Abstract
The gene coding for ribose-5-phosphate isomerase (Rpi) from Thermotoga lettingae TMO was cloned and expressed in E. coli. The recombinant enzyme was purified by Ni-affinity chromatography. It converted d-psicose to d-allose maximally at 75 °C and pH 8.0 with a 32 % conversion yield. The k m, turnover number (k cat), and catalytic efficiency (k cat k −1m ) for substrate d-psicose were 64 mM, 6.98 min−1 and 0.11 mM−1 min−1 respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
d-Allose is a non-caloric sweetener with no toxicity (Iga et al. 2010). It is an aldohexose that is rarely found in nature and has numerous health and medical benefits, especially its anti-tumor activity (Hoshikawa et al. 2010; Jeong et al. 2011; Sui et al. 2005; Yamaguchi et al. 2008a). Moreover, the combination of d-allose and traditional cancer treatments, including radiation (Hoshikawa et al. 2011) and chemical treatments (Yamaguchi et al. 2008b), has been effective.
Because of these properties, d-allose bioproduction has attracted a great deal of attention. Interconversion between d-allose and d-psicose by isomerization has been focused on as a commercially attractive enzymatic reaction for d-allose production. Owing to its broad substrate specificity, ribose-5-phosphate isomerase (Rpi) B has been suggested as a practical solution for d-allose production, as it can catalyze the interconversion of d-ribose 5-phosphate to d-ribulose 5-phosphate and a variety of monosaccharide substrates that are configured similarly to monosaccharide phosphate substrates (Lim and Oh 2011). Another enzyme involved in d-allose bioproduction is l-rhamnose isomerase (l-Rhi) from Pseudomonas stutzeri (Bhuiyan et al. 1998). l-Rhi from Caldicellulosiruptor saccharolyticus has the highest activity against d-allose (Lin et al. 2011). While almost all l-Rhis require divalent cations to increase activity, a metal-independent enzyme would be better for industrial applications. In addition to metal independence, converting d-allose from d-psicose using Rpi has the advantages of a high yield and lack of byproduct formation (Lim and Oh 2011). Thus far, d-allose-producing Rpi has been characterized only from Clostridium thermocellum (Park et al. 2007), Clostridium difficile and Thermotoga maritima (Yeom et al. 2010).
The elucidation of the Thermotoga lettingae TMO genome sequence (NCBI accession number: CP000812.1) in 2009 opened the door for genome scale research in to this thermophilic anaerobic bacterium. In this paper, the putative Rpi gene from T. lettingae was cloned and expressed in E. coli, and the recombinant T. lettingae Rpi was purified and characterized as a potential d-allose producer.
Materials and methods
Bacterial strains and plasmid
Escherichia coli BL21 (DE3) (Novagen) and pET-22b (+) plasmid were used as the host and vector for expression, respectively. The Rpi gene from Thermotoga lettingae was synthesized (Sangong, Shanghai, China) and cloned into the vector, resulting in a recombinant plasmid designated pET-22b-TL-Rpi.
Expression and purification of recombinant T. lettingae Rpi
The recombinant E. coli for protein expression was cultivated in an LB medium containing 50 μg ml−1 ampicillin at 37 °C. When the OD600 reached 0.6, IPTG was added to 1 mM and the Rpi was induced and expressed at 28 °C for 6 h. The T. lettingae Rpi was expressed as a 6× his-tagged fusion protein and purified by Ni-affinity chromatography (GE Healthcare) according to the manufacturer’s protocol (pET His Taq System; Novagen). The purity and integrity of the recombinant T. lettingae Rpi were checked using 15 % (w/v) SDS–PAGE.
Enzyme assay
One unit of enzyme activity was defined as the amount of enzyme required to produce 1 μmol d-allose per min. The reaction mixture in 1 ml contained a 50 mM sodium phosphate buffer (pH 8.0), 10 mM d-psicose and 0.15 U enzyme. The enzyme assay was performed at 75 °C for 10 min and then terminated by adding HCl to give 200 mM. The Rpi activity was determined using the cysteine-carbazole method (Dische and Borenfreund 1951).
