitrile did not support the biomass, and 4-chlorophenylacetonitrile, terephthalonitrile, tetrachloroterephthalonitrile and ε-caprolactam could increase the nitrilase activity. Tetrachloroterephthalonitrile significantly enhanced nitrilase activity from 20.9 to 36.3 U/(g DCW). ε-Caprolactam is a good inducer for the formation of nitrilase (He et al. 2010) though tetrachloroterephthalonitrile was clearly the best inducer in this study but nitrilase activity was practically unchanged upon addition of terephthalonitrile or caprolactam although 4-chlorophenylacetonitrile did result in a slight improvement.
Effects of different inducer on the terephthalonitrile-hydrolyzing activity. Rhodococcus sp. was cultured in 100 ml growth medium containing 5 mM inducer (Fig. 1a–n) at 30 °C for 48 h. Cells (0.04 g DCW) harvested by centrifugation (8,000×g, 6 min) were suspended in 2 ml phosphate buffer (100 mM, pH 6.8) containing 100 mM of terephthalonitrile for biotransformation
To enhance the induction efficiency, effects of inducer concentration and addition time of inducer were investigated. As shown in Fig. 3a, addition of 0.5 mM tetrachloroterephthalonitrile increased the nitrilase activity by about 3.5-fold. Using tetrachloroterephthalonitrile (1 mM) as inducer, 196 U/(g DCW) was obtained. Tetrachloroterephthalonitrile >1 mM was detrimental to nitrilase activity and the growth of the organism. Consequently, tetrachloroterephthalonitrile (1 mM) as inducer was employed in the following study. As shown in Fig. 3b, the time of adding the inducer had significant effect on nitrilase activity, and hyperinduction of Rhodococcus sp. nitrilase was obtained in an appropriate incubation time (24 h) at 30 °C. At 24 h of incubation, tetrachloroterephthalonitrile (1 mM) was added into growth medium and the highest nitrilase induction (311 U/g DCW) and growth (6.7 g DCW/l) were achieved.
Effects of inducer concentration and adding time on the terephthalonitrile-hydrolyzing activity. a This strain was cultured in the growth medium containing tetrachloroterephthalonitrile (0–10 mM). b Tetrachloroterephthalonitrile (1 mM) was added into the growth medium at 0–36 h, respectively. After the strain was cultured for 48 h, the cells were harvested by centrifugation (8,000×g, 6 min). Biotransformations of terephthalonitrile were carried out at 30 °C and 180 rpm in 2 ml potassium phosphate buffer (100 mM, pH 6.8) containing 0.04 g DCW cells
Nitrilase can synthesize important intermediates (Yeom et al. 2008; Cowan et al. 1998). To investigate the substrate specificity of Rhodococcus sp. nitrilase induced by tetrachloroterephthalonitrile, various nitriles (Fig. 1a–m) were hydrolyzed by resting cells. Nitrilase from Rhodococcus sp. had a broad substrate specificity in hydrolyzing both aliphatic and aromatic nitriles (Table 1) and into the corresponding acids without formation of amides. Dinitriles could also be hydrolyzed at high conversion rates. Terephthalic acid and its derivatives are available in substantial quantities and present a useful building-block in synthesizing various chemical products. Tetrachloroterephthalic acid and tetrachloroisophthalic acid can be employed to synthesize monoesters. Moreover, tetrachloroterephthalic acid and tetrafluoroterephthalic acid are important intermediates of pesticide. Significantly, Rhodococcus sp. CCZU10-1 may have potential scientific and commercial applications in synthesizing these dicarboxylic acids.
Optimization of reaction conditions
Hydrolysis reactions of terephthalonitrile were carried out in 100 mM KH2PO4/K2HPO4 buffers from pH 6 to 8 (data not shown). Nitrilase activity was maximal at pH 6.8. The effects of temperature from 20 to 40 °C on the nitrilase activity were also investigated (data not shown). Nitrilase activity was maximal at 30 °C and above 30 °C activity decreased considerably possibly due to thermal deactivation of enzymes in the resting cells during the reaction. Substrate concentrations have significant effects on the nitrilase activity (Fig. 4), and the highest reaction rate [434 U/(g DCW)] was recorded at 400 mM. Substrate inhibition resulted in that the terephthalonitrile-hydrolyzing rates were significantly decreased at 500 and 600 mM terephthalonitrile. The effects of various metal ions additive (0.1 mM), including CaCl2, CoCl2, CuCl2, FeCl2, MgCl2, MnCl2 and ZnCl2, on the nitrilase activity of Rhodococcus sp. were investigated (data not shown). Ca2+ and Fe2+ could significantly increase in the nitrilase activity, and 516 and 469 U/(g DCW) were obtained, respectively. However, the addition of Mn2+ and Zn2+ caused decreased activity. Therefore, Ca2+ (0.1 mM) was chosen as the optimum metal ion additive.
Based on the above experiments, the optimum reaction temperature, reaction pH, metal ion additive and substrate concentration were 30 °C, 6.8, Ca2+ (0.1 mM) and 400 mM, respectively. Under these optimum reaction conditions, 400 mM terephthalonitrile and isophthalonitrile were hydrolyzed by Rhodococcus sp. (Fig. 5). Biotransforming terephthalonitrile for 12 h, terephthalic acid was synthesized in the yield of 98.3 %. However, isophthalic acid was synthesized in the yield of 96.8 % after 24 h. During the hydrolysis, no cyanocarboxylic acids and amides were detected.
