Abstract
A nitrilase from Hoeflea phototrophica DFL-43 (HpN) demonstrating excellent catalytic activity towards benzoylacetonitrile was identified from a nitrilase tool-box, which was developed previously in our laboratory for (R)-o-chloromandelic acid synthesis from o-chloromandelonitrile. The HpN was overexpressed in Escherichia coli BL21 (DE3), purified to homogeneity by nickel column affinity chromatography, and its biochemical properties were studied. The HpN was very stable at 30–40 °C, and highly active over a wide range of pH values (pH 6.0–10.0). In addition, the HpN could tolerate against several hydrophilic organic solvents. Steady-state kinetics indicated that HpN was highly active towards benzoylacetonitrile, giving a KM of 4.2 mM and a kcat of 170 s−1, the latter of which is ca. fivefold higher than the highest record reported so far. A cascade reaction for the synthesis of optically pure (S)-β-phenylalanine from benzoylacetonitrile was developed by coupling HpN with an ω-transaminase from Polaromonas sp. JS666 in toluene-water biphasic reaction system using β-alanine as an amino donor. Various (S)-β-amino acids could be produced from benzoylacetonitrile derivatives with moderate to high conversions (73–99%) and excellent enantioselectivity (> 99% ee). These results are significantly advantageous over previous studies, indicating a great potential of this cascade reaction for the practical synthesis of (S)-β-phenylalanine in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optically pure β-amino acids occur in numerous natural products with potent biological activities and represent an important class of chiral building blocks for a variety of pharmaceuticals, agrochemicals, and other fine chemicals (Jin et al. 2006; Ruf et al. 2012). They can also be incorporated into proteins and peptide mimics to improve their stability and therapeutic properties (Steer et al. 2002). Due to the significance of enantiomerically pure β-amino acids, great efforts have been made towards their efficient synthesis (Weiner et al. 2010). However, chemical methods are usually performed in kinetic resolution mode whose theoretical yield is only 50% and typically require the acquisition of chiral precursors, high loads of expensive catalysts or chiral auxiliaries, and careful control of the reaction conditions. All of the abovementioned drawbacks limit the practical application of chemical methods.
As an alternative, biocatalytic methods are attracting tremendous attention because of the environmentally benign reaction conditions and high selectivities (chemoselectivity, regioselectivity, and enantioselectivity) (Bornscheuer et al. 2012; Xu et al. 2017). Therefore, several enzymatic routes for the synthesis of optically pure β-amino acids have been developed, including kinetic resolution of racemic β-amino acids or their ester/amide derivatives (Bea et al. 2011; Li et al. 2013; Weise et al. 2017; Wu et al. 2010; You et al. 2013) and asymmetric amination/transamination of respective keto acids or asymmetric addition of ammonia to arylacrylates (Kim et al. 2007; Weise et al. 2015; Zhang et al. 2015a). In the case of kinetic resolution, the theoretical yield of only 50% limits their practical application, while in the case of asymmetric synthesis, the biocatalysts involved suffer from either low activity (Kim et al. 2007; Weise et al. 2015; Zhang et al. 2015a) or poor regioselectivity/enantioselectivity (Weise et al. 2015), resulting in low yields of the target product. Additionally, the preparation of racemic β-amino acids and their derivatives are relatively complicated and highly expensive, which would significantly increase the cost of the process. Therefore, there is still great need for the development of new methods for the asymmetric synthesis of optically pure β-amino acids from cheap and easily available starting materials.
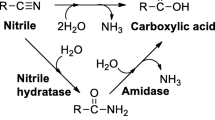
Nitrilase, which catalyzes the hydrolysis of nitriles to carboxylic acids, is considered as one of the most promising industrial-relevant biocatalysts and has been employed for the production of optically pure (R)-(–)-mandelic acid at BASF in an industrial scale (Ress-Loschke et al. 2005). Nitrilases from different origins have also been applied for the synthesis of various bulk chemicals and fine chemicals, such as acrylic acid, lactams, glycolic acid, nicotinic acid, (R)-2-chloromandelic acid, (R)/(S)-4-cyano-3-hydroxybutyric acid, S-ibuprofen, (3S)-3-cyano-5-methyl hexanoic acid, (R)-ethyl-3-hydroxyglutarate, as well as (R)/(S)-β-hydroxy acids (Ankati et al. 2009; DeSantis et al. 2003; Dong et al. 2010; Gavagan et al. 1998; Nagasawa et al. 1990; Stepanek et al. 1999; Wu et al. 2008; Xie et al. 2006; Yamamoto et al. 1990; Zhang et al. 2012). The synthetic applications and future perspectives of nitrilases were reviewed elsewhere (Gong et al. 2017; Martinkova and Kren 2010; Martinkova and Mylerova 2003). Recently, we also reported the application of nitrilases from Alcaligenes sp. ECU0401 and Labrenzia aggregata for the efficient synthesis of (R)-(–)-mandelic acid and (R)-o-chloromandelic acid, respectively (Zhang et al. 2010, 2012, 2015b).