Determination of kinetic parameters
The kinetic parameters of the recombinant T. lettingae Rpi were determined under standard conditions using d-psicose from 5–75 mM. Kinetic parameters such as the Michaelis–Menten constant (k m) and turnover number (k cat) values for d-psicose were determined by nonlinear regression and quantification of the enzyme concentration.
Analytical methods
The protein concentration was determined using the Bradford method with bovine serum albumin as a standard. The recombinant enzyme molecular weight was measured using a Superdex 75 column equilibrated to an ÄKTA Purifier System according to the manufacturer’s protocol. The concentrations of d-psicose and d-allose were determined by HPLC with a Waters Sugar-Pak 1 Ca2+ carbohydrate column eluted with water at 0.4 ml min−1 and 85 °C.
Results and discussion
Gene cloning and expression
The whole T. lettingae TMO genome was released in GenBank (NCBI accession number: CP000812.1) and an Rpi B gene was shown to encode a polypeptide of 145 amino acid residues with a calculated molecular mass of 16,015 Da. The putative Rpi gene from T. lettingae was synthesized with NdeI and XhoI restriction sites and cloned in to a vector, obtaining a recombinant plasmid pET-22b-TL-Rpi. The plasmid was then transformed into E. coli BL21 (DE3) to express the Rpi. The gene product was confirmed to exhibit an isomerase activity for d-allose production.
The expressed T. lettingae Rpi was purified to electrophoretic homogeneity (Fig. 1) by metal chelating affinity chromatography with a final purification of 9.1-fold, a yield of 75 % and a specific activity of 0.2 U mg−1. The enzyme exhibited 16.8 and 35.2 kDa as apparent molecular mass through SDS–PAGE and gel filtration, respectively, indicating that the enzyme is a dimer with two identical subunits.
Effects of temperature and pH
The recombinant enzyme had optimal activity at pH 8.0. More than 80 % of its maximal activity was maintained at pH from 6 to 9 (Fig. 2a). A broad pH range made the conversion reaction conditions more flexible to meet the needs of industrial production, such as the provision of a slightly acidic reaction environment to reduce browning reactions, especially at high temperatures. The relatively higher activity of the T. lettingae Rpi at pH 6.0 presented a potential advantage for industrial application, and no similar properties of other Rpis were reported. The enzyme showed relatively high stability at pH 8.0, indicating it could be stored and used in the same pH environment (Fig. 2b). The optimal temperature for the T. lettingae Rpi was 75 °C (Fig. 3a). A thermal inactivation of the recombinant enzyme followed first-order kinetics, and the half-life at 75 °C was 3.3 h (Fig. 3b). The activation energies for the isomerase reaction and thermal inactivation calculated from Arrhenius equation were 68.7 (Fig. 3a) and 235.9 kJ mol−1 (Supplementary Fig. 1), respectively. Compared with other d-allose-producing Rpis reported thus far (Table 1), these results suggest that T. lettingae Rpi is remarkably thermostable and could provide higher reaction rates, higher substrate solubility and fewer contamination problems in biocatalysis.
