Abstract
The Atlantic Forest is one of the most threatened biomes in the world. Here, we use a common set of microsatellite markers to assess the genetic diversity and population structure of three species from the genus Psidium (P. guajava, P. macahense, and P. guineense), located in a disturbed environment of the Atlantic Forest, the restinga, in Espírito Santo, Brazil. Psidium guajava populations presented the highest number of alleles (95) followed by P. guineense (81) and P. macahense (68). The genetic variability was high (P. guajava = 0.71; P. guineense = 0.74; P. macahense = 0.63), with greater variation within populations (72 to 84%) than among populations (15 to 27%), reflecting elevated values of genetic differentiation (P. guajava, FST: 0.15; P. macahense, ØST: 0.27; P. guineense, ØST: 0.21). The populations were clustered into two main groups and considered moderately structured. This is the first report of genetic studies and evidence of polyploidy to P. macahense. Our results may provide information that can be used in management and conservation strategies, to preserve the diversity of Psidium populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Atlantic Forest is a complex ecosystem, with singular geological and climatic variables, mainly influenced by the rainfall regime, altitude variation, and dry season (IBGE 2012). The Atlantic Forest vegetation comprises six categories based on species distribution: ombrophilous forest (evergreen), semideciduous (seasonally dry forest), deciduous forest, mixed ombrophilous forest, rocky outcrop, and restinga (i.e., scrub vegetation on sandy coastal plains) (IBGE 2012). These categories cover all major plant habitat types across the Brazilian Atlantic Forest, and each vegetation type is associated with a distinct set of environmental conditions (IBGE 2012).
The heterogeneity of geographical characteristics, and the large range in altitude, have favored the high diversity and endemism of the Atlantic Forest, including more than 14,000 species (Stehmann et al. 2009). Despite the high levels of diversity and endemism, the degree of threat to the Atlantic Forest conservation is alarming (Ribeiro et al., 2011). Currently, the Atlantic Forest survives in small degraded patches and protected areas (only c. 8%) making it a global priority for biodiversity conservation, i.e., a biodiversity hotspot (Mittermeier et al. 2005).
Myrtaceae is the sixth most represented family (BFG, 2015) in the Atlantic Forest, with 710 species and a high number of endemics (Myrtaceae in Flora do Brasil 2020). Within the Myrtaceae family, the genus Psidium L., which presents at least 92 species, is detached (WCSP 2020), with approximately 60 taxa occurring in Brazil. The Atlantic Forest is particularly rich in Psidium species with a total of 35 species, from which sixteen are endemic in this biome (Proença et al. 2020).
The genus Psidium is reported to be dominant in particular vegetation types, such as restingas (Assis et al. 2004). Restingas consist of coastal ecosystems, which suffer constant anthropic actions (Cunha, 2005). Its vegetation is found on sandy and immensely leached soils. Due to the constant vegetation removal from these sites, the deposition of organic matter and the addition of nutrients are interrupted, which lead to the exposure of some areas, characterizing the fragility of these ecosystems (Guedes et al., 2006). These environments, which are extremely fragile, susceptible to disturbance and that present low resilience, allow changes in the genetic structure of natural populations (Assis et al., 2004; Guedes et al., 2006).
The genetic variability is the basis for species conservation, and studies aiming to elucidate populations’ genetic structure are important, either for demonstrating the natural environments fragmentation or for assisting the planning of goals that seek sustainable use and conservation of species (Forest et al., 2015).
Microsatellite markers are highly used in wild populations studies (Li et al. 2017; Nawaz et al. 2017). Nevertheless, the use of microsatellites to evaluate genetic diversity in different species, in the same environment, is not usual. This fact may help comprehend the species diversity patterns in a certain location. There are no species-specific microsatellite markers developed for the native species P. guineense and P. macahense; however, an elevated number of conserved microsatellites have been reported in Psidium species, revealing distribution patterns among groups of species (Tuler et al. 2015). These transferring microsatellites markers allow us to work with non-model organisms and their natural populations. These markers are abundant in the genome, have codominant nature, are highly polymorphic (Ramu et al. 2013), and are intensively indicated to investigate the genetic diversity among and within populations of different species.