In the present study, a nitrilase from Hoeflea phototrophica DFL-43, which shows very high activity towards benzoylacetonitrile, was identified by screening the nitrilase tool-box previously developed in our laboratory for (R)-o-chloromandelic acid production from o-chloromandelonitrile (Zhang et al. 2012), and its biochemical properties were characterized in detail. In addition, a new strategy for the asymmetric synthesis of optically pure β-phenylalanine from benzoylacetonitrile was developed by coupling the nitrilase-catalyzed benzoylacetonitrile hydrolysis with an ω-transaminase-mediated transamination of benzoylacetic acid (Scheme 1). Although β-amino acids could also be produced from β-nitriles by a one-pot two-step process employing a nitrilase and an amino acid dehydrogenase, however, a third enzyme (glucose dehydrogenase) is needed to regenerate the expensive NADPH (Zhang et al. 2015a). Recently, a similar process coupling a nitrilase and an ω-transaminase for the synthesis of β-amino acids from β-nitriles was reported (Mathew et al. 2017). The weakness of this process is that the expensive amino donor, optically pure (S)-phenethylamine, was needed to achieve a moderate conversion. In addition, the byproduct acetophenone served as a strong inhibitor for ω-transaminase, which would reduce the total yield of the product (Bea et al. 2011; Kim et al. 2007).
Materials and methods
Materials
Benzoylacetonitrile (98%) was obtained from Energy Chemicals (Shanghai, China), racemic β-phenylalanine (99%) was purchased from Accela ChemBio Co., Ltd. (Shanghai, China), β-alanine (98%), pyridoxal 5′-phosphate (PLP, 98%), and 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate (GITC, 98%) were provided by Aladdin (Shanghai, China). Cation resin D001 was kindly donated by Shanghai Huazhen Sci. & Tech. Co., Ltd. All other chemicals with the highest purity were also obtained commercially and used without further purification unless otherwise stated.
The cloning and overexpression of nitrilases from different origins were based on our previous studies (Zhang et al. 2012). Gene sequence of the ω-transaminase from Polaromonas sp. JS666 (GenBank accession number: ABE43415.1) as described previously (Bea et al. 2011) was synthesized by Genscript (Nanjing, China) and cloned into the vector pET28a (+). The ω-transaminase was overexpressed in E. coli BL21 (DE3) as described previously (Bea et al. 2011).
Purification of recombinant nitrilase HpN
Recombinant E. coli cells expressing HpN were harvested by centrifugation (8000×g) at 4 °C for 10 min, washed twice by physiological saline, and resuspended in buffer A (20 mM sodium phosphate, 500 mM NaCl, 10 mM imidazole, pH 8.0), then the cells were disrupted by sonication with an ultrasonic oscillator (JY92-II, Scientz Biotech. Co., Ltd.). The cell debris was removed by centrifugation (12,000×g) at 4 °C for 40 min. Then the supernatant was loaded onto a His-trap Ni-NTA FF column (5 mL, GE Healthcare Corp.) pre-equilibrated with buffer A, and the proteins were eluted with an increasing gradient of imidazole from 10 to 500 mM in buffer A at a flow rate of 5 mL/min. The fractions containing the target protein were concentrated to about 1 mL and exchanged with potassium phosphate buffer (20 mM, pH 8.0; 4 × 10 mL) for reducing the imidazole concentration using centrifugal filter units with 10 kDa cutoff (Millipore, USA). Protein purity was confirmed by SDS-PAGE analysis, and protein concentration was determined by the Bradford method using bovine serum albumin as the standard (Bradford 1976).
Activity assay
For nitrilase: the reaction mixture (1 mL) containing 10 mM benzoylacetonitrile (200 mM stock solution in DMSO), 10 μg of enzyme in potassium phosphate buffer (100 mM, pH 8.0), was incubated at 30 °C and 1000 rpm on a Thermomixer Compact (Eppendorf, Germany). Samples (100 μL) were withdrawn at 10 min, and the reaction was stopped by adding 10 μL HCl (2 M) and extracted with 500 μL ethyl acetate for GC analysis. The conversion was determined by measuring the amount of benzoylacetonitrile consumed in the reaction. One unit of enzyme activity was defined as the amount of enzyme consuming 1 μmol of benzoylacetonitrile per minute under the standard assay conditions.
For ω-TA: the reaction mixture (10 mL) containing 20 mM benzoylacetonitrile in 5 mL toluene, 0.1 mM PLP, 200 mM β-alanine, 50 mg lyophilized nitrilase powder, and 150 mg of lyophilized transaminase powder in 5 mL potassium phosphate buffer (100 mM, pH 8.0) was incubated at 37 °C and 180 rpm. The aqueous layer (100 μL) was withdrawn at 30 min for HPLC analysis. The conversion was determined by measuring the amount of β-phenylalanine produced in the reaction. One unit of enzyme activity was defined as the amount of enzyme producing 1 μmol of β-phenylalanine per minute under the coupled reaction conditions.