Effects of pH on the activity and stability of recombinant T. lettingae Rpi. a The relative activity of T. lettingae Rpi was investigated at 65 °C and different pH values. Three buffer systems, sodium citrate (50 mM, pH 5.0–5.5), sodium phosphate (50 mM, pH 6.0–7.5) and Tris/HCl (50 mM, pH 8.0–9.0) were used. All the experiments were conducted in triplicate; the data were expressed as mean ± standard deviation. Hundred percent activity = 93 ± 1.1 nmol mg−1 min−1. b The residual activities were assayed at 75 °C and pH 8.0 after preincubated recombinant T. lettingae at pH 6.0 (solid circle), 7.0 (open squares), and 8.0 (solid squares) at room temperature for different time intervals. Data were plotted as ln (relative activity %) versus time. All the experiments were conducted in triplicate; hundred percent activity = 155 ± 3.1 nmol mg−1 min−1
Effects of temperature on the activity and thermal stability of recombinant T. lettingae Rpi. a The relative activity of T. lettingae Rpi was investigated at temperatures varying from 55–85 °C in 50 mM sodium phosphate (pH 8.0) buffer. Data were plotted as ln (relative activity %) versus T−1. All the experiments were conducted in triplicate; 100 % activity = 158 ± 1.7 nmol mg−1 min−1. b The residual activities were assayed after preincubated the recombinant T. lettingae Rpi at 55 °C (solid squares), 65 °C (open squares), 75 °C (solid circle) and 85 °C (open circle) for different time intervals. Data were plotted as ln (relative activity %) versus time. All the experiments were conducted in triplicate; 100 % activity = 161 ± 2.3 nmol mg−1 min−1
Effects of metal ions
The effects of 1 mM Co2+, Mn2+, Mg2+, Ni2+, Zn2+, Ca2+, Cu2+ and EDTA were investigated but no obvious enhancement or inhibition was observed. T. lettingae Rpi is a metal-independent enzyme, which is consistent with other sugar phosphate isomerases (Lim and Oh 2011).
Kinetic parameters
The kinetic parameters of the recombinant T. lettingae Rpi were determined and compared with other Rpis (Table 1). The k m, turnover number (k cat) and catalytic efficiency (k cat k −1m ) for the substrate D-psicose were 64 mM, 6.98 min−1 and 0.11 mM−1 min−1 respectively. Although the active-site amino acid sequences were conserved (Supplementary Fig. 2), the overall kinetic activity of the T. lettingae Rpi was higher than that of C. difficile Rpi and T. maritima Rpi and much lower than that of C. thermocellum Rpi (Table 1). In agreement with a report by Jung et al. (2011), a homology modeling result showed that the T. lettingae Rpi substrate binding pocket (SBP) was 13.3 % larger than the C. thermocellum Rpi SBP (Supplementary Fig. 3) and that a smaller SBP may contribute to a high catalytic efficiency. This SBP volume difference sheds light on the direction of molecular modification in improving T. lettingae Rpi catalytic efficiency.
Conversion of d-psicose to d-allose
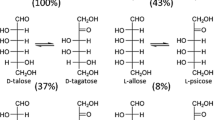
To determine the isomerization products and equilibrium ratio of the recombinant T. lettingae Rpi, 100 mM d-psicose was incubated with 0.3 U ml−1 enzyme at 55 °C for 0–6 h. The concentrations of d-allose increased with the reaction time and then reached maximal values at 4 h when the reaction was approaching equilibrium. Around 32 % d-allose was formed without any detectable byproducts (Fig. 4a,b). Furthermore, when 2 g dry cell wt recombinant E. coli harboring T. lettingae Rpi was used to convert 100 g d-psicose l−1 in a 1 l reaction system at 55 °C and pH 8.0, 28 g d-allose l−1 was obtained after 24 h.
Bioconversion of d-psicose into d-allose by recombinant T. lettingae Rpi. a Time courses of d-allose (solid squares) production from d-psicose (open squares) by the recombinant T. lettingae Rpi. Data represent the mean ± SD from triplicate experiments. b HPLC analysis of isomerization reaction products of the recombinant T. lettingae Rpi against d-psicose. 0 h indicates 100 mM d-psicose containing enzyme inactivated by boiling; 4 h indicates enzyme reaction after 4 h
Conclusions
The recombinant T. lettingae Rpi was characterized as a thermostable and metal-independent enzyme with a relatively broad pH value range. It converted d-psicose to d-allose with a 32 % conversion yield. All of these results indicated the potential advantages of biocatalyst in industrial application. With a successful activity improvement brought on by site-specific mutagenesis, T. lettingae Rpi could be a good candidate for large scale d-allose production.