Here, we used the same set of microsatellite markers to assess P. guajava, P. guineense, and P. macahense genetic diversity and structure in restinga environments of the Atlantic Forest in the state of Espírito Santo, Brazil. The results will allow the evaluation of necessary conservation strategies for the natural populations and will provide genetic basis for the species management for breeding and conservation purposes. In addition, the study of three species of a genus considered dominant in restinga can reflect the status of the natural environment fragmentation of this vegetation type, which is extremely fragile, susceptible to disturbance and that presents low resilience, hence, can allow changes in the genetic structure of natural populations.
Material and Methods
Study Area and Population Sampling
The genetic diversity of the abundant Psidium species in Espírito Santo, P. guajava L. (28), P. macahense DC. (73), and P. guineense Sw. (59) was evaluated (Supplemental file 1). Our studied site encompassed four areas of restinga and one of native fields in Reserva Natural Vale, which presents restinga (Table 1). Leaves from 10 populations were collected, packed in paper bags, and lyophilized for posterior DNA extraction. Besides the leaves, fertile branches were collected from the individuals of the sampled populations and were pressed and herborized.
Microsatellite Genotyping
For the DNA extraction, 200 mg of the dried leaves was macerated with MagnaLyser (Roche). The CTAB 2% method was used (Doyle and Doyle 1990). DNA quantification and integrity verification were carried out by agarose gel electrophoresis (0.9%) and nanodrop 2000/2000c (Thermo Scientific).
Nine microsatellites obtained from P. guajava were used for genotyping P. guajava, P. macahense, and P. guineense (Supplemental file 2). The polymerase chain reaction (PCR) was conducted with the following amplification conditions: 60 ng of DNA, 1X PCR buffer, 1 U Taq DNA polymerase, 0.2 µM from each primer (forward and reverse), 0.2 mM of dNTP, and 1.8 of mM MgCl2 completing with water for a final volume of 15 µL. The following programming was used in the thermocycler (Thermo Scientific): four minutes at 94 ºC, 30 cycles of 45 s at 94 ºC, one minute at 55 ºC, and 2 min at 72 ºC, with final extension of four minutes at 72 ºC (Risterucci et al. 2005). The PCR products were separated and visualized by agarose gel electrophoresis (1.5%) and when amplification was confirmed, capillary electrophoresis was carried out (Fragment Analyzer—Advanced Analytical Technologies).
Analysis in P. guajava Populations
For the descriptive analysis of genetic diversity in P. guajava, the GDA program (Lewis and Zaykin 2001) was used for the obtention of the following estimates: average number of alleles per loci (A); number of private alleles (APRIV.); expected heterozygosity (He); observed heterozygosity (Ho); inbreeding coefficient (f) (Wright 1951); and the deviation from zero of the genotype proportions that were in Hardy–Weinberg (HW) equilibrium, which was evaluated through allelic permutations, within the populations, according to Fisher’s exact test described by Weir (1996).
The Arlequin software version 3.1 (Excoffier et al. 2006) was used for the analysis of molecular variance (AMOVA) and to obtain a matrix of FST pairs among the populations (Weir and Hill 2002).
The number of genetic groups clustered among the individuals from P. guajava populations was inferred with the STRUCTURE software version 2.3.4. STRUCTURE is an inference software extensively used to detect the populational genetic structure (Kaeuffer et al. 2007), which uses a Bayesian Monte Carlo Markov Chain (MCMC) approach (Pritchard et al. 2000). Twenty runs were carried out, the burn-in consisted of a set of 250,000 replications, followed by 500,000 steps of MCMC for a K = 6 (chosen according to the number of populations). The ΔK criterion was used for the best K obtention (Evanno et al. 2005).
Analysis in P. macahense and P. guineense Populations
Due to the polyploid nature observed through the molecular data from P. macahense and P. guineense, these populations’ data were analyzed with the F-DASH program (Obbard et al. 2006) and with the POLYSAT software (Clark and Jasieniuk 2011).