Effect of temperature and pH on the activity of HpN and stability
The effects of temperature and pH on HpN were determined by measuring the enzyme activity at different temperatures (20–60 °C) and in buffers with different pH values (pH 5.0–10.0): 100 mM sodium citrate buffer, pH 5.0–6.0; 100 mM potassium phosphate buffer, pH 6.0–8.0; 100 mM Tris-HCl buffer, pH 8.0–9.0; and 100 mM glycine-NaOH, pH 9.0–10.0.
Thermostability of the nitrilase was investigated by measuring the residual activity under the standard assay conditions after being incubated at different temperatures (30, 40, 50, and 60 °C) for a required period of time.
Influence of metal ions and EDTA on nitrilase activity
The influences of different metal ions or EDTA on nitrilase activity were studied by assaying the enzyme activity in the presence of specific metal ion or EDTA at final concentrations of 1 and 10 mM. The activity measured in the absence of metal ions and EDTA was taken as 100% and used as a reference.
Effect of organic solvents on nitrilase activity
The effects of various organic solvents on nitrilase activity were investigated by measuring the enzyme activity in the presence of specific organic solvent at a final concentration of 5% (v/v). The activity measured in the absence of organic solvent was taken as 100% and used as a reference.
Substrate spectrum
For substrate specificity investigation, the nitrilase activity was assayed under the standard assay conditions with 10 mM each substrate (from 200 mM stock solution in DMSO). The conversion was determined by measuring the ammonia formed in the reaction as described previously (Fawcett and Scott 1960). A control experiment, in which the nitrilase was replaced by reaction buffer, was performed for each substrate, and the ammonia produced in the control experiment was subtracted from the value found in the enzyme reaction.
Kinetic constants
To determine the steady-state kinetic constants of the nitrilase towards benzoylacetonitrile, the reactions (1 mL) were carried out at 50 °C and 1000 rpm with substrate concentrations ranging from 1 to 20 mM using purified HpN in potassium phosphate buffer (100 mM, pH 8.5). Aliquots (100 μL) were taken at fixed time intervals, and the reactions were stopped by adding 10 μL HCl (2 M) and extracted with 500 μL ethyl acetate for GC analysis. The best fit of the data to the Michaelis-Menten equation found using the solver function of Microsoft excel gave the respective kinetic constants.
Two-step one-pot synthesis of (S)-β-phenylalanine from benzoylacetonitrile
Asymmetric synthesis of (S)-β-phenylalanine from benzoylacetonitrile was carried out by a cascade reaction coupling the nitrilase with an ω-transaminase. The reaction mixture (10 mL) containing 200 mM β-alanine, 1 mM PLP, 50 mg lyophilized nitrilase powder (0.6 U, determined in the biphasic system), and 150 mg lyophilized ω-transaminase powder (0.75 U, based on the coupled reaction) in 5 mL potassium phosphate buffer (100 mM, pH 8.0), and 20 mM benzoylacetonitrile in 5 mL toluene was incubated at 37 °C and 180 rpm. At different time intervals, samples were withdrawn for HPLC and GC analysis. For preparative synthesis, the reaction was performed in 100-mL scale under the same reaction conditions described above. The product was isolated by cation exchange resin column as described previously using D001 instead (Wu et al. 2010).
Analytic methods
Quantitative analysis of β-phenylalanine was analyzed by HPLC with an ODS-C18 column (5 μm, 4.6 × 200 mm, Shimadzu, Japan), eluted with methanol:water:trifluoroacetic acid (55:45:0.1, v/v/v) at a flow rate of 0.8 mL/min and detected at 254 nm. For enantiomeric excess (ee) determination, (S)-β-phenylalanine was derivatized by GITC as described previously (Ito et al. 1992) before HPLC analysis. Conversion of benzoylacetonitrile and the concentration of byproduct acetophenone were determined by GC analysis with Rxi®-5Sil MS Capillary Column (0.25 μm, 0.25 mm × 30 m, RESTEK, USA) using n-dodecane as an internal standard. The temperature for injector and detector was 280 °C, and the column temperature was 100 °C for 2 min, rise to 160 °C at 10 °C/min and hold for 2 min.
Results
Screening of nitrilases for benzoylacetonitrile hydrolysis
To identify nitrilases capable of catalyzing benzoylacetonitrile hydrolysis, a nitrilase tool-box previously developed in our laboratory for the synthesis of (R)-o-chloromandelic acid from o-chloromandelonitrile (Zhang et al. 2012) was subjected to a primary screening using benzoylacetonitrile as a substrate (10 mM) under the standard assay conditions. As shown in Table 1, a nitrilase from Hoeflea phototrophica DFL-43, abbreviated as HpN, showed the highest hydrolytic activity towards benzoylacetonitrile with a relatively high activity of 2.35 U mg−1 cell-free extract. Three other nitrilases (SpwN, LaN, and ShwN) showed moderate to low activities, ranging from 0.02 to 0.92 U mg−1 cell-free extract. In contrast, three more nitrilases (ArN, EbN, and JpE) could not accept benzoylacetonitrile as substrate at all. It is noteworthy that all these nitrilases could be expressed very well in E. coli in soluble form. Therefore, HpN was chosen as a promising nitrilase for benzoylacetonitrile hydrolysis and was used for the following research.