Efficient biocatalyst recycling
To increase the stability of biocatalyst and obtain an efficient biocatalyst recycling, immobilized cells are preferred, thereby potentially reducing production costs (He et al. 2010, 2012). In this study, immobilized cells in calcium alginate beads were used for hydrolyzing terephthalonitrile and isophthalonitrile in batch mode. As shown in Fig. 6, conversions of over 90 % were obtained with immobilized cells even after 20 cycles. High biocatalyst productivity (776 g terephthalic acid/(g DCW) cell 629 g isophthalic acid/(g DCW) cell) and volumetric productivity [2.7 g terephthalic acid/(l h) and 2.6 g isophthalic acid/(l h)] were achieved using immobilized cells. Significantly, an efficient reusability of the biocatalyst was achieved by immobilization.
Batch reaction of immobilized cells. The reaction, consisting of 400 mM substrate and 10 g immobilized beads (containing 2 g DCW cells) or 2 g DCW free cells in 100 ml Tris/HCl buffer (100 mM, pH 6.8), was carried out for 12 h at 30 °C and 180 rpm. After each cycle, the beads were washed with physiological saline (0.85 % NaCl) and transferred into a fresh Tris/HCl buffer (100 mM, pH 6.8)
Conclusion
Rhodococcus sp. CCZU10-1 harboring nitrilase activity can synthesize terephthalic acid, isophthalic acid and their derivatives from the corresponding nitriles, without formation of amides or cyanocarboxylic acids. High yields of terephthalic acid and isophthalic acid were obtained. Furthermore, the application of stabilized, alginate-immobilized cells for synthesizing dicarboxylic acids may be an alternative in future application. These data suggest that use of this strain of Rhodococcus sp. may have potential scientific and commercial applications for production of aromatic dicarboxylic acids.
References
Banerjee A, Kaul P, Banerjee UC (2006) Enhancing the catalytic potential of nitrilase from Pseudomonas putida for stereoselective nitrile hydrolysis. Appl Microbiol Biotechnol 72:77–87
Bayer S, Birkemeyer C, Ballschmiter M (2011) A nitrilase from a metagenomic library acts regioselectively on aliphatic dinitriles. Appl Microbiol Biotechnol 89:91–98
Bengis-Garber C, Gutman AL (1989) Selective hydrolysis of dinitriles into cyano-carboxylic acids by Rhodococcus rhodochrous N.C.I.B. 11216. Appl Microbiol Biotechnol 32:11–16
Bramucci MG, McCutchen CM, Nagarajan V, Thomas SM (2001) Microbial production of terephthalic acid and isophthalic acid. US Patent 6,187,569
Cowan D, Cramp R, Pereira R, Graham D, Almatawah Q (1998) Biochemistry and biotechnology of mesophilic and thermophilic nitrile metabolizing enzymes. Extremophiles 2:207–216
Gavagan JE, DiCosimo R, Eisenberg A, Fager SK, Folsom PW, Hann EC, Schneider KJ, Fallon RD (1999) A Gram-negative bacterium producing a heat-stable nitrilase highly active on aliphatic dinitriles. Appl Microbiol Biotechnol 52:654–659
He YC, Xu JH, Su JH, Zhou L (2010) Bioproduction of glycolic acid from glycolonitrile with a new bacterial isolate of Alcaligenes sp. ECU0401. Appl Biochem Biotechnol 37:741–750
He YC, Zhou Q, Ma CL, Cai ZQ, Wang LQ, Zhao XY, Chen Q, Gao DZ, Zheng M, Wang XD, Sun Q (2012) Biosynthesis of benzyolformic acid from benzyol cyanide by a newly isolated Rhodococcus sp. CCZU10-1 in toluene-water biphasic system. Bioresour Technol 115:88–95
Kobayashi M, Nagasawa T, Yamada H (1988) Regiospecific hydrolysis of dinitrile compounds by nitrilase from Rhodococcus rhodochrous J1. Appl Microbiol Biotechnol 29:231–233
Vejvoda V, Sveda O, Kaplan O, Prikrylová V, Elisáková V, Himl M, Kubác D, Pelantová H, Kuzma M, Kren V, Martínková L (2007) Biotransformation of heterocyclic dinitriles by Rhodococcus erythropolis and fungal nitrilases. Biotechnol Lett 29:1119–1124
Wang J, Tian J, Xu JH (2006) A method for producing terephthalic acid by Comamonas testosterone DSM6577. Chin J Catal 27:297–298
Yeom SJ, Kim HJ, Lee JK, Kim DE, Oh DK (2008) An amino acid at position 142 in nitrilase from Rhodococcus rhodochrous ATCC 33278 determines the substrate specificity for aliphatic and aromatic nitriles. Biochem J 415:401–407
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21102011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, YC., Wu, YD., Pan, XH. et al. Biosynthesis of terephthalic acid, isophthalic acid and their derivatives from the corresponding dinitriles by tetrachloroterephthalonitrile-induced Rhodococcus sp.. Biotechnol Lett 36, 341–347 (2014). https://doi.org/10.1007/s10529-013-1367-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1367-3