The F-DASH program possesses a genetic statistic of differentiation denominated F’ST, which is based on the diversity of allelic phenotypes. This program is suggested for polyploid species (allopolyploids or autopolyploids) in which is possible to count alleles and loci, although it may not be possible to obtain the dosage effect of alleles and the respective genotypes (Obbard et al. 2006). With this program, it was possible to verify the number of allelic-phenotypes, number of alleles identified among the loci, to calculate the diversity statistics (H’) (based on allelic-phenotypes analogous to He), and the differentiation statistics (F’ST) (based on allelic phenotypes analogous to FST).
The POLYSAT software, used through the R program platform (R Core Team 2016), analyzes microsatellite data from auto and allopolyploids (Clark and Jasieniuk 2011). With this software, the F’ST and G’ST indexes were calculated among population pairs (Polysat 2017). In addition, a Bruvo’s distance matrix, among the pairs of samples, was also calculated (Bruvo et al. 2004). The Bruvo’s distance is used for polyploids, since it considers the distances between microsatellite alleles, when the number of allele’s copies is unknown (Clark and Jasieniuk 2011); however, it is known that Bruvo’s distance can cause some biases in polyploid analysis as others distance/dissimilarity metrics (Meirmans et al. 2018). The distance matrix was used to calculate AMOVA through the Genes program (Cruz, 2013). The Bruvo’s distance matrix was also used in the PCA (principal components analysis) in POLYSAT.
The STRUCTURE software version 2.3.4 (Pritchard et al. 2000) was also used for the analysis of the polyploids’ data, with the same methodology used for P. guajava. The structure harvester is used to get the exact population number for each species independently. (http://taylor0.biology.ucla.edu/structureHarvester/, it is a web-based program for collating results generated by the program STRUCTURE.
Results and Discussion
Psidium guajava, P. macahense and P. guineense Populations Analysis
This study is one of the first to explore genetic data using populations of three species of Psidium. Psidium guajava populations presented the highest number of alleles (95) even though the lowest number of individuals (28), with average of 10.3 alleles per locus. We detected 68 alleles in 73 individuals of P. macahense (average of 7.5 alleles per locus) and 81 alleles in 59 P. guineense individuals (average of 9.0 alleles). The locus mPgCIR 253 presented the highest number of alleles for P. guajava and P. guineense (16 alleles for each species), and the locus mPgCIR 10 presented the highest number of alleles for P. macahense (11 alleles). The loci mPgCIR 29, mPgCIR 253, and mPgCIR 162 presented the lowest number of alleles for P. guajava, P. macahense, and P. guineense (7, 3, and 5, respectively) (Supplemental file 3).
The allelic richness is used to measure the genetic diversity and is considered a key parameter for the genetic resource management. A higher number of alleles were identified in P. guajava populations; even P. macahense and P. guineense had approximately twice as many sampled individuals in relation to P. guajava. Evidences of P. guineense tetraploid origin from P. guajava were reported (Marques et al. 2016), as the genetic proximity of these two species by microsatellites (Tuler et al. 2015), phylogenetic markers (Tuler et al., 2015), and genomic SNPs approaches (Grossi et al.; 2021). In addition, the microsatellite markers used here are species specific for P. guajava and cross-species for P. guineense and P. macahense. Some studies related that the origin of the microsatellite markers may impact the estimates of genetic diversity (Fischer et al., 2017; Queirós et al. 2015). However, there are no species-specific microsatellite markers developed for the native species P. guineense and P. macahense.
The high number of alleles by microsatellites locus in P. guajava has already been demonstrated in the literature, as 9.56 alleles per locus detected in 70 accessions (Mehmood et al. 2016) and 8.9 alleles per locus were detected in 35 American P. guajava accessions and three related species, P. guineense, P. sartorianum, and P. friedrichsthalianum (Sitther et al. 2014). The average He of P. guajava was 0.83, and the Ho was 0.68. The average inbreeding coefficient was 0.17 (Supplemental file 3). Although the allelic richness of Psidium guajava populations, the inbreeding is relevant for the breeding approaches. In addition, Grossi et al (2021), also showed a higher diversity of P. guajava individuals of natural occurrence in relation to commercial/breeding genotypes.