Enzyme purification
Due to the N-terminal His-tag, HpN was easily purified to homogeneity by nickel column affinity chromatography (Fig. 1), and the purified enzyme migrated as a single band on SDS-PAGE with a molecular size of about 40 kDa, which is consistent with that predicted from the amino acid sequence. The purified nitrilase showed a very high specific activity of 35 U mg−1 protein towards benzoylacetonitrile, indicating that the enzyme was purified by 15-fold from the cell-free extract.
Effects of pH and temperature
The activity of the purified HpN was investigated at various pH values ranging from 5.0 to 10.0. Interestingly, the nitrilase was active over a wide range of pH values from 6.0 to 10.0, and more than 70% of the maximum activity was retained between pH 6.0 and 9.0. The maximum activity was observed at 100 mM Tris-HCl buffer with a pH value of 8.5 (Fig. 2a). The optimum temperature of HpN was 50 °C, lower or higher temperatures would significantly decrease the nitrilase activity, indicating that the nitrilase was highly temperature-sensitive, especially at higher temperatures (Fig. 2b).
The pH and temperature optima of the purified enzyme. (a) pH optima. Diamond, 100 mM sodium citrate buffer (pH 5.0−6.0); Square, 100 mM potassium phosphate buffer (pH 6.0−8.0); Triangle, 100 mM Tris-HCl buffer (pH 8.0−9.0); Circle, 100 mM glycine-NaOH buffer (pH 9.0−10.0). (b) Temperature optima: The enzyme activity was measured at various temperatures (20−60 °C) in 100 mM potassium phosphate buffer (pH 8.0)
Thermostability of the purified nitrilase was then determined at temperatures of 30, 40, 50, and 60 °C, respectively, in potassium phosphate buffer (100 mM, pH 8.0). It should be noted that the enzyme was very stable at 30 °C, and no activity loss was observed after 24-h incubation (Fig. 3). The nitrilase was relatively stable at 40 °C with a half-life of 12 h, while at 50 and 60 °C, the enzyme was more labile, giving half-lives of only 3 and 1 h, respectively.
Influences of metal ions and EDTA
The effects of metal ions and EDTA on the activity of HpN were investigated at concentrations of 1 and 10 mM. As shown in Table 2, the nitrilase activity was obviously inhibited by the thiol-binding metal ions, such as Ag+, Hg2+, and Cu2+, and as low as 1 mM Ag+ or Hg2+ could result in completely loss of enzyme activity, confirming that the thiol group is essential for nitrilase activity; similar results were also observed by previous studies (Zhang et al. 2011a, 2012). Meanwhile, Ni2+ and Fe2+ could also serve as effective inhibitors. The other metal ions and the metal chelating agent EDTA did not show obvious effect on the nitrilase activity.
Effect of organic solvents
Since most of the nitriles are highly hydrophobic, and in the bioreaction process, organic solvents are usually added to help the dispersion of substrate in the reaction mixture. However, organic solvents especially highly hydrophilic organic solvents have also been proven to be detrimental to biocatalysts (Zhang et al. 2011b). Therefore, the effects of various organic solvents on the nitrilase activity were examined with a concentration of 5% (v/v). As shown in Fig. 4, most of the organic solvents tested displayed good biocompatibility with the nitrilase, and more than 80% of the activity could be retained. The only exception was acetone, in which more than 60% of the nitrilase activity was inhibited, demonstrating that acetone might not be a suitable organic solvent for the nitrilase-catalyzed reaction.
Substrate spectrum
The substrate specificity of the purified nitrilase HpN towards a series of nitriles including arylacetonitriles, aromatic nitriles, and aliphatic nitriles were investigated (Table 3). It is not surprising that the nitrilase showed moderate to high activities towards arylacetonitriles since the nitrilase tool-box from which HpN was identified was developed previously in our laboratory using an arylacetonitrilase gene sequence as a template for blasting in NCBI database (Zhang et al. 2012). The HpN exhibited very high activity towards phenylacetonitrile and benzoylacetonitrile. However, substitution on the aromatic ring of phenylacetonitrile and benzoylacetonitrile significantly decreased the nitrilase activity, probably due to steric hindrance. It should be noted that the HpN displayed very low activities towards aliphatic nitriles with the exception of diaminomaleonitrile, which could not be hydrolyzed by HpN. In addition, the nitrilase HpN does not accept aromatic nitriles as substrates.