References
Bhuiyan SH, Itami Y, Rokui Y, Katayama T, Izumori K (1998) d-Allose production from d-psicose using immobilized l-rhamnose isomerase. J Ferment Bioeng 85:539–541
Dische Z, Borenfreund E (1951) A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem 192:583–587
Hoshikawa H, Mori T, Mori N (2010) In vitro and in vivo effects of d-allose: up-regulation of thioredoxin-interacting protein in head and neck cancer cells. Ann Otol Rhinol Laryngol 119:567–571
Hoshikawa H, Indo K, Mori T, Mori N (2011) Enhancement of the radiation effects by d-allose in head and neck cancer cells. Cancer Lett 306:60–66
Iga Y, Nakamichi K, Shirai Y, Matsuo T (2010) Acute and sub-chronic toxicity of d-allose in rats. Biosci Biotechnol Biochem 74:1476–1478
Jeong RU, Lim S, Kim MO, Moon MH (2011) Effect of d-allose on prostate cancer cell lines: phospholipid profiling by nanoflow liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 401:689–698
Jung J, Kim JK, Yeom SJ, Ahn YJ, Oh DK, Kang LW (2011) Crystal structure of Clostridium thermocellum ribose-5-phosphate isomerase B reveals properties critical for fast enzyme kinetics. Appl Microbiol Biotechnol 90:517–527
Lim YR, Oh DK (2011) Microbial metabolism and biotechnological production of d-allose. Appl Microbiol Biotechnol 91:229–235
Lin CJ, Tseng WC, Fang TY (2011) Characterization of a thermophilic l-Rhamnose isomerase from Caldicellulosiruptor saccharolyticus ATCC 43494. J Agric Food Chem 59:8702–8708
Park CS, Yeom SJ, Kim HJ, Lee SH, Lee JK, Kim SW, Oh DK (2007) Characterization of ribose-5-phosphate isomerase of Clostridium thermocellum producing d-allose from d-psicose. Biotechnol Lett 29:1387–1391
Sui L, Dong YY, Watanabe Y, Yamaguchi F, Hatano N, Izumori K, Tokuda M (2005) Growth inhibitory effect of d-allose on human ovarian carcinoma cells in vitro. Anticancer Res 25:2639–2644
Yamaguchi F, Kamitori K, Sanada K, Horii M, Dong YY, Sui L, Tokuda M (2008a) Rare sugar d-Allose enhances anti-tumor effect of 5-fluorouracil on the human hepatocellular carcinoma cell line HuH-7. J Biosci Bioeng 106:248–252
Yamaguchi F, Takata M, Kamitori K, Nonaka M, Dong Y, Sui L, Tokuda M (2008b) Rare sugar d-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of P27 (kip1). Int J Oncol 32:377–385
Yeom SJ, Kim BN, Park CS, Oh DK (2010) Substrate specificity of ribose-5-phosphate isomerases from Clostridium difficile and Thermotoga maritima. Biotechnol Lett 32:829–835
Yeom SJ, Seo ES, Kim YS, Oh DK (2011) Increased d-allose production by the R132E mutant of ribose-5-phosphate isomerase from Clostridium thermocellum. Appl Microbiol Biotechnol 89:1859–1866
Acknowledgments
This work was supported by the 973 Project (No. 2012CB720802), NSFC Project (No. 31171705 and 21276001), the 863 Project (No. 2011AA100904), and the Support Project of Jiangsu Province (No. BE2010678 and BE2010626).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, Z., Mu, W. & Jiang, B. Characterization of ribose-5-phosphate isomerase converting d-psicose to d-allose from Thermotoga lettingae TMO. Biotechnol Lett 35, 719–724 (2013). https://doi.org/10.1007/s10529-013-1136-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1136-3