The tetraploid nature of P. guineense reported by Marques et al. (2016) and Tuler et al. (2015) was corroborated by the alleles profile in different microsatellite loci (Supplemental file 4). An important appointment for P. macahense concerns the frequent detection of genotypes with 3 to 4 alleles suggesting the species polyploidy. In addition, this species occurs in the entire coastal region of Espírito Santo, with predominance in restinga areas, different from the other two species that are widely distributed in several environments (Tuler et al. 2015; Tuler et al., 2015). The restriction in environment numbers may result in lower genetic variability of alleles per locus (Loveless and Hamrick, 1984), what may have occurred with this species.
Psidium macahense and P. guineense were analyzed as polyploids (Supplemental file 4). The analyses considered allelic phenotypes, due to the impossibility of identifying all the possible genotypes. Altogether, 134 allelic phenotypes were identified in four P. macahense populations and 151 in the three P. guineense populations. Values of H’ per locus (analogous to He) ranged from 0.40 to 0.89, with an average of 0.79 in P. macahense, and for P. guineense ranged from 0.77 to 0.92 with an average of 0.84. The average genetic differentiation (F’ST) estimative was 0.19 for P. macahense and 0.12 for P. guineense (Supplemental file 3).
Regarding the populations, the average number of alleles in P. guajava ranged from 3.11 (PRK) to 7.22 (IOC) and a great number of private alleles (48) were detected in the populations, with notability to the IOC population (31) (Supplemental file 5). In P. guajava populations, He values ranged from 0.58 in (PRK) to 0.79 (IOC). Similar results were found in the literature, as He = 0.70 detected for P. guajava accessions in the United States (Sitther et al. 2014). Similar values of genic diversity were also found for other Myrtaceae species, as He = 0.70 for Myrtus communis (Nora et al. 2015) and He = 0.71 for Eugenia francavilleana (Ferreira et al. 2014). The Ho ranged from 0.61 (IOC) to 0.82 (PRK). The inbreeding coefficients were positive for the GUA and IOC populations (f = 0.03 and f = 0.24, respectively) indicating the presence of homozygotes. Nevertheless, this coefficient was negative for the PRK population (f = − 0.49) indicating partiality to the heterozygotes in this population (Supplemental file 5).
The diversity estimates based in allelic phenotypes for the P. macahense and P. guineense populations showed a wide genic diversity within populations from each species, where the H’ average for P. macahense populations was of 0.63, ranging from 0.55 (ITV) to 0.69 (PRK), and for P. guineense, 0.74, ranging from 0.69 (ITA) to 0.81 (GUA) (Supplemental file 5). Polyploidy may be associated with elevated variability (Hamrick 1979). This explains why P. macahense and P. guineense presented elevated diversity estimates. Similar results were found for the Myrtaceae family, showing that the elevated diversity detected by the microsatellites is a characteristic in Myrtaceae species (Ferreira et al. 2014; Sitther et al. 2014; Nora et al. 2015).
An average of 49.25 allelic phenotypes was observed for P. macahense populations, with the highest variation from allelic phenotypes detected in ITA (65), local which also presented the highest total number of alleles (Ae = 49). The lowest number of allelic phenotypes was presented by ITV (33), which also presented the lowest number of alleles (32). A higher average number of allelic phenotypes were detected in P. guineense (69.3) populations, with notability to the GUA population that presented a higher number of allelic phenotypes (74), with the highest number of alleles (63). The lowest average number of allelic phenotypes was observed for the PRK population (61) (Supplemental file 5).
The loci showed the high deviation from HW equilibrium (Supplemental file 6). The deviation in the HW proportions may indicate the presence of selection, mixture in the population or that the mating is not occurring randomly (Rousset and Raymond 1995).