Steady-state kinetics
The steady-state kinetic constants of the purified nitrilase towards benzoylacetonitrile were studied with substrate concentrations ranging from 1 to 20 mM, giving Vmax, kcat, and KM of 256 U mg−1, 170 s−1, and 4.2 mM, respectively. Although nitrilases have found applications in the synthesis of various pharmaceuticals, agrochemicals, and fine chemicals, their activity levels (Vmax) if available are usually below 50 U mg−1 (Banerjee et al. 2006; Zhang et al. 2011a; Zhu et al. 2007), indicating that the nitrilase presented in this study represents a highly active and promising biocatalyst.
Two-step one-pot synthesis of (S)-β-amino acids from benzoylacetonitrile derivatives
Due to the significant inhibitory effect of carbonyl compounds on ω-transaminase (Bea et al. 2011; Kim et al. 2007) and the poor solubility of benzoylacetonitrile derivatives in aqueous buffer, a toluene-aqueous biphasic reaction system was adopted for the two-step one-pot synthesis of (S)-β-amino acids from benzoylacetonitrile derivatives since this biphasic system has been proven very useful in relieving substrate inhibition (Zhang et al. 2011a, 2012). Meanwhile, toluene is also a good solvent for benzoylacetonitrile derivatives. In the toluene-aqueous biphasic reaction system (1:1, v/v), 20 mM benzoylacetonitrile in the organic phase could be completely transformed into (S)-β-phenylalanine within 12 h with an optical purity of > 99% ee (Fig. 5 and Table 4). However, only moderate conversions (73–89%) of (S)-β-amino acids were obtained from benzoylacetonitrile derivatives even though the biocatalyst loading was twofold higher than that for benzoylacetonitrile, and the reaction time was also extended to 24 h, which was ascribed to the much lower specific activity of the nitrilase towards benzoylacetonitrile derivatives (Table 3). A preparative scale reaction (100-mL) was then carried out under the same reaction conditions, and enantiomerically pure (S)-β-phenylalanine was obtained with an isolated yield of 82 and > 99% ee after purification by column chromatography using a cation exchange resin (D001).
Discussion
Due to the important role of optically pure β-amino acids in synthetic organic chemistry and the common limitations including poor catalytic activity, unsatisfactory selectivity, and the low product yield of currently available biocatalytic synthesis methods (Bea et al. 2011; Kim et al. 2007; Li et al. 2013; Weise et al. 2015, 2017; Wu et al. 2010; You et al. 2013; Zhang et al. 2015a), there is still a great need to develop new synthetic routes, especially asymmetric synthetic methods starting from cheap raw materials. ω-Transaminase has been shown to be a group of versatile biocatalyst in the synthesis of chiral amines or amino acids due to its widespread in nature and broad substrate spectrum (Koszelewski et al. 2010). Some progresses have been made towards the asymmetric amination of β-ketoacids for the synthesis of optically pure β-phenylalanine; however, the inherent inhibitory effect of β-ketoacids on ω-transaminase and the spontaneous decarboxylation to acetophenone and carbon dioxide would greatly reduce the product yield (Bea et al. 2011; Kim et al. 2007). Although this could be partially alleviated by coupling the transamination with lipase, in which the more stable β-ketoacid esters were used instead of the labile β-ketoacids (Bea et al. 2011; Kim et al. 2007), the preparation of the ester precursors is relatively complicated and costly, since either harsh reaction conditions (e.g., − 78 °C) (Galliford and Scheidt 2008) or highly expensive catalysts (e.g., palladium) (Chen et al. 2013) were required.
To this end, we focused on the cascade reaction coupling nitrilase with ω-transaminase, in which the cheap and easily available benzoylacetonitrile was hydrolyzed by nitrilase into benzoylacetic acid which was then aminated by ω-transaminase forming β-phenylalanine (Scheme 1). Although nitrilases have been employed for the synthesis of various carboxylic acids from nitriles, there was only one nitrilase (NitBJ) reported to be active towards benzoylacetonitrile so far. Most importantly, the specific activity of NitBJ towards benzoylacetonitrile was merely 0.36 U mg−1 protein (Mathew et al. 2017; Zhang et al. 2015a). By screening the nitrilase tool-box developed in our laboratory (Zhang et al. 2012), a Hoeflea phototrophica nitrilase (HpN) was identified, exhibiting excellent catalytic activity towards benzoylacetonitrile with a Vmax of 256 U mg−1 protein, which is about fivefold higher than the highest nitrilase activity reported so far (Zhu et al. 2007). Substrate spectrum investigation showed that HpN was highly active towards arylacetonitriles, while very low activities were observed with aliphatic nitriles, and aromatic nitriles could not be hydrolyzed by HpN. A BLASTp search revealed that the amino acid sequence of HpN shows 67% identity to a nitrilase from an uncultured organism (GenBank accession number: AAR97377.1) (Robertson et al. 2004), which is the highest among the homologous nitrilases, indicating that HpN might represent a novel nitrilase. The nitrilase HpN was then chosen for the following research due to its highest activity and good expression level in E. coli.
Biochemical property characterization indicated that the nitrilase was very stable between 30 and 40 °C (Fig. 3), which are frequently employed temperatures for the biotransformation process. It should be noted that the nitrilase was highly active over a wide pH range (6.0–10.0), with an optimum pH around 8.5 (Fig. 2a). The optimum temperature (37 °C) and pH (8.5) of ω-transaminase from Polaromonas sp. JS666 (Bea et al. 2011) are similar to those of HpN, which is beneficial for the cascade reaction since coordination of the inter-compatibility between different enzymes in the cascade process is a great challenge. In addition, the nitrilase could tolerate against a wide range of hydrophilic organic solvents (Fig. 4), which is very important for the biotransformation of highly hydrophobic nitriles, in which organic solvents are usually added to the reaction mixture to help the distribution of substrates.
A cascade reaction coupling nitrilase with ω-transaminase for the enzymatic synthesis of optically pure β-phenylalanine from benzoylacetonitrile was thereby firstly performed in an aqueous monophasic system. However, only little amount of β-phenylalanine was detected, while benzoylacetic acid and acetophenone accounted the majority of products (data not shown), indicating that benzoylacetonitrile was successfully hydrolyzed by nitrilase to form benzoylacetic acid, and acetophenone was formed by the spontaneous decarboxylation of benzoylacetic acid. The low yield of β-phenylalanine might be ascribed to the inhibitory effect of carbonyl compounds on ω-transaminase, which was also observed in previous studies (Bea et al. 2011; Kim et al. 2007). In addition, when benzoylacetonitrile or acetophenone was used as the substrate for the ω-transaminase-catalyzed transamination, neither decrease of substrate nor formation of new product was detected, demonstrating that these two compounds could not be accepted as substrates by the ω-transaminase.
To relieve the substrate inhibition, a toluene-water biphasic system (1:1, v/v) was then adopted for the cascade reaction. Meanwhile, excess amount of ω-transaminase as compared with nitrilase was added to make sure that benzoylacetic acid produced at the first step could be transformed into β-phenylalanine by ω-transaminase as soon as its formation and the spontaneous decarboxylation to acetophenone could be avoided. Under these reaction conditions, various optically pure (S)-β-amino acids could be produced from benzoylacetonitrile derivatives in the cascade reaction employing nitrilase and ω-transaminase with moderate to high yields (Table 4). Although similar cascade reactions coupling lipase/nitrilase with ω-transaminase have also been employed for the production of a series of (S)-β-amino acids, only low to moderate yield of the product could be obtained (Table 5), probably due to the much lower catalytic activity of the nitrilase towards the nitrile compounds than that used in this study (Bea et al. 2011; Kim et al. 2007; Mathew et al. 2016a, b, 2017). Another possible reason might be the aqueous-organic biphasic reaction system adopted in this work, in which the organic phase would serve as a reservoir of the keto nitriles and regulating the substrate concentration around the biocatalyst and minimizing the substrate inhibition. The only limitation of the aqueous-toluene biphasic system might be the mass transfer problem (partially due to high biocatalyst loading in this study); therefore, selection of suitable organic solvents for the biphasic system or using extraction reagents is still needed in the future studies. It should also be noted that the much cheaper amino donor β-alanine was used in this study instead of the highly expensive (S)-phenethylamine employed in the previous studies (Bea et al. 2011; Kim et al. 2007; Mathew et al. 2016a, b, 2017), which would significantly reduce the cost of the bioprocess.
In summary, a novel nitrilase from Hoeflea phototrophica was identified from a home-made nitrilase tool-box, with an excellent activity towards benzoylacetonitrile which enables the development of a cascade reaction coupling nitrilase with ω-transaminase for the enzymatic synthesis of optically pure (S)-β-phenylalanine from the cheap and widely available benzoylacetonitrile. It should be noted that an in-depth optimization of the reaction conditions or protein engineering of the involved nitrilase and especially ω-transaminase to improve their catalytic efficiencies is still needed to further enhance the productivity of the cascade enzymatic process. However, the much better results in this work than the previous studies indicate that the cascade reaction we developed in this study might serve as a promising alternative for the green production of optically pure (S)-β-phenylalanine in the future.