Moderated genetic differentiation was observed for three populations of P. guajava (GST = 0.16, FST = 0.14), as well as inbreeding (FIS = 0.089). Psidium guajava is a species, which cross-pollination is the most frequent reproductive form (Alves and Freitas 2007); however, self-pollination has been reported, which may favor the appearance of homozygotes (Nimisha et al. 2013). The AMOVA for P. guajava populations indicated 84.93% of variation within populations (FST = 0.15, P > 0.01) and 15.07% of variation among populations. The elevated number of private alleles in P. guajava populations may be related to the diversity evidence within populations, with notability to the IOC population, which presented the highest number of private alleles. The FST values among P. guajava pairs of populations were all significant, showing divergence among all the populations. The lowest divergence occurred among IOC and GUA (0.11) populations and the highest among PRK and IOC (0.18) populations (Supplemental file 8).
For P. macahense, 72.55% of variation was indicated within populations (ØST = 0.27, P > 0.01) and 27.45% among populations and in P. guineense, 78.77% within populations (ØST = 0.21, P > 0.01) and 21.23% among populations (Supplemental file 7). These values may indicate elevated differentiation, which may be related to the presence of historic gene flow among populations and that may consequently be associated to the migration among regions (Fuchs and Hamrick 2010). For P. macahense populations, all the F’ST comparisons among populations were significant, with higher divergence observed among GUA and ITV (0.24) and lower between ITA and ITV (0.06). In the P. guineense populations, comparisons were not significant (Supplemental file 8). Collectively these results reflect the distribution pattern in the PCA analysis for the two species (Supplemental file 9). Also, we included a morphological analysis that sowed the proximity between P. guajava and P. guineense and the divergence of P. macahense in relation to the other two species (Supplemental file 10). This proximity of P. guajava and P. guineense and their divergence in relation the native Psidium species of occurrence in Brazil was also shown by SNPs markers analysis (Grossi et al., 2021).
Genetic Structure in P. guajava, P. macahense, and P. guineense Populations
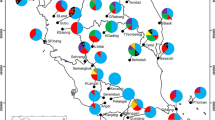
Regarding the genetic structure analysis, K = 2 was detected and this was considered the model that best described the genetic structure of the populations from the three species (Fig. 1 and Supplemental file 9). This structure reveals the existence of a defined pattern of geographical relations. For P. macahense and P. guineense, two groups were formed: the South and North regions populations. For P. guajava, all the populations came from the south region and also was the formation of two groups (GUA and PRK; IOC). The set of SSR markers used was enough to differentiate all the individuals.
For Psidium guajava, two groups are noticed (GUA and PRK; IOC); however, when K = 3 is used, IOC population was divided into two distinct groups. The population PRK was more homogeneous than the other populations (Fig. 1). The GUA population proceeds from an ecological reservation area (Paulo Cézar Vinha State Park) probably less injured by human actions. The PRK population, despite a little distant from GUA, was clustered into the same group. This probably happened because despite suffering intense human actions, PRK populations still present forestry remainders and well-preserved restinga shrubby formations, as well as wide areas that possess vegetation in distinct rehabilitation stages (Braz et al. 2013). The IOC population is located in an area closer to the coastal region and has been suffering an extensive real-state agency speculation, which unleashed a higher degradation throughout the years in the characteristic restinga vegetation of this local. When K = 3 was considered, the IOC population was divided into two distinct groups, what shows the great variability within this population, which showed higher number of private alleles and higher expected heterozygosity within P. guajava populations.
For P. macahense using K = 2, the populations of the south region (GUA and PRK) were clustered and the populations of the north region (ITV and ITA) were grouped into another group. With K = 4, GUA population formed a distinct group. With K = 5, populations ITV and ITA were differentiated into two other groups and with K = 6, and the populations shared even more alleles (Fig. 1).
When K = 2, two main groups were observed for P. guineense. The first group comprises GUA and IOC populations (south region), and the second group comprises ITA (north region). When K = 3, GUA forms a distinct group. With K = 4, GUA population still constitutes a separated group from IOC, however, sharing several alleles. As for the other species, as long as the K value was elevated, higher variability among populations was detected (Fig. 1 and Supplemental file 9).