References
Ankati H, Zhu D, Yang Y, Biehl ER, Hua L (2009) Asymmetric synthesis of both antipodes of β-hydroxy nitriles and β-hydroxy carboxylic acids via enzymatic reduction or sequential reduction/hydrolysis. J Org Chem 74:1658–1662
Banerjee A, Kaul P, Banerjee UC (2006) Purification and characterization of an enantioselective arylacetonitrilase from Pseudomonas putida. Arch Microbiol 184:407–418
Bea HS, Park HJ, Lee SH, Yun H (2011) Kinetic resolution of aromatic β-amino acids by transaminase. Chem Commun 47:5894–5896
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485:185–194
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen S, Yuan F, Zhao H, Li B (2013) tert-BuOK-Catalyzed condensation of ethyl diazoacetate to aldehydes and palladium-catalyzed 1,2-hydrogen migration for the synthesis of β-ketoesters under solvent-free conditions. RSC Adv 3:12616–12620
DeSantis G, Wong K, Farwell B, Chatman K, Zhu Z, Tomlinson G, Huang H, Tan X, Bibbs L, Chen P, Kretz K, Burk MJ (2003) Creation of a productive, highly enantioselective nitrilase through gene site saturation mutagenesis (GSSM). J Am Chem Soc 125(38):11476–11477
Dong HP, Liu ZQ, Zheng YG, Shen YC (2010) Novel biosynthesis of (R)-ethyl-3-hydroxyglutarate with (R)-enantioselective hydrolysis of racemic ethyl 4-cyano-3-hydroxybutyrate by Rhodococcus erythropolis. Appl Microbiol Biotechnol 87:1335–1345
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Galliford CV, Scheidt KA (2008) An unusual dianion equivalent from acylsilanes for the synthesis of substituted β-keto esters. Chem Commun 16:1926–1928
Gavagan JE, Fager SK, Fallon RD, Folsom PW, Herkes FE, Eisenberg A, Hann EC, DiCosimo R (1998) Chemoenzymatic production of lactams from aliphatic α,ω-dinitriles. J Org Chem 63:4792–4801
Gong JS, Shi JS, Lu ZM, Li H, Zhou ZM, Xu ZH (2017) Nitrile-converting enzymes as a tool to improve biocatalysis in organic synthesis: recent insights and promises. Crit Rev Biotechnol 37:69–81
Ito S, Ota A, Yamamoto K, Kawashima Y (1992) Resolution of the enantiomers of thiol compounds by reversed-phase liquid chromatography using chiral derivatization with 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate. J Chromatogr A 626:187–196
Jin M, Fischbach MA, Clardy J (2006) A biosynthetic gene cluster for the acetyl-CoA carboxylase inhibitor andrimid. J Am Chem Soc 128:10660–10661
Kim J, Kyung D, Yun H, Cho BK, Seo JH, Cha M, Kim BG (2007) Cloning and characterization of a novel β-transaminase from Mesorhizobium sp. strain LUK: a new biocatalyst for the synthesis of enantiomerically pure β-amino acids. Appl Environ Microbiol 73:1772–1782
Koszelewski D, Tauber K, Faber K, Kroutil W (2010) ω-Transaminases for the synthesis of non-racemic α-chiral primary amines. Trends Biotechnol 28:324–332
Li D, Ji L, Wang X, Wei D (2013) Enantioselective acylation of β-phenylalanine acid and its derivatives catalyzed by penicillin G acylase from Alcaligenes faecalis. Prep Biochem Biotechnol 43:207–216
Martinkova L, Kren V (2010) Biotransformations with nitrilase. Curr Opin Chem Biol 14:130–137
Martinkova L, Mylerova V (2003) Synthetic applications of nitrile-converting enzymes. Curr Org Chem 7:1279–1295
Mathew S, Jeong SS, Chung T, Lee SH, Yun H (2016a) Asymmetric synthesis of aromatic β-amino acids using ω-transaminase: Optimizing the lipase concentration to obtain thermodynamically unstable β-keto acids. Biotechnol J 11:185–190
Mathew S, Nadarajan SP, Chung T, Park HH, Yun H (2016b) Biochemical characterization of thermostable ω-transaminase from Sphaerobacter thermophilus and its application for producing aromatic β- and γ-amino acids. Enzyme Microb Technol 87–88:52–60
Mathew S, Nadarajan SP, Sundaramoorthy U, Jeon H, Chung T, Yun H (2017) Biotransformations of β-keto nitriles to chiral (S)-β-amino acids using nitrilase and ω-transaminase. Biotechnol Lett 39:535–543
Nagasawa T, Nakamura T, Yamada H (1990) Production of acrylic acid and methacrylic acid using Rhodococcus rhodochrous J1 nitrilase. Appl Microbiol Biotechnol 34:322–324
Ress-Loschke M, Friedrich T, Hauer B, Mattes R, Engels D (2005) Method for producing chiral carboxylic acids from nitriles with the assistance of a nitrilase or microorganisms which contain a gene for the nitrilase. US Patent, US 6,869,783 B1
Robertson DE, Chaplin JA, DeSantis G, Podar M, Madden M, Chi E, Richardson T, Milan A, Miller M, Weiner DP, Wong K, McQuaid J, Farwell B, Preston LA, Tan X, Snead MA, Keller M, Mathur E, Kretz PL, Burk MJ, Short JM (2004) Exploring nitrilase sequence space for enantioselective catalysis. Appl Environ Microbiol 70:2429–2436