When K = 3 was considered for P. guineense and K = 4 was considered for P. macahense, GUA population was separated into a distinct group. Because GUA population origins from a conservation area, these species may be more preserved than the others, demonstrating the importance of Conservation Units for the maintenance of the genetic diversity of the species.
Restingas are the coastal ecosystems that most suffer pressure from agricultural, industrial, and urban development, highlighting the need for in situ conservation strategies (Cunha 2005). In the State of Espírito Santo, an intense degradation with consequent reduction on several species populations is observed. Here, a set of microsatellite markers revealed inbreeding of three species of Psidium, moderately structure for P. guajava and elevated structure for native species (P. macahense and P. guineense). The Psidium genus has dominant species in this vegetation type, which is a disturbed environment of the Atlantic Forest, in Espírito Santo, Brazil. The same profile of structuration of the studied species suggests fragmentation of restinga in ES. Another information is the evidence of the poliployd nature of P. macahense, an endemic species, was the first time showed. This species had a more dissimilar profile of diversity detected. In addition, a higher structuration and lower diversity were showed for the considered polyploid native species (P. guineense and P. macahense) in relation to P. guajava, a cultivated specie. The higher polyploidy is not necessarily an indicative of genetic diversity, as indicated for P. cattleyanum (Machado et al. 2021). Then, our results may provide information that can be used in management and conservation strategies, to preserve the diversity of Psidium populations.
In conclusion, despite the high levels of intrapopulation genetic diversity, Psidium species are discontinued or heterogeneous in restingas, with at least two large populations distributed between the north and south of Espírito Santo for native species. The populations of the conserved area (GUA), for the three species, showed genetic differences from the remain populations, from not conserved areas, suggesting that degradation in the restingas also contributes with changes in the genetic structure of natural populations of Psidium, both for native and cultivated species. This suggests that management and conservation strategies, sustainable commercial exploitation, and breeding programs for the three Psidium species should aim at preserving the diversity of their populations. Thus, a more rigorous legislative agenda would guarantee the maintenance of existing biodiversity and protection of restingas, a fragile, low resilient, coastal, and disturbed ecosystem.
Data availability
The data file will be supplied at a new Supplementary File S10 if necessary.
References
Alves JE, Freitas BM (2007) Requerimentos de polinização da goiabeira. Ciência Rural 37:1281–1286
Assis AM, Thomas LD, Pereira OJ (2004) Florística de um trecho de floresta de restinga no município de Guarapari. Espírito St Acta Bot Bras 18:191–201
BFG (The Brazil Flora Group) (2015) Growing knowledge: an overview of seed Plant diversity in Brazil. Rodriguésia 66:1085–1113
Braz DM, Jacques EL, Somner GV, Sylvestre LS, Rosa MMT, Pereira-Moura MVL, Filho PG, Couto AVS, Amorim TA (2013) Restinga de Praia das Neves. ES. Brasil: caracterização fitofisionômica. florística e conservação. Biota Neotrop 13:315–331
Bruvo R, Michiels NK, D’souza TG, Schulenburg HA (2004) Simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol Ecol 13:2101–2106
Clark LV, Jasieniuk M (2011) Polysat: a R package for polyploid microsatellite analysis. Mol Ecol Res 11:562–566
R Core (2016) Team R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cruz CD (2013) GENES—A software package for analysis in experimental statistics and quantitative genetics. Acta Sci Agron 35(3):271–276