Ruf S, Buning C, Schreuder H, Horstick G, Linz W, Olpp T, Pernerstorfer J, Hiss K, Kroll K, Kannt A, Kohlmann M, Linz D, Hubschle T, Rutten H, Wirth K, Schmidt T, Sadowski T (2012) Novel β-amino acid derivatives as inhibitors of cathepsin A. J Med Chem 55:7636–7649
Steer DL, Lew RA, Perlmutter P, Smith AI, Aguilar MI (2002) β-Amino acids: versatile peptidomimetics. Curr Med Chem 9:811–822
Stepanek E, Kubicek M, Marek M, Adler PM (1999) Optimal design and operation of a separating microreactor. Chem Eng Sci 54:1493–1498
Weiner B, Szymanski W, Janssen DB, Minnaard AJ, Feringa BL (2010) Recent advances in the catalytic asymmetric synthesis of β-amino acids. Chem Soc Rev 39:1656–1691
Weise NJ, Parmeggiani F, Ahmed ST, Turner NJ (2015) The bacterial ammonia lyase EncP: a tunable biocatalyst for the synthesis of unnatural amino acids. J Am Chem Soc 137:12977–12983
Weise NJ, Ahmed ST, Parmeggiani F, Turner NJ (2017) Kinetic resolution of aromatic β-amino acids using a combination of phenylalanine ammonia lyase and aminomutase biocatalysts. Adv Synth Catal 359:1–8
Wu S, Fogiel AJ, Petrillo KL, Jackson RE, Parker KN, DiCosimo R, Ben-Bassat A, O’Keefe DP, Payne MS (2008) Protein engineering of nitrilase for chemoenzymatic production of glycolic acid. Biotechnol Bioeng 99:717–720
Wu B, Szymanski W, de Wildeman S, Poelarends GJ, Feringa BL, Janssen DB (2010) Efficient tandem biocatalytic process for the kinetic resolution of aromatic β-amino acids. Adv Synth Catal 352:1409–1412
Xie Z, Feng J, Garcia E, Bernett M, Yazbeck D, Tao J (2006) Cloning and optimization of a nitrilase for the synthesis of (3S)-3-cyano-5-methyl hexanoic acid. J Mol Catal B-Enzym 41:75–80
Xu P, Zheng GW, Zong MH, Li N, Lou WY (2017) Recent progress on deep eutectic solvents in biocatalysis. Bioresour Bioprocess 4:34
Yamamoto K, Ueno Y, Otsubo K, Kawakami K, Komatsu K (1990) Production of S-(+)-ibuprofen from a nitrile compound by Acinetobacter sp. strain AK226. Appl Environ Microbiol 56:3125–3129
You P, Qiu J, Su E, Wei D (2013) Carica papaya lipase catalyzed resolution of β-amino esters for the highly enantioselective synthesis of (S)-Dapoxetine. Eur J Org Chem 2013:557–565
Zhang ZJ, Xu JH, He YC, Ouyang LM, Liu YY, Imanaka T (2010) Efficient production of (R)-(–)-mandelic acid with highly substrate/product tolerant and enantioselective nitrilase of recombinant Alcaligenes sp. Process Biochem 45:887–891
Zhang ZJ, Xu JH, He YC, Ouyang LM, Liu YY (2011a) Cloning and biochemical properties of a highly thermostable and enantioselective nitrilase from Alcaligenes sp. and its potential for (R)-(–)-mandelic acid production. Bioprocess Biosyst Eng 34:315–322
Zhang ZJ, Pan J, Liu JF, Xu JH, He YC, Liu YY (2011b) Significant enhancement of (R)-mandelic acid production by relieving substrate inhibition of recombinant nitrilase in toluene-water biphasic system. J Biotechnol 152:24–29
Zhang CS, Zhang ZJ, Li CX, Yu HL, Zheng GW, Xu JH (2012) Efficient production of (R)-o-chloromandelic acid by deracemization of o-chloromandelonitrile with a new nitrilase mined from Labrenzia aggregata. Appl Microbiol Biotechnol 95:91–99
Zhang D, Chen X, Zhang R, Yao P, Wu Q, Zhu D (2015a) Development of β-amino acid dehydrogenase for the synthesis of β-amino acids via reductive amination of β-keto acids. ACS Catal 5:2220–2224
Zhang ZJ, Yu HL, Imanaka T, Xu JH (2015b) Efficient production of (R)-(–)-mandelic acid by isopropanol-permeabilized recombinant E. coli cells expressing Alcaligenes sp. nitrilase. Biochem Eng J 95:71–77
Zhu D, Mukherjee C, Biehl ER, Hua L (2007) Discovery of a mandelonitrile hydrolase from Bradyrhizobium japonicum USDA110 by rational genome mining. J Biotechnol 129:645–650
Funding
This study was funded by National Natural Science Foundation of China (nos. 21406067 and 21536004), Natural Science Foundation of Shanghai (no. 18ZR1408400), Fundamental Research Funds for the Central Universities (no. 22221818014) and Shanghai Commission of Science and Technology (no. 15JC1400403).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zhang, ZJ., Cai, RF. & Xu, JH. Characterization of a new nitrilase from Hoeflea phototrophica DFL-43 for a two-step one-pot synthesis of (S)-β-amino acids. Appl Microbiol Biotechnol 102, 6047–6056 (2018). https://doi.org/10.1007/s00253-018-9057-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9057-7