Cunha I (2005) Desenvolvimento sustentável na costa brasileira. Rev Galega De Econo 14:1–14
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2006) Arlequin version 3.01: an integrated software package for population genetics data analysis. Evol Bioinforma 1:47–50
Ferreira RR, Accoroni KAG, Rossi A, Guidugli MC, Mestriner MA, Martinez CA, Alzate-Marin AL (2014) Genetic diversity assessment for Eugenia unifloraL.E. pyriformis Cambess. E. brasiliensis Lam. and E. francavilleana O. Berg neotropical tree species (Myrtaceae) with heterologous SSR markers. Genet Resour Crop Evol 61:267–272
Fischer MC, Rellstab C, Leuzinger M et al (2017) Estimating genomic diversity and population differentiation—an empirical comparison of microsatellite and SNP variation in Arabidopsis halleri. BMC Genomics 18:69
Forest F, Crandall KA, Chase MW, Faith DP (2015) Phylogeny, extinction and conservation: embracing uncertainties in a time of urgency. Philos Trans R Soc B370:1–8
Fuchs EJ, Hamrick JL (2010) Spatial genetic structure within size classes of the endangered tropical tree Guaiacum sanctum (Zygophyllaceae). Am J Bot 97:1200–1207
Grossi LL, Fernandes M, Silva MA, Bernardes CO, Tuler AC, Santos PHD, Ferreira A, Ferreira MFS (2021) DArTseq-derived SNPs for the genus Psidium reveal the high diversity of native species. Tree Genet Genomes 17:23
Guedes D, Barbosa LM, Martins SE (2006) Composição florística e estrutura fitossociológica de dois fragmentos de floresta de restinga no Município de Bertioga.SP.Brasil. Acta Bot Bras 20:299–311
Hamrick JL (1979) Relationships between life history characteristics and electrophoretically detectable genetic variation in plants. Annu Rev Ecol Evol Syst 10:173–200
IBGE (Instituto Brasileiro de Geografia e Estatística). Manual Técnico da Vegetação Brasileira. Rio de Janeiro. 2012.ftp://geoftp.ibge.gov.br/documentos/recursos_naturais/manuais_tecnicos/manual_tecnico_vegetacao_brasileira.pdf. Accessed01 December2014
Kaeuffer R, Réale D, Coltman DW, Pontier D (2007) Detecting population structure using STRUCTURE software: effect of background linkage disequilibrium. Heredity 99:374–380
Lewis PO and E Zaykin D (2001) GDA – Genetic Data Analysis: Computer program for the analysis of allelic data. Version 1.0 (d16c). http://lewis.eeb.uconn.edu/lewishome/software.html. Accessed12 December 2016
Li L, Lin H, Tang W, Liu D, Bao B, Yang J (2017) Population genetic structure in wild and aquaculture populations of Hemibarbus maculates inferred from microsatellites markers. Aquac Fish 2:78–83
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Syst 15:65–95
Machado RM, Oliveira FA, Alves FM, Souza AP, Forni-Martins ER (2021) Population Genetics of Polyploid Complex Psidium cattleyanum Sabine (Myrtaceae): Preliminary Analyses Based on New Species-Specific Microsatellite Loci and Extension to Other Species of the Genus. Biochem Genet 59:219–234
Marques AM, Tuler AC, Carvalho CR, Carrijo TT, Ferreira MFS, Clarindo WR (2016) Refinement of the karyological aspects of Psidium guineense (Swartz. 1788): a comparison with Psidium guajava (Linnaeus. 1753). Comp Cytogenet 10:117–128
Mehmood A, Luo S, Ahmad NM, Dong C, Mahmood T, Sajjad Y, Jaskani MJ, Sharp P (2016) Molecular variability and phylogenetic relationships of guava (Psidium guajava L.) cultivars using inter-primer binding site (iPBS) and microsatellite (SSR) markers. Genet Resour Crop Evol 63:1345–1361
Meirmans PG, Liu S, van Tienderen PH (2018) The analysis of polyploid genetic data. J Heredity 109:283–296
Mittermeier RA, Gil RP, Hoffman M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, Fonseca GAB (2005) Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions, 2nd edn. University of Chicago Press, Boston
Myrtaceae in Flora do Brasil 2020.Jardim Botânico do Rio de Janeiro. Disponível em: <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB171>. Acesso em: 27 out. 2021
Nawaz MA, Yang SH, Rehman HM, Baloch FS, Lee JD, Park JH, Chung G (2017) Genetic diversity and population structure of Korean wild soybean (Glycine soja Sieb. and Zucc.) inferred from microsatellite markers. Biochem Syst Ecol 71:87–96
Nimisha S, Kherwar D, Ajay KM, Singh B, Usha K (2013) Molecular breeding to improve guava (Psidium guajava L.): Current status and future prospective. Sci Hortic 164:578–588
Nora S, Albaladejo RG, Aparicio A (2015) Genetic variation and structure in the Mediterranean shrubs Myrtus communis and Pistacia lentiscus in different landscape contexts. Plant Biol 17:311–319
Obbard DJ, Harris SA, Pannell JR (2006) Simple allelic-phenotype diversity and differentiation statistics for allopolyploids. Heredity 97:296–303
Polysat. Tools for polyploidy microsatellite analysis. calcFst: Estimate Population Differentiation Statistics. https://rdrr.io/cran/polysat/man/calcFst. html. Accessed 15 January2017
Pritchard JK, Stephens M, Donnelly PJ (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Proença CEB, Costa IR and Tuler AC (2020) Psidium in Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro. Available em: <http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB10853>. Access: 05 out. 2021
Queirós J, Godinho R, Lopes S, Gortazar C, de la Fuente J, Alves PC (2015) Effect of microsatellite selection on individual and population genetic inferences: anempirical study using cross-specific and species-specific amplifications. Mol Ecol Resour 15:747–760
Ramu P, Billot C, Rami JF, Ve SS, Upadhyaya HD, Reddy LA, Hash CT (2013) Assessment of genetic diversity in the sorghum reference set using EST-SSR markers. Theor Appl Genet 126:2051–2064
Ribeiro MC, Martensen AC, Metzger JP, Tabarelli M, Scarano FR, Fortin MJ (2011) The Brazilian Atlantic forest: a shrinking biodiversity hotspot. In: Zachos FE, Habel JC (eds) Biodiversity hotspots. Springer, Heidelberg, pp 405–434
Risterucci AM, Duval MF, Rohde W, Billotte N (2005) Isolation and characterization of microsatellite loci from Psidium guajava L. Mol Ecol Notes 4:745–748
Rousset F, Raymond M (1995) Testing heterozygote excess and deficiency. Genetics 140:1413–1419
Sitther V, Zhang D, Harris DL, Yadav AK, Zee FT, Meinhardt LW, Dhekney SA (2014) Genetic characterization of guava (Psidium guajava L.) germplasm in the United States using microsatellite markers. Genet Resour Crop Evolut 61:829–839
Stehmann J, Forzza RC, Sobral MandKamino LHY (2009) Plantas da Floreta Atlântica. Jardim Botânico do Rio de Janeiro Press, Rio de Janeiro
Tuler AC, Carrijo TT, Nóia LR, Ferreira A, Peixoto AL, Ferreira MFS (2015) SSR markers: a tool for species identification in Psidium (Myrtaceae). Mol Biol Rep 42:1501–1513
WCSP (2020) World Checklist of Selected Plant Families. https:// apps.kew.org/wcsp/. Accessed 06 January 2020
Weir BS (1996) Genetic data analysis II: methods for discrete population genetic data. Sunderland. Sinauer Associates, Massachusetts
Weir BS, Hill WG (2002) Estimating F-statistics. Annu Rev Genet 36:721–750
Wright S (1951) The genetical structure of populations. Annu Eugen 15:223–354
Acknowledgements
The authors thank Conselho Nacional de Pesquisa (CNPq, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil)—Finance Code 001, Fundação de Amparo à Pesquisa do Espírito Santo FAPES, Vitória—ES, Brazil), and VALE for providing financial support to this study.
Funding
The CNPq, FAPES/VALE and CAPES provided funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Oliveira Bernardes, C., Tuler, A.C., Canal, D. et al. Genetic Diversity and Population Structure of Psidium Species from Restinga: A Coastal and Disturbed Ecosystem of the Brazilian Atlantic Forest. Biochem Genet 60, 2503–2514 (2022). https://doi.org/10.1007/s10528-022-10222-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-022-10